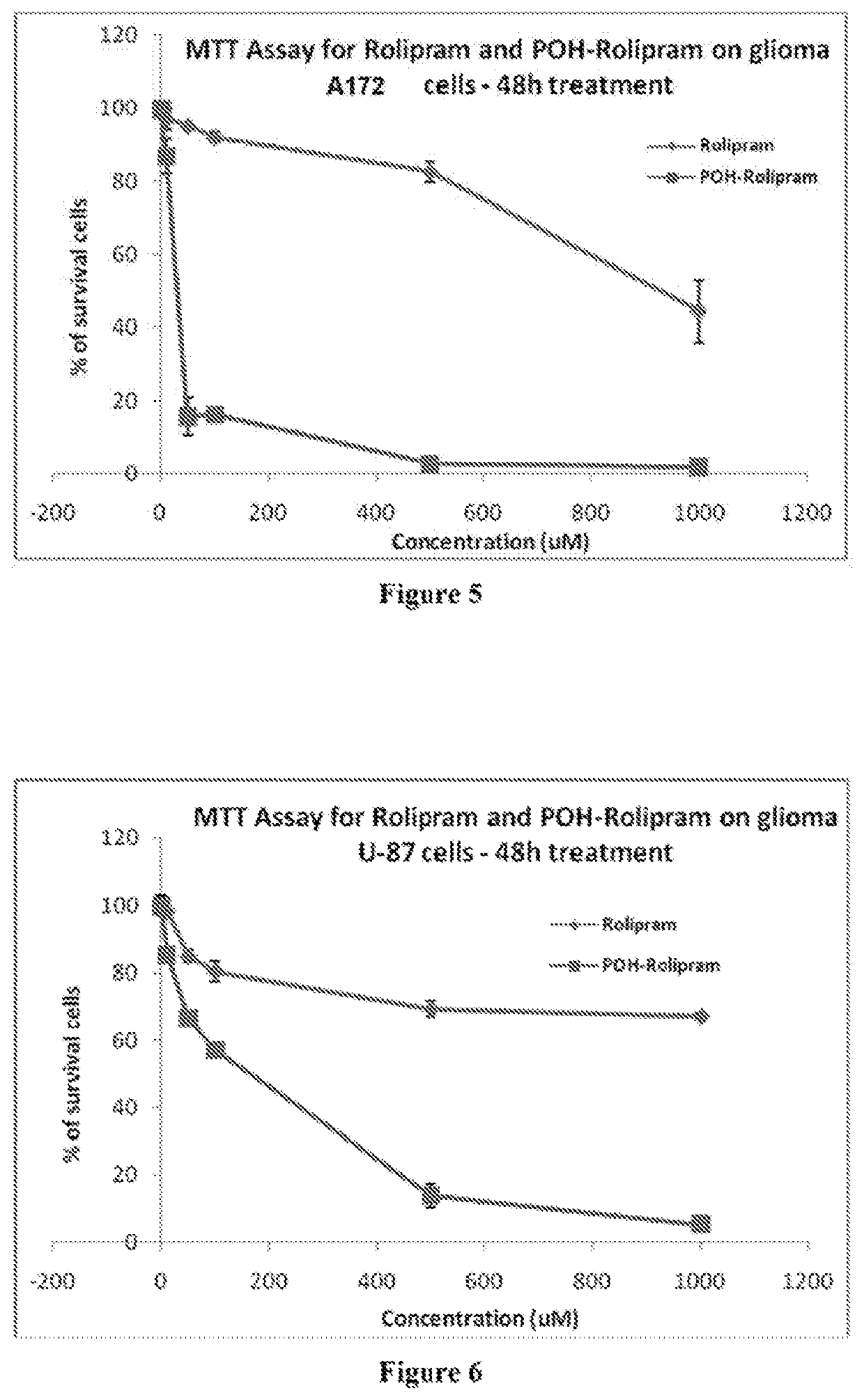
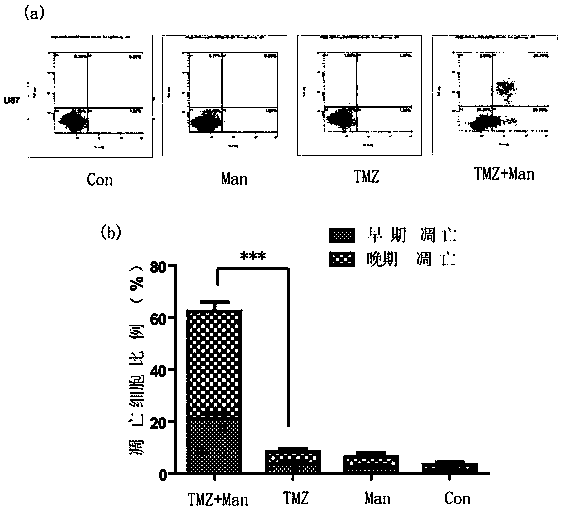
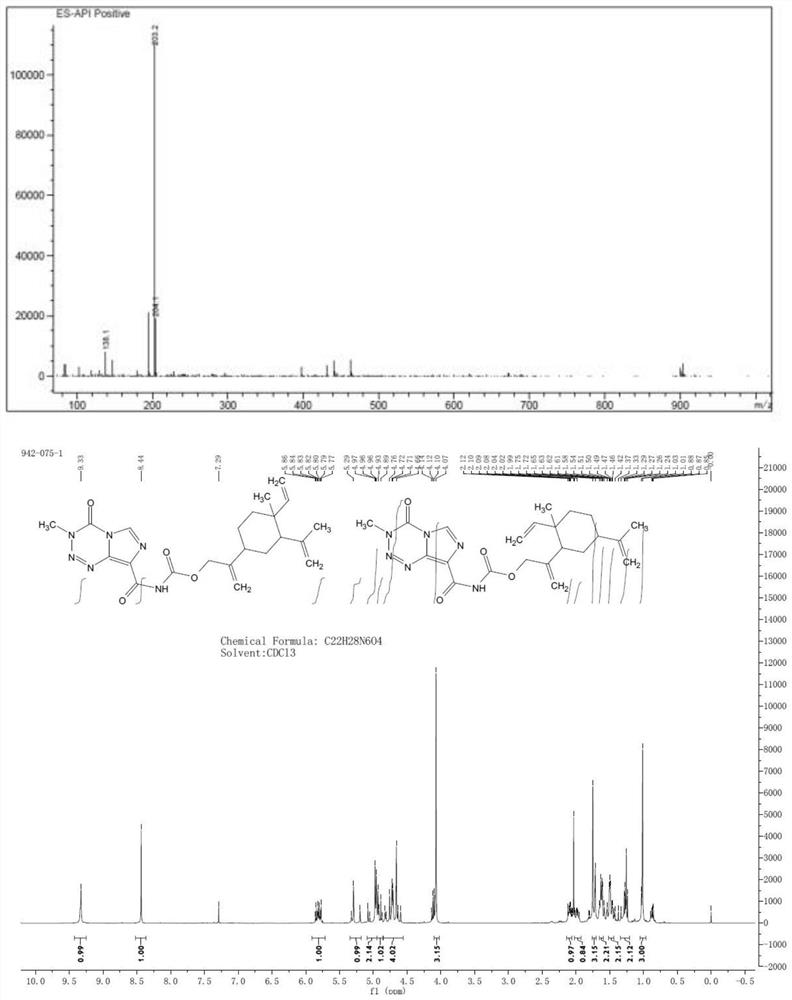
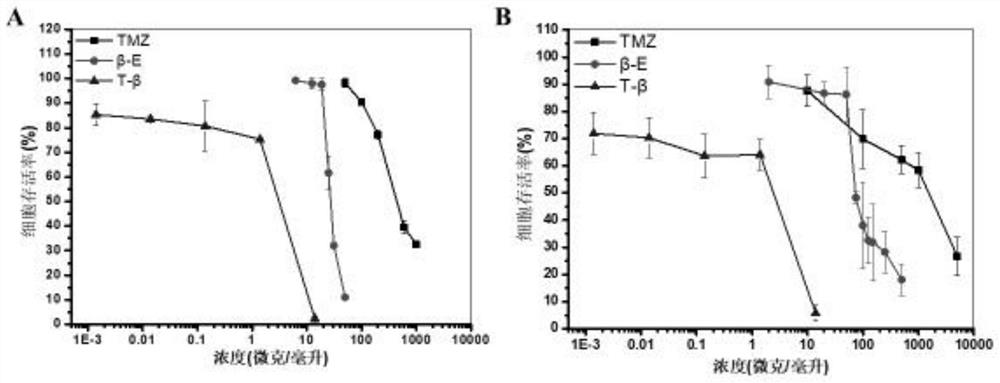
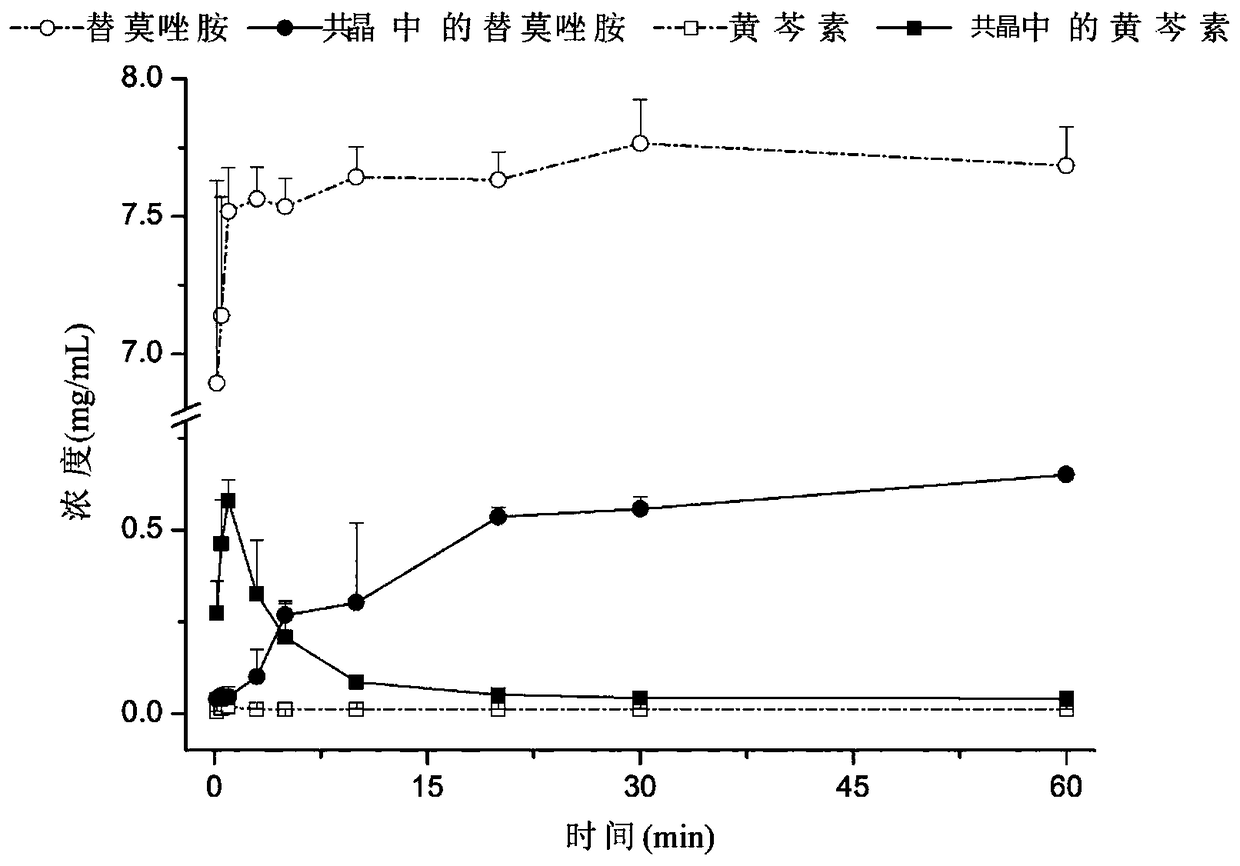
Patents
Literature
90 results about "Muzolimine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Muzolimine is a High-ceiling loop diuretic. It is a pyrazole diuretic which was used for treatment of hypertension but was withdrawn worldwide because of severe neurological side effects.
Methoxyamine potentiation of temozolomide anti-cancer activity
InactiveUS6465448B1Prevents APE cleavageDisrupting DNA repairBiocideAnimal repellantsMethoxyamineTemozolomida
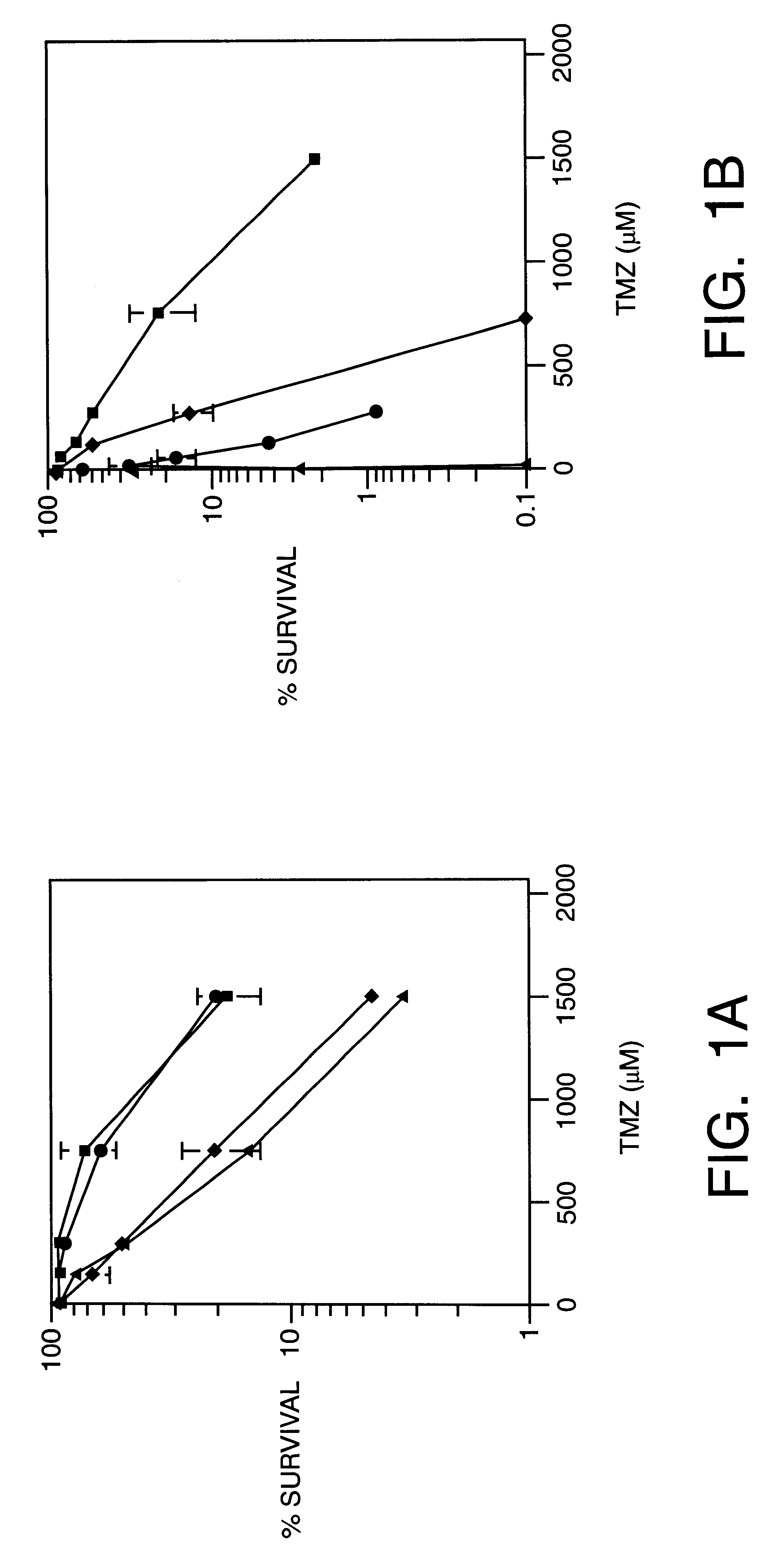
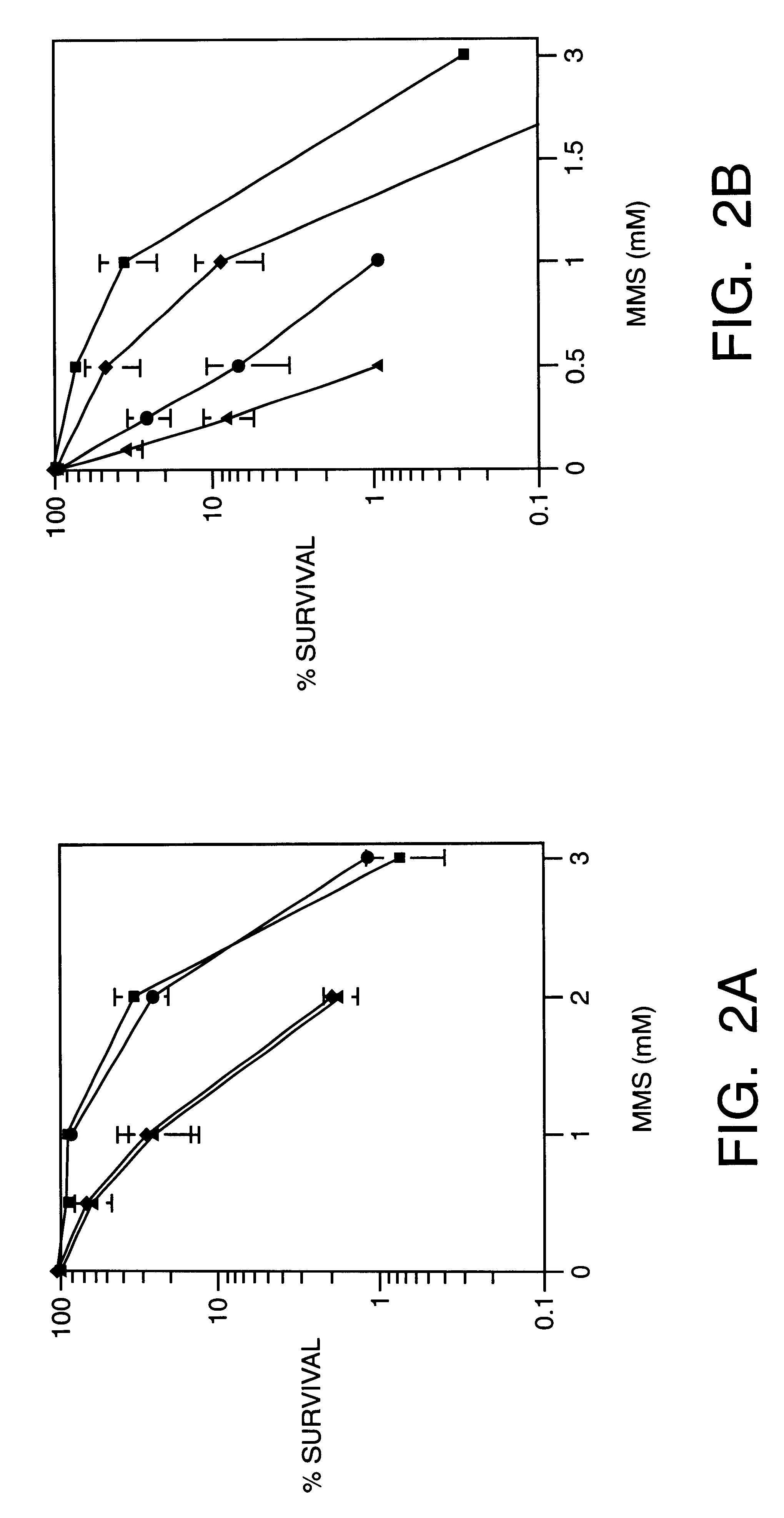
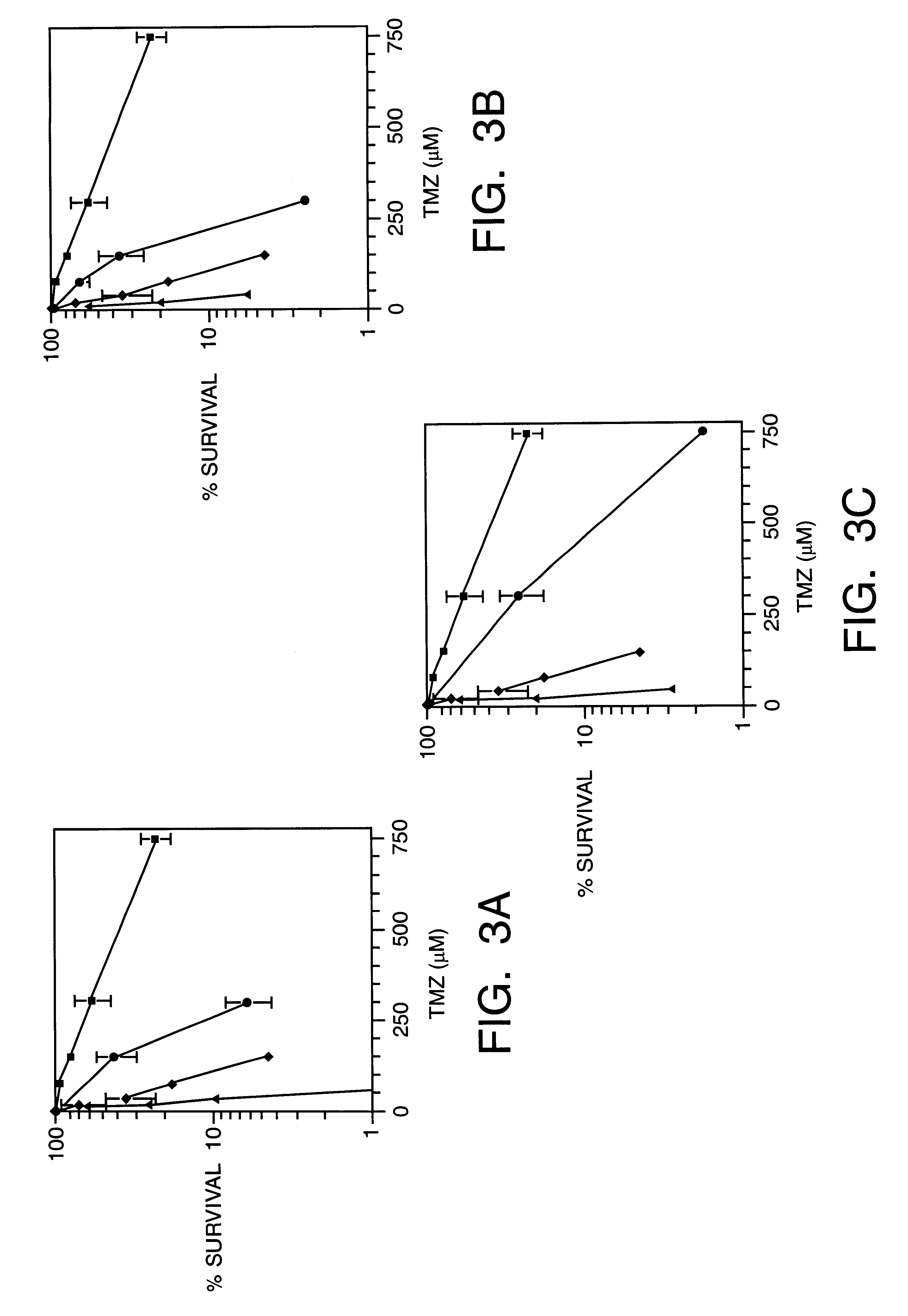
This invention generally relates to novel compositions and methods for the treatment of certain cancers. Additionally, this invention relates to novel compositions and methods to screen drugs for the treatment of certain cancers. Specifically, the invention contemplates that temozolomide and methoxyamine, in combination or in sequence, shall be used as a treatment for certain tumors that are resistant to treatment by temozolomide alone.
Owner:CASE WESTERN RESERVE UNIV
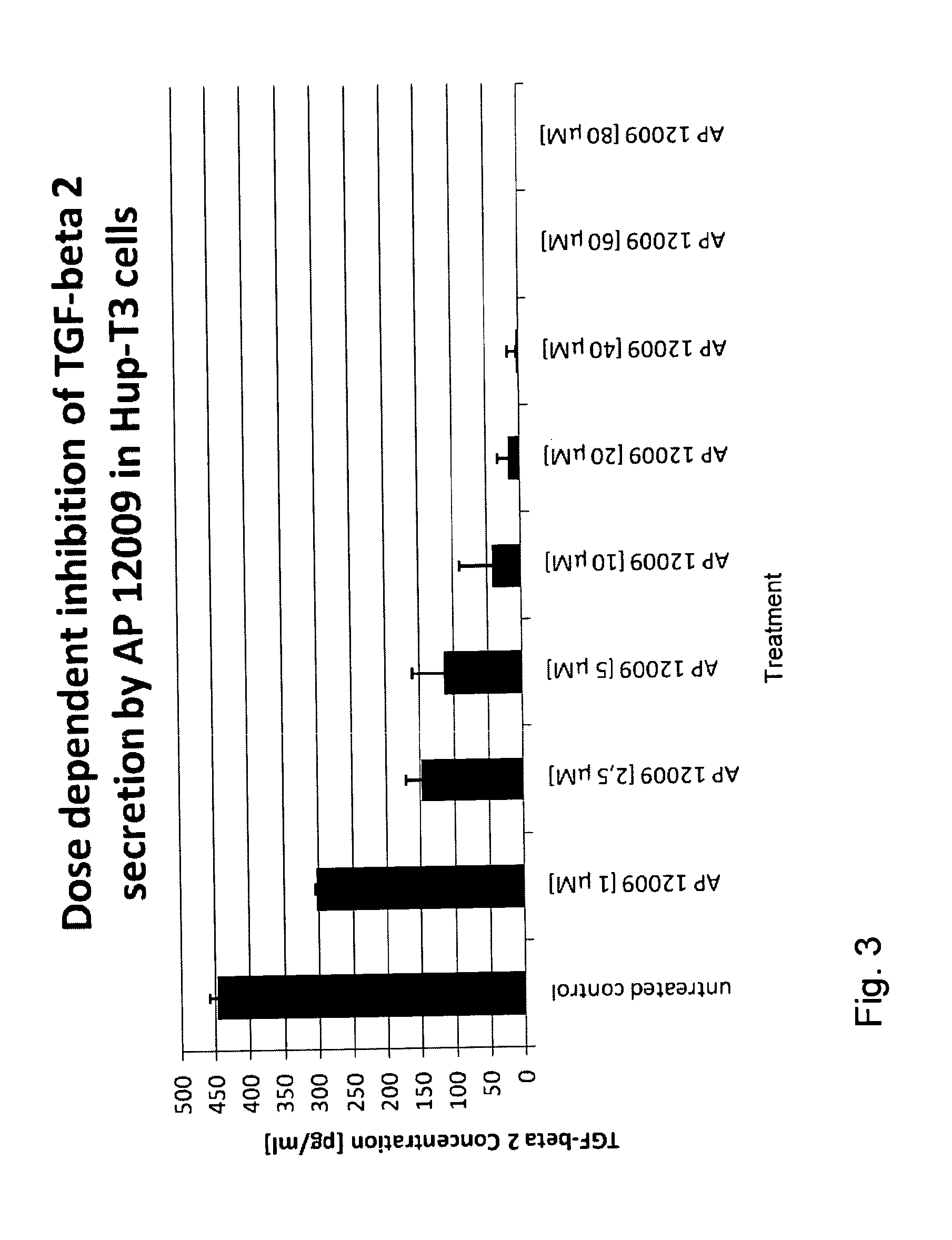
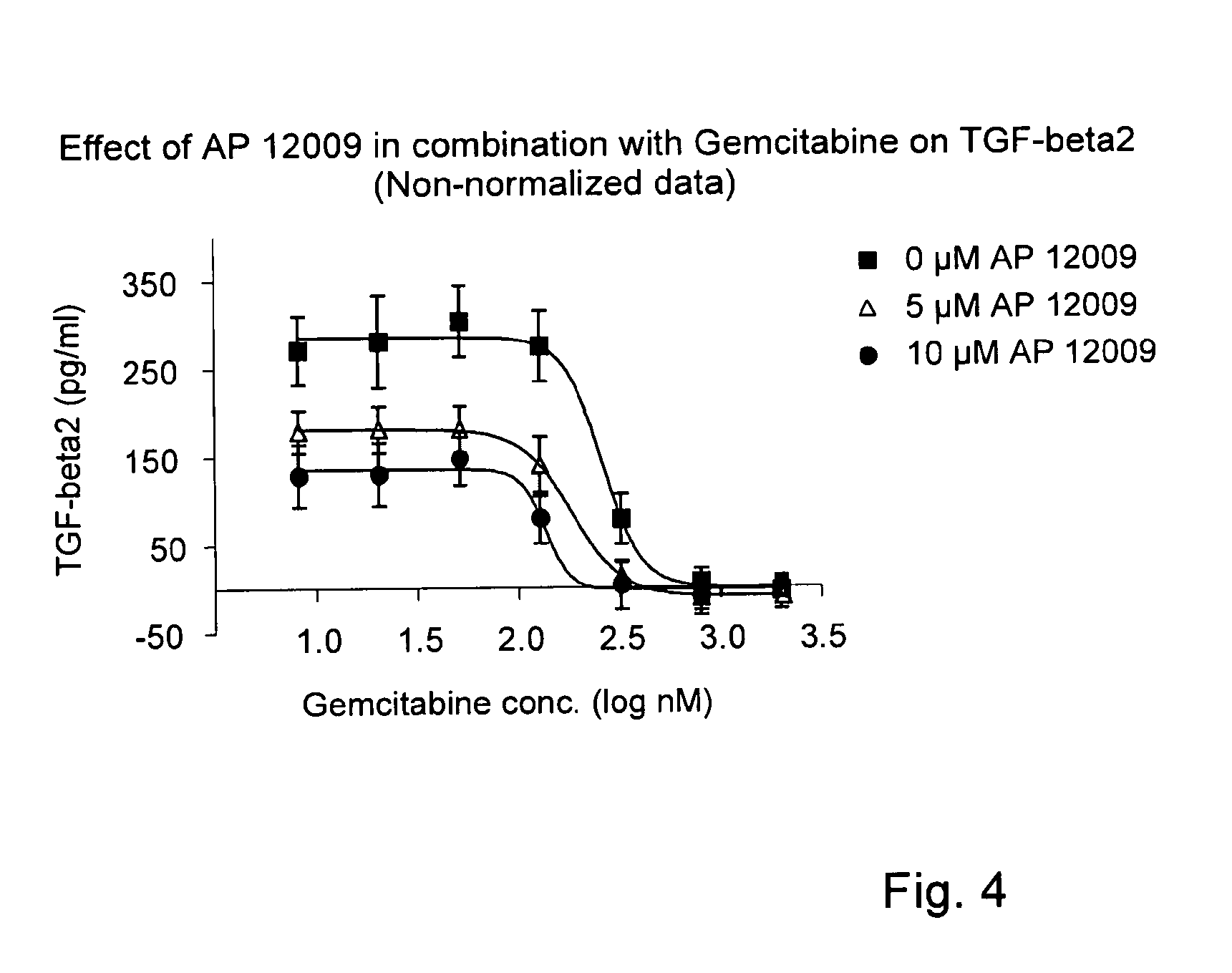
Combination of a chemotherapeutic agent and an inhibitor of the TGF-beta system
ActiveUS8476246B2Reduce IC50Improve efficiencyBiocideOrganic active ingredientsDocetaxel-PNPDocetaxel
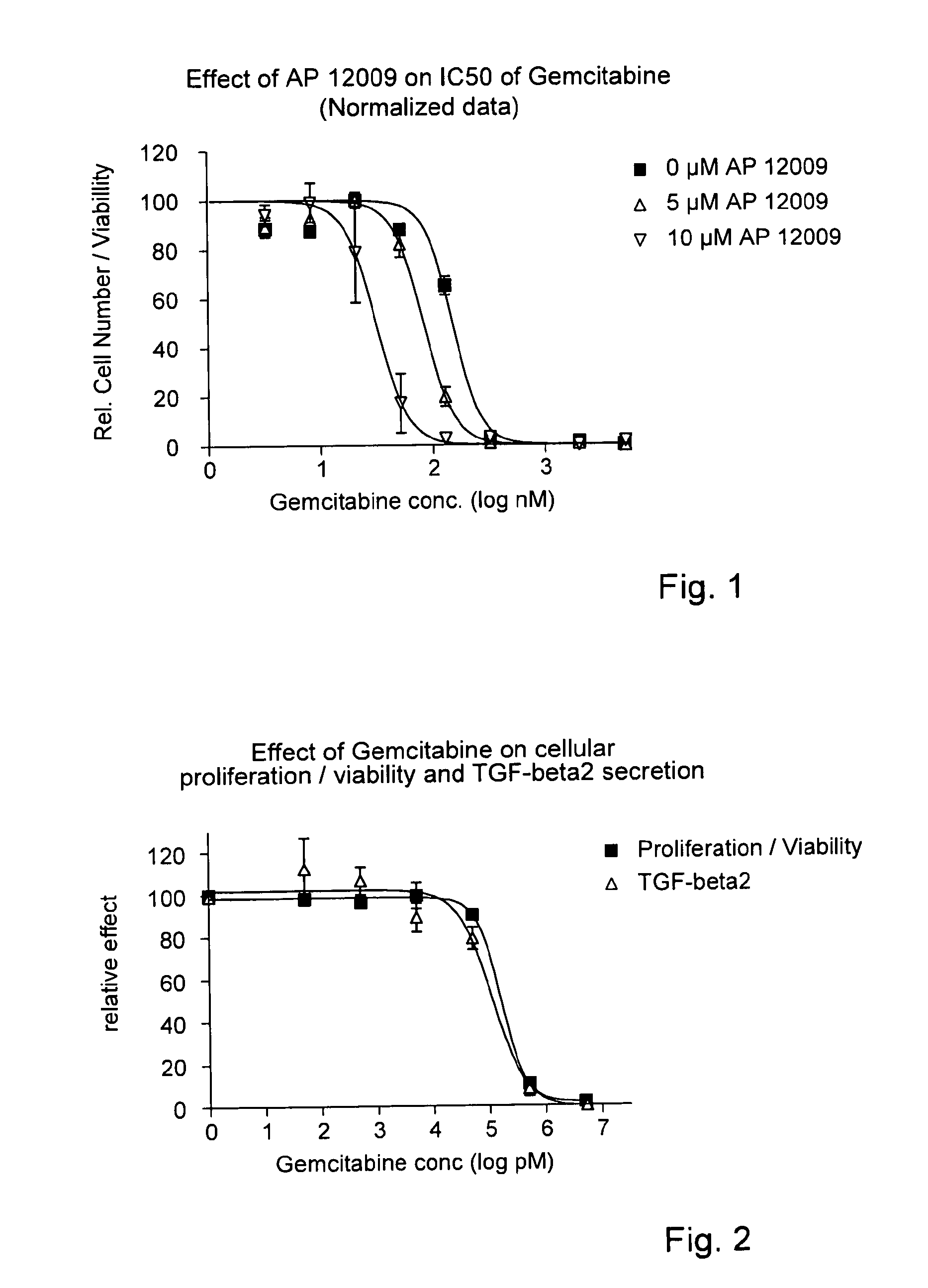
Pharmaceutical composition comprising a chemotherapeutic agent and a TGF-beta antisense oligonucleotide, wherein the antisense oligonucleotide reduces the sensitivity and IC50, respectively, of the cytotoxicity of the chemotherapeutic agent. Preferably, the antisense oligonucleotide is a TGF-beta 1, 2, and / or 3 antisense oligonucleotide and the chemotherapeutic agent is preferably gemcitabine, 5-fluorouracil, temozolomide, dacarbacine, docetaxel, cisplatin, oxaliplatin, tamoxifen, or irinotecan.
Owner:ANTISENSE PHARMA GMBH
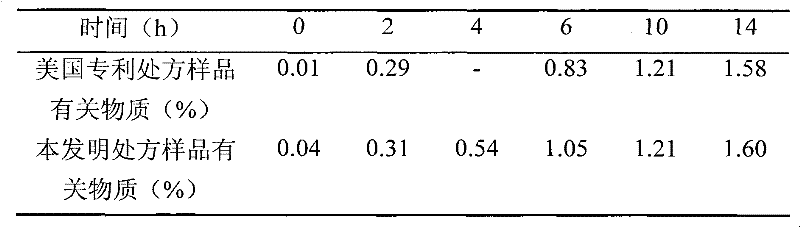
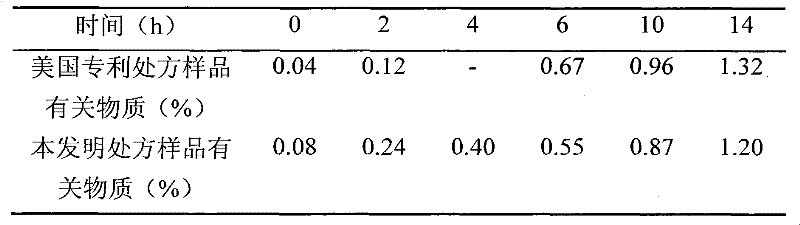
Injectable parenteral medicinal preparation of temozolomide and preparation method thereof
ActiveCN102342931AImprove stabilityEasy to acceptOrganic active ingredientsPowder deliveryVitamin CPharmaceutical formulation
The invention relates to an injectable parenteral medicinal preparation of temozolomide and a preparation method thereof. The medicinal preparation comprises (1) temozolomide or pharmaceutically acceptable salt thereof, (2) at least one stabilizer, and (3) at least one aqueous diluent, wherein the stabilizer is selected from L-alanine, L-glycine, L-cysteine, L-cysteine hydrochloride anhydride, L-cysteine hydrochloride monohydrate, acetyl cysteine, S-carboxymethyl-L-cysteine, L-ethyl cysteine hydrochloride, L-methyl cysteine hydrochloride, vitamin C or a mixture thereof. The invention further relates to lyophilized power containing the medicinal preparation and products thereof.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Controlled releases system containing temozolomide
InactiveUS8821913B2Eliminate inconvenienceBioactivity can be achievedPill deliveryAntineoplastic agentsControl releaseTemozolomida
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
Taxane analogs for the treatment of brain cancer
ActiveUS20110318334A1High activityImprove solubilityBiocideOrganic active ingredientsBevacizumab InjectionMammal
Provided herein are compounds and methods for the treatment of brain cancer in a mammal, wherein the method comprises the administration to the mammal a compound that stabilizes tubulin dimers or microtubles at G2-M interface during mitosis but is not a substrate for MDR protein. In particular, the present application relates to the use of an orally effective abeo-taxane, alone or in combination with temozolomide or bevacizumab, for the treatment of brain cancer.
Owner:TAPESTRY PHARMACEUTICALS INC
Double-element solution type preparation for intravenous injection and intracerebral injection
InactiveCN101467967AAddress long-term stabilityUse meets the requirementsOrganic active ingredientsPharmaceutical delivery mechanismActive componentRoom temperature
The invention discloses a binary solution type formulation for intravenous and intracerebral injection, shown as right. The main active component thereof is temozolomide with the binary system and having temozolomide sterile powder and solvent for dissolving medicaments. The operating temozolomide solution agent can be obtained through the combination of the solvent. The invention is characterized in that, 1, the binary formulation is stable at the room temperature for over 2 years; 2, the prepared medicaments liquid is stable at the room temperature at least for 48h to meet the operating requirement of injection administration; 3, the solvent is nontoxic and non-irritant, and the prepared medicaments can be used for the intracerebral injection during the operation and stereotaxic intraturmor injection of local chemotherapy.
Owner:北京京卫燕康药物研究所有限公司
Unit dosage forms of temozolomide
InactiveUS20080319039A1Reduced pill burdenPatient compliance is goodOrganic active ingredientsBiocideDiseaseTemozolomida
This invention relates to unit dosage forms of temozolomide. These unit dosage forms are particularly well-suited for decreasing the pill burden and increasing patient compliance. The invention also relates to methods of treating proliferative disorders in a patient with these unit dosage forms. The invention additionally relates to kits comprising these unit dosage forms.
Owner:MERCK SHARP & DOHME CORP
Pharmaceutical composition for restraining tumors
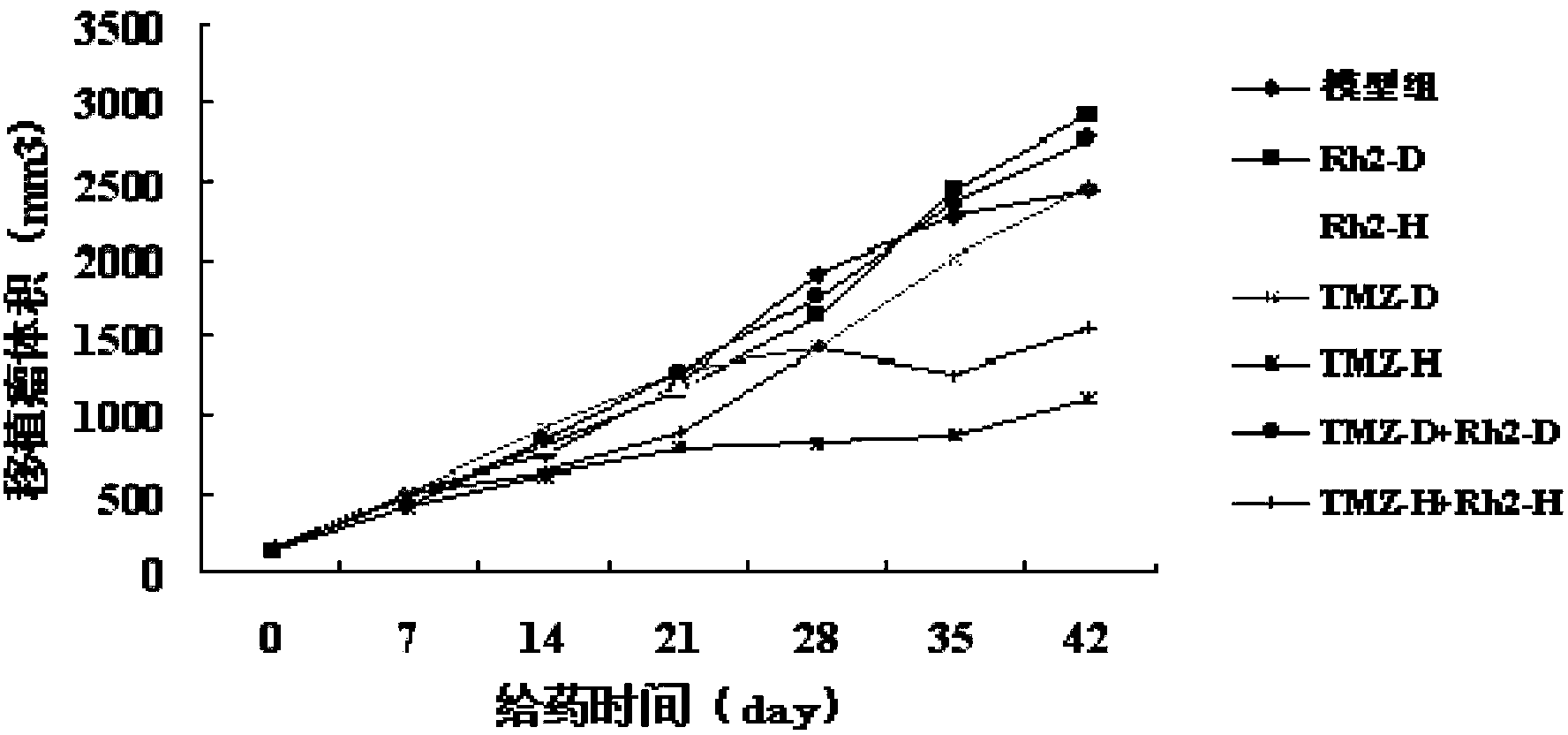
The invention relates to a pharmaceutical preparation, in particular to a compound pharmaceutical preparation with ginsenoside Rh2 and temozolomide as medicine active ingredients.
Owner:TIANJIN TASLY PHARMA CO LTD
Treatment of cancers using a combination comprising parp inhibitors, temozolomide and/or radiation therapy
InactiveUS20200155567A1More responseOrganic active ingredientsPharmaceutical delivery mechanismTemozolomidaPharmaceutical drug
Disclosed herein is a method for the prevention, delay of progression or treatment of cancer in a subject, comprising administering to the subject in need thereof a PARP inhibitor, particularly, (R)-2-fluoro-10a-methyl-7, 8, 9, 10, 10a, 11-hexahydro-5, 6, 7a, 11-tetraazacyclohepta [def] cyclopenta [a] fluoren-4 (5H)-one, a sesqui-hydrate thereof, or a pharmaceutically acceptable salt thereof, in combination with temozolomide and / or radiation therapy. Also, disclosed a pharmaceutical combination comprising a PARP inhibitor, particularly, (R)-2-fluoro-10a-methyl-7, 8, 9, 10, 10a, 11-hexahydro-5, 6, 7a, 11-tetraazacyclohepta [def] cyclopenta [a] fluoren-4 (5H)-one, a sesqui-hydrate thereof, or a pharmaceutically acceptable salt thereof, in combination with temozolomide and the use thereof.
Owner:BEIGENE SWITZERLAND GMBH
Sustained release temozolomide capsules and preparation method thereof
ActiveCN108014097AConstant dissolutionConstant dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsAntioxidantDissolution
The invention relates to sustained release temozolomide capsules and a preparation method thereof, and belongs to the technical field of medicine. Each sustained release temozolomide capsule comprises2-20 sustained release micro-tablets and a capsule shell, wherein each micro-tablet contains 2-20 mg of temozolomide. The sustained release micro-tablets comprise raw and auxiliary materials in percentage by weight as follows: 5%-50% of temozolomide, 5%-40% of a diluent, 10%-70% of a sustained release agent, 0.5%-2% of an antioxidant, 0-5% of an adhesive and 0.5%-2% of a lubricant. The inventionfurther discloses the preparation method of the sustained release temozolomide capsules. The materials are pelletized or mixed and pressed into the micro-tablets directly, and the micro-tablets are loaded to hollow capsules quantitatively. The sustained release temozolomide capsules have the advantages of being good in dissolution, convenient to use, stable in plasma concentration and long in acting time, compliance of patients is improved, the preparation process is simple and lower in cost, and industrial production is easy.
Owner:BEIJING SL PHARMA +1
Methods of treating neurofibromatosis with perillyl alcohol
ActiveUS11147809B2Hydroxy compound active ingredientsPharmaceutical delivery mechanismCarbamateNeurofibra
Owner:UNIV OF SOUTHERN CALIFORNIA
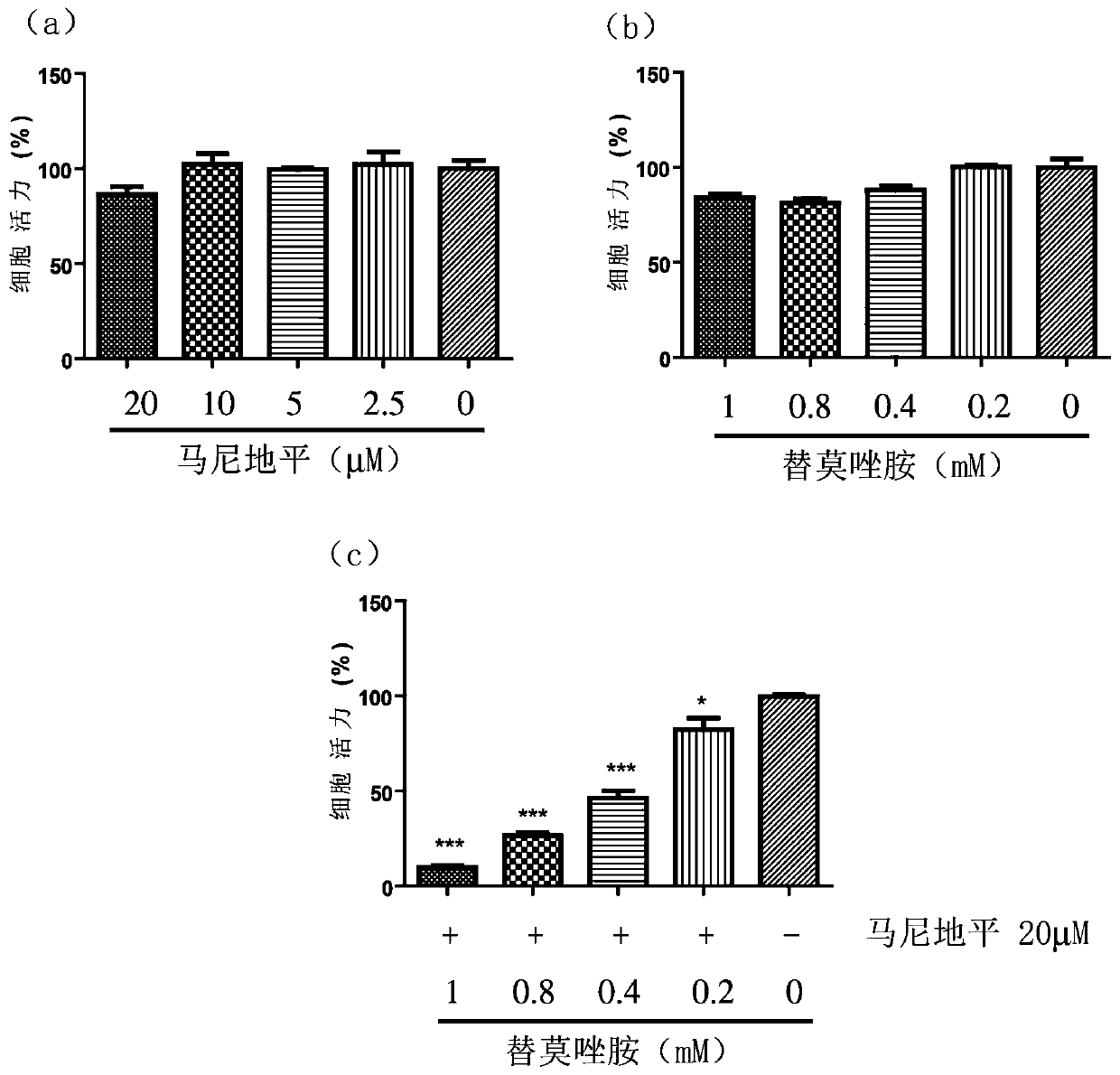
Application of manidipine combined temozolomide and pharmaceutical composition thereof in preparation of drug for treating brain glioma
InactiveCN110960533AGood treatment effectLower doseOrganic active ingredientsNervous disorderPharmaceutical drugTherapeutic effect
Owner:SUZHOU QINGYA QIRUI BIOTECHNOLOGY CO LTD
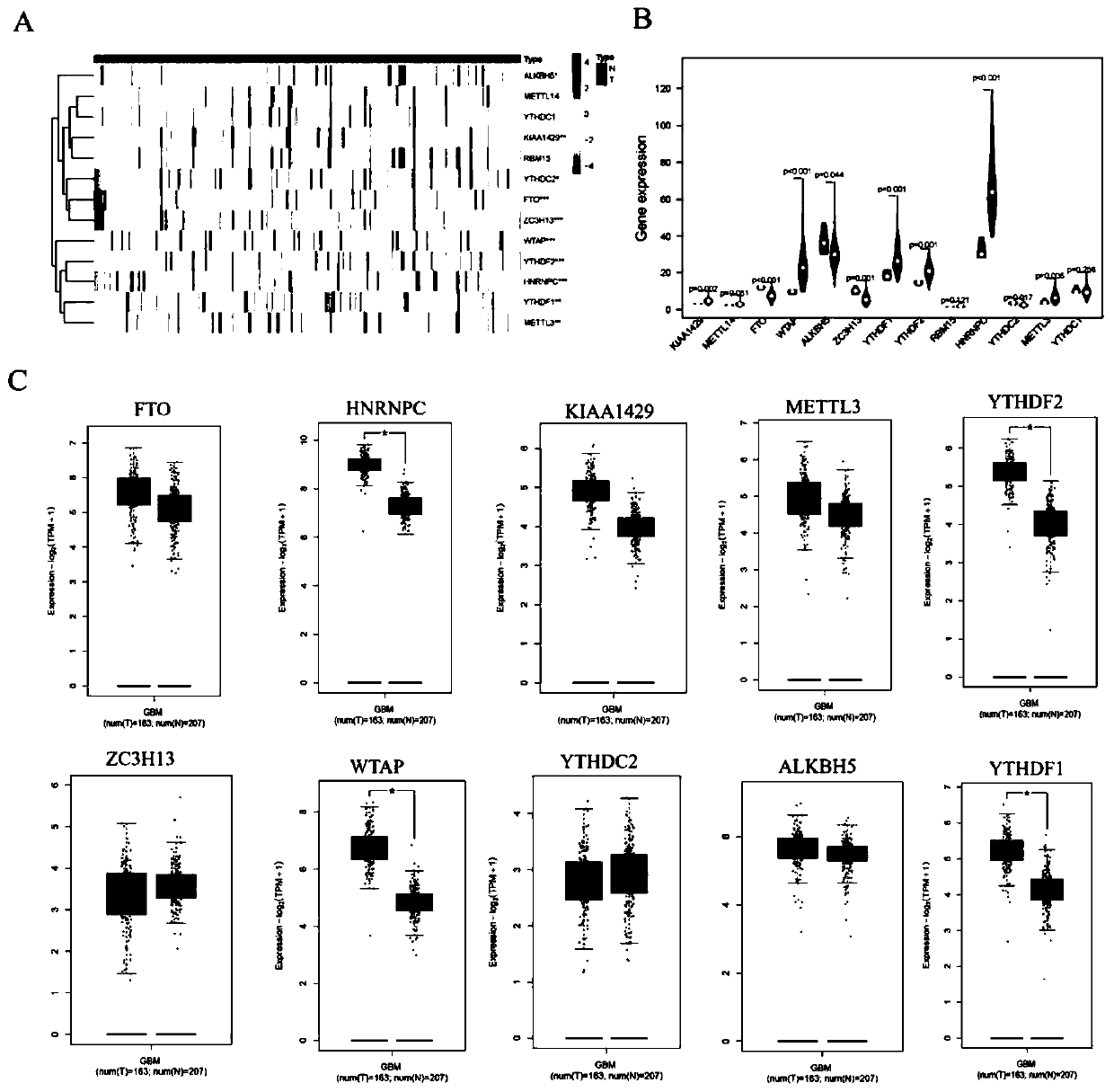
Glioma malignant progress and survival prognosis detection molecular marker, temozolomide drug resistance detection target and application
PendingCN111534596AIncrease drug sensitivitySurvival predictionMicrobiological testing/measurementMalignancyMalignant progression
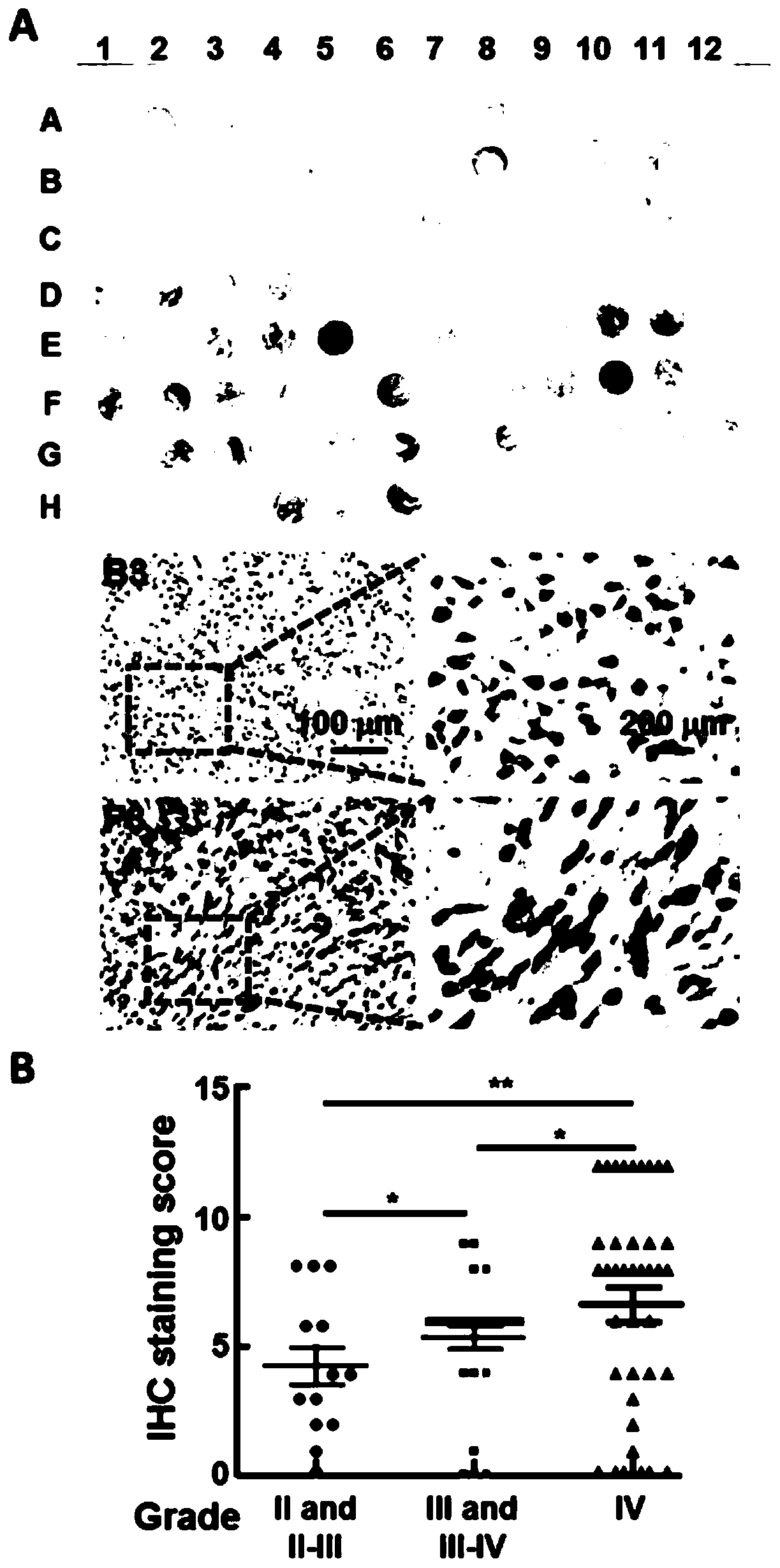
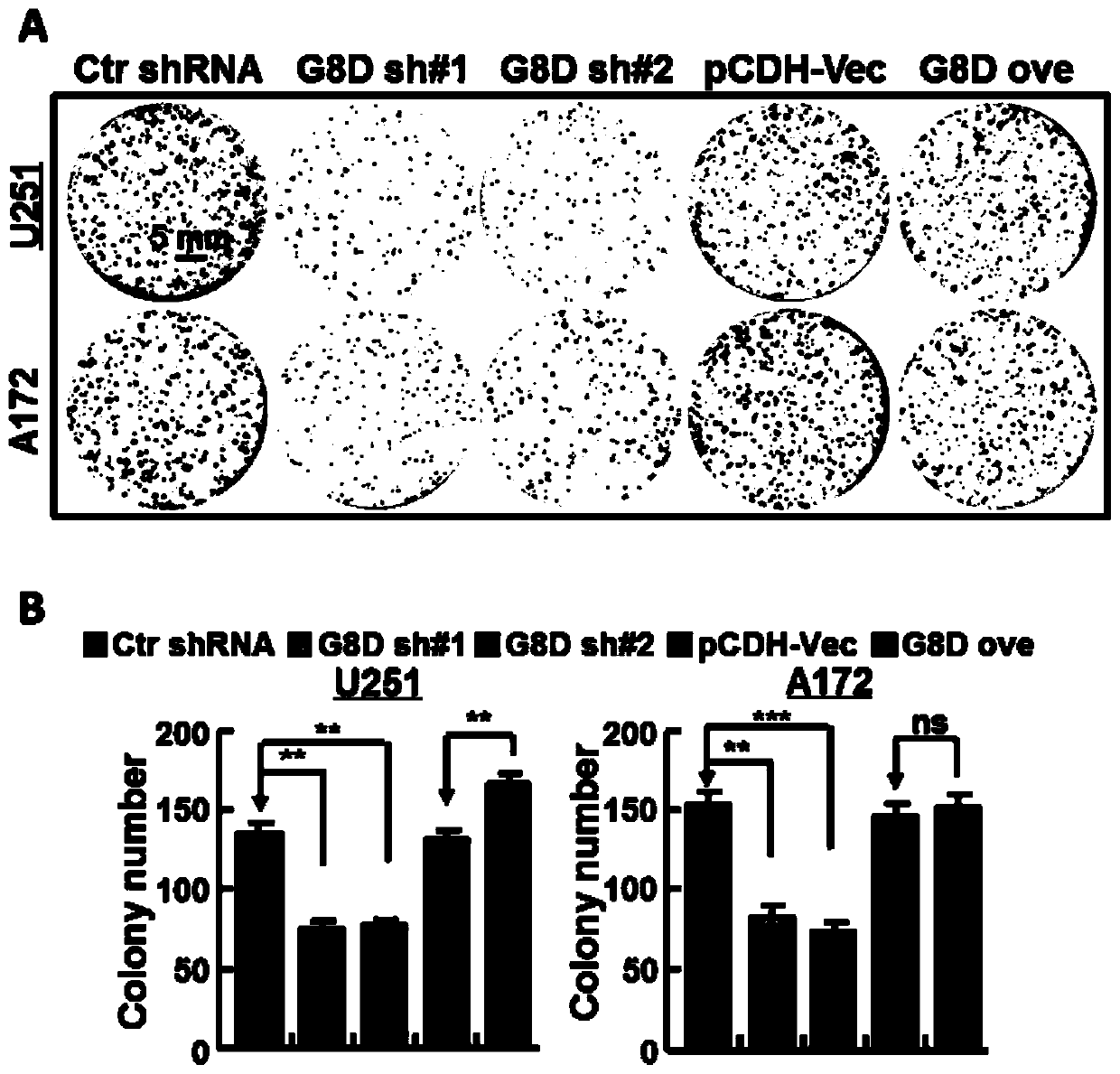
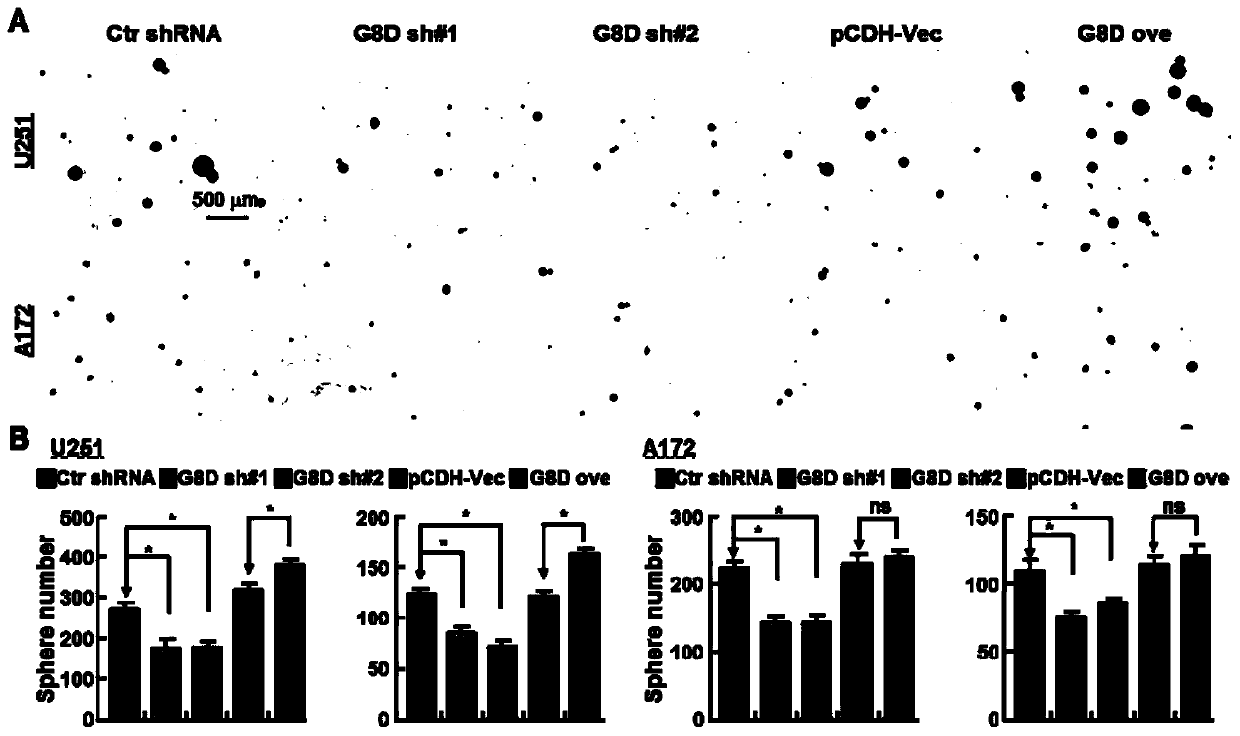
The invention belongs to the technical field of biomedicine, and particularly relates to a glioma malignant progress and survival prognosis detection molecular marker, a temozolomide drug resistance detection target and application. An m6A methylation reader HNRNPC which is highly expressed in glioma tissue and closely related to the tumor occurrence and development process is screened out by a bioinformatics method, further analysis finds that the expression level of the m6A methylation reader HNRNPC is closely related to the survival rate and prognosis of patients, and through clinical masstumor tissue sample verification, the m6A methylation reader HNRNPC can be used as a molecular marker and an independent survival prognosis factor for glioma malignant progress. Meanwhile, it is foundthat knockout of the HNRNPC can increase medicine sensitivity of glioma cells to temozolomide. A new diagnosis, treatment and prognosis predicting target is provided for glioma treatment, and a new molecular target can be provided for improving temozolomide drug resistance.
Owner:江西省肿瘤医院
Pharmaceutical Composition Comprising Temozolomide Ester
InactiveUS20080044457A1Inhibition effectGood killing effectBiocideOrganic chemistryTransdermal patchAbnormal tissue growth
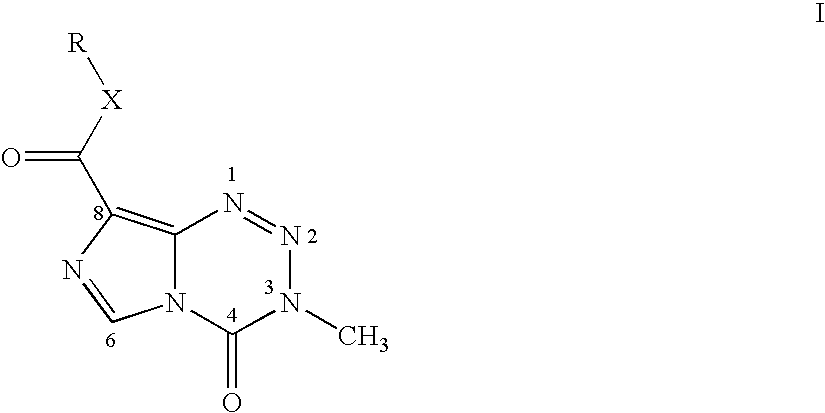
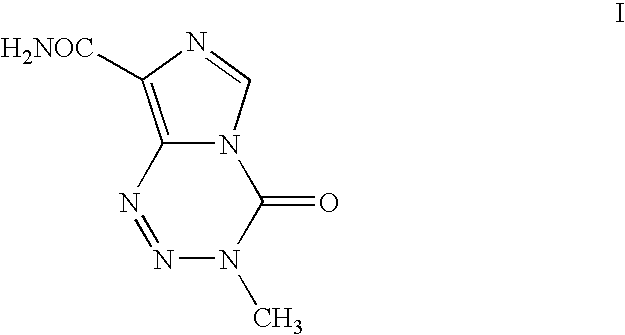
The present invention discloses general formula I of Temozolomide-8-carboxylate compounds, the process for preparation, pharmaceutical compositions comprising the compounds and the use of the compounds and pharmaceutical compositions for the manufacture of an antitumor medicament. The said pharmaceutical composition comprises one or more general formula I Temozolomide-8-carboxylate compounds as active ingredient, together with conventional pharmaceutical carriers. The composition also comprises one or more pharmaceutically acceptable acidic material, optionally second or tertiary alcohol or ester or ether derivatives thereof. The said pharmaceutical composition can be made into various common formulations, particularly oral formulations as well as topically transdermal patches. The present invention also discloses the application of the compounds and the compositions to treat tumor.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD +1
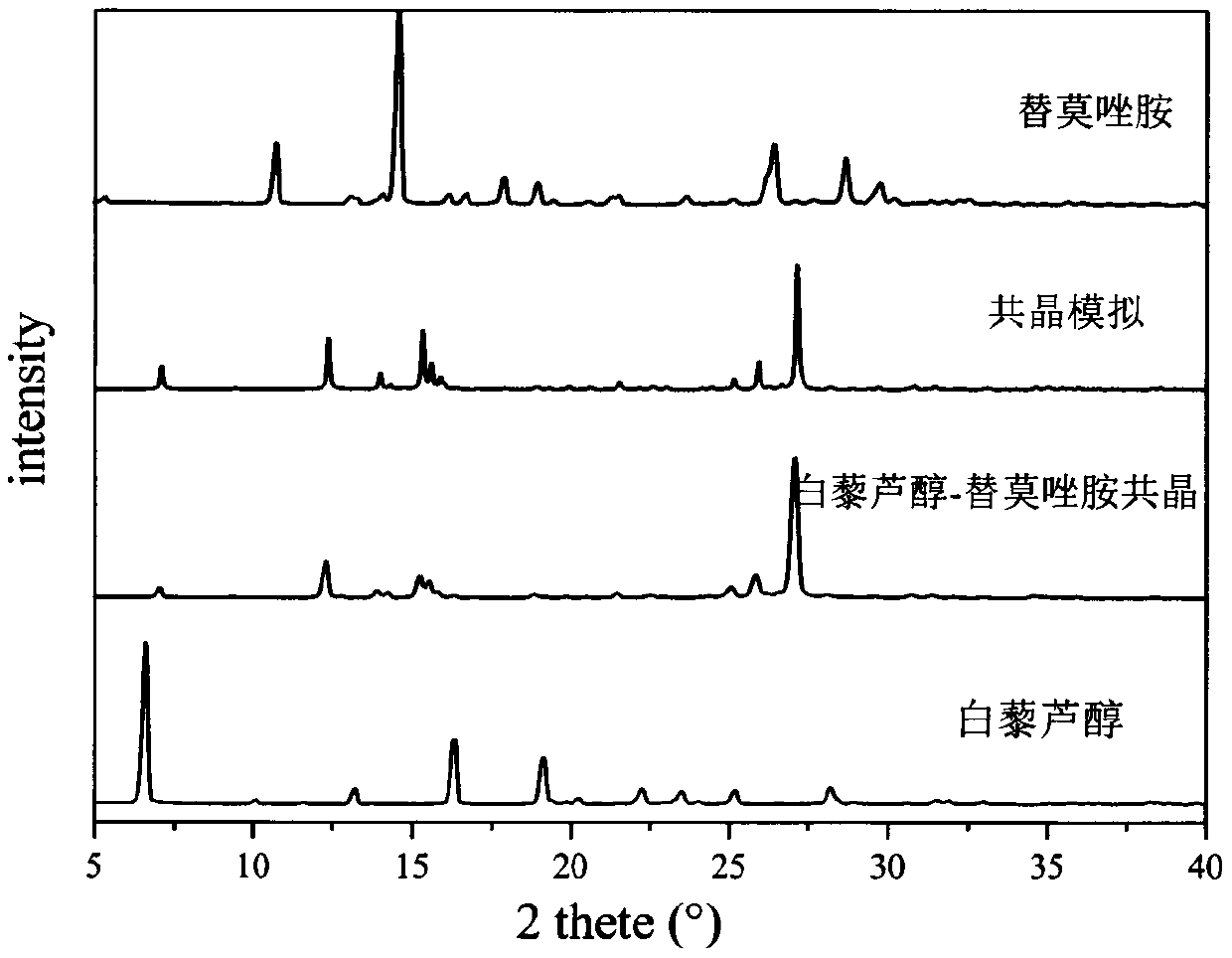
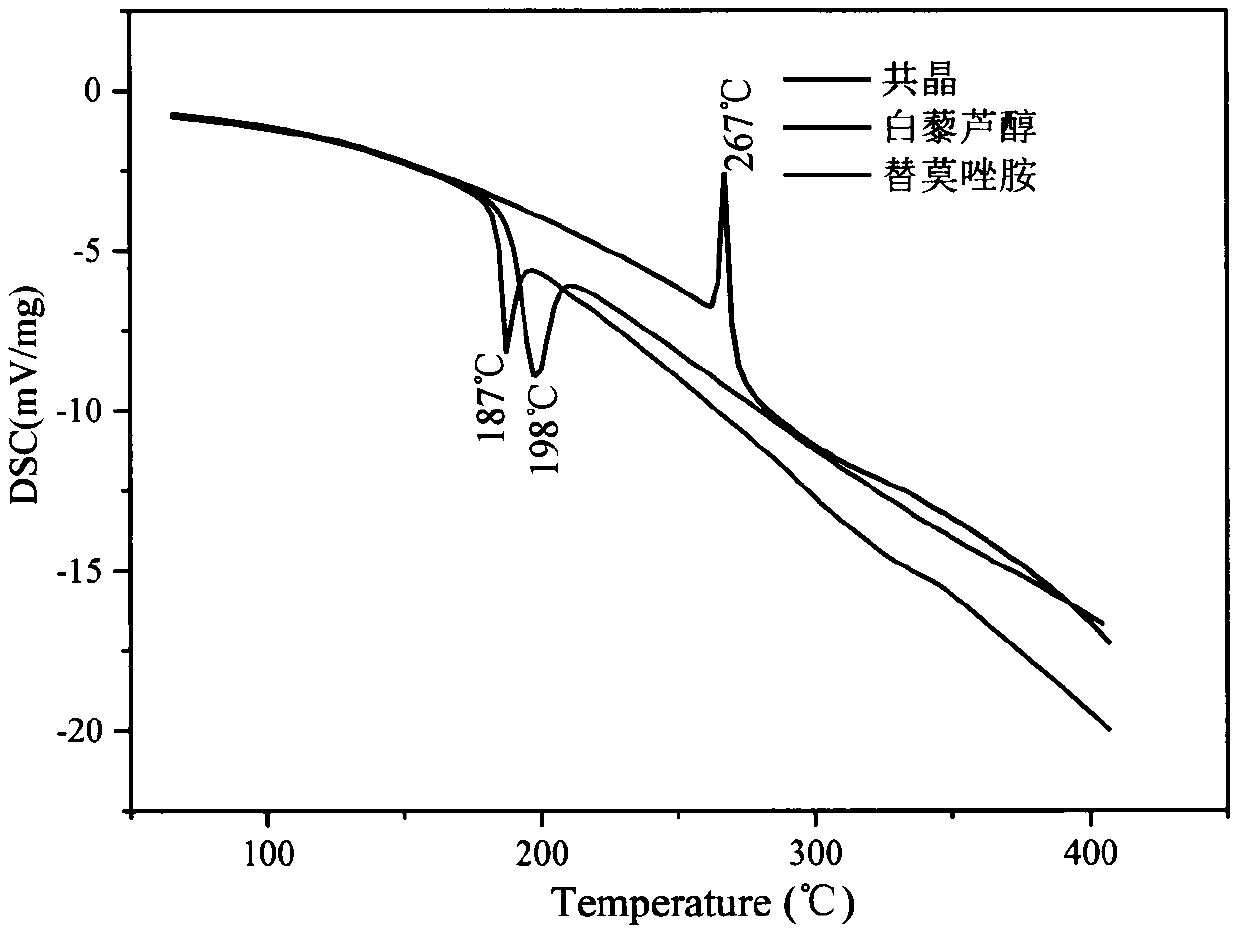
Resveratrol-temozolomide eutectic crystal and preparation method and application thereof
PendingCN111423444AGood curative effectImprove securityOrganic compound preparationOrganic chemistry methodsTemozolomidaPharmaceutical drug
The invention aims to provide a resveratrol-temozolomide eutectic crystal, which can improve the solubility of a medicine, improve the stability and improve the bioavailability, and also provides a preparation method and application of the resveratrol-temozolomide eutectic crystal. The invention relates to the resveratrol-temozolomide eutectic crystal. Resveratrol is used as an active pharmaceutical ingredient and is combined with another drug temozolomide through hydrogen bond weak action to form a resveratrol-temozolomide drug-drug co-crystal, the resveratrol-temozolomide drug-drug co-crystal is crystallized in a monoclinic system, the space group is P21 / c, the axial long axis angle alpha is 90.00 degrees, beta is 95.9755 (1) degrees and gamma is 90.00 degrees.
Owner:GUANGXI UNIV OF CHINESE MEDICINE
Injectable temozolomide powder injection preparation and preparation method thereof
InactiveCN103845294AGood resolubilityQuality is easy to controlPowder deliveryOrganic active ingredientsTemozolomidaEngineering
The invention discloses an injectable anticancer drug temozolomide freeze-dried powder injection preparation and its preparation method. The powder injection preparation is stable, controllable in quality, simple in preparation process and strong in operability, and a prepared product has the characteristics of good solubility, convenient clinical use, a long period of storage and the like.
Owner:杭州容立医药科技有限公司
Temozolomide sustained-release implant for treating solid tumor
InactiveCN101185630AIncreased sensitivityHigh clinical application valueOrganic active ingredientsPharmaceutical delivery mechanismPoly dl lactideTherapeutic effect
A temozolomide sustained release implant for treating solid tumors is characterized in that the sustained release implant includes effective dose of anticancer temozolomide, sustained release excipients and a certain quantity of sustained release moderator. Solid tumors include pancreatic cancer, lung cancer, liver cancer, breast cancer, cerebroma, ovarian cancer, prostatic carcinoma, esophageal carcinoma, lymphadenoma, osteosarcoma and colorectal cancer. Sustained release excipients mainly are one or combination of copolymer of glycolic acid and hydroxyacetic acid, polifeprosan, and poly(L-lactide-co-ethyl phosphate); in the process of decompression, temozolomide can be slowly released to local tumor, thus maintaining effective drug concentration of local tumor as well as significantly reducing overall toxic reaction. Being placed in local tumor, the sustained release implant not only reduces overall toxic reaction of temozolomide, but also selectively improves drug concentration in local tumor, enhancing the therapeutic effects of non-operative therapy such as chemotherapy drugs and radiotherapy.
Owner:SHANDONG LANJIN PHARMA +1
Temozolomide injectable composition and preparation method thereof
ActiveCN107875396AShorten the timeLower levelOrganic active ingredientsMacromolecular non-active ingredientsGlycineTemozolomida
The invention relates to a temozolomide injectable composition and a preparation method thereof. Concretely, the invention discloses a temozolomide composition and a preparation method thereof. The composition is an injectable composition. Glycine and sulfobutylether-beta-cyclodextrin are used as solubilizing agents; organic dissolvants are not added. The temozolomide injectable composition has various advantages of obviously improving the dissolution speed, reducing the solution state existing time of temozolomide during the industrial production, reducing the relevant substance level and thelike.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD +1
Temozolomide powder formulation
ActiveUS9949967B2Good and consistent flowabilityTitrated readily and accuratelyOrganic active ingredientsDispersion deliveryOral medicationTemozolomida
The present invention relates to a solid pharmaceutical composition of temozolomide that has good and consistent flowability as a powder and taste masking and is readily dispersible in an aqueous solution suitable for oral administration, e.g., as a dry sprinkle. This permits patients and healthcare workers to accurately measure doses and safely dispense the drug.
Owner:AMPLIPHARM PHARMA LLC
Treatment cancers using combination comprising parp inhibitors, temozolomide and/or radiation therapy
InactiveCN110891576AOrganic active ingredientsOrganic chemistryCancer preventionRadical radiotherapy
Disclosed herein is a method for the prevention, delay of progression or treatment of cancer in a subject, comprising administering to the subject in need thereof a PARP inhibitor, particularly, (R) -2-fluoro-10a-methyl-7, 8, 9, 10, 10a, 11-hexahydro-5, 6, 7a, 11-tetraazacyclohepta [def] cyclopenta [a] fluoren-4 (5H) -one, a sesqui-hydrate thereof, or a pharmaceutically acceptable salt thereof, incombination with temozolomide and / or radiation therapy. Also, disclosed a pharmaceutical combination comprising a PARP inhibitor, particularly, (R) -2-fluoro-10a-methyl-7, 8, 9, 10, 10a, 11-hexahydro-5, 6, 7a, 11-tetraazacyclohepta [def] cyclopenta [a] fluoren-4 (5H) -one, a sesqui-hydrate thereof, or a pharmaceutically acceptable salt thereof, in combination with temozolomide and the use thereof.
Owner:百济神州(苏州)生物科技有限公司
Temozolomide powder formulation
ActiveUS10098874B2Good and consistent flowabilityTitrated readily and accuratelyPowder deliveryOrganic active ingredientsOral medicationTemozolomida
The present invention relates to a solid pharmaceutical composition of temozolomide that has good and consistent flowability as a powder and taste masking and is readily dispersible in an aqueous solution suitable for oral administration, e.g., as a dry sprinkle. This permits patients and healthcare workers to accurately measure doses.
Owner:AMPLIPHARM PHARMA LLC
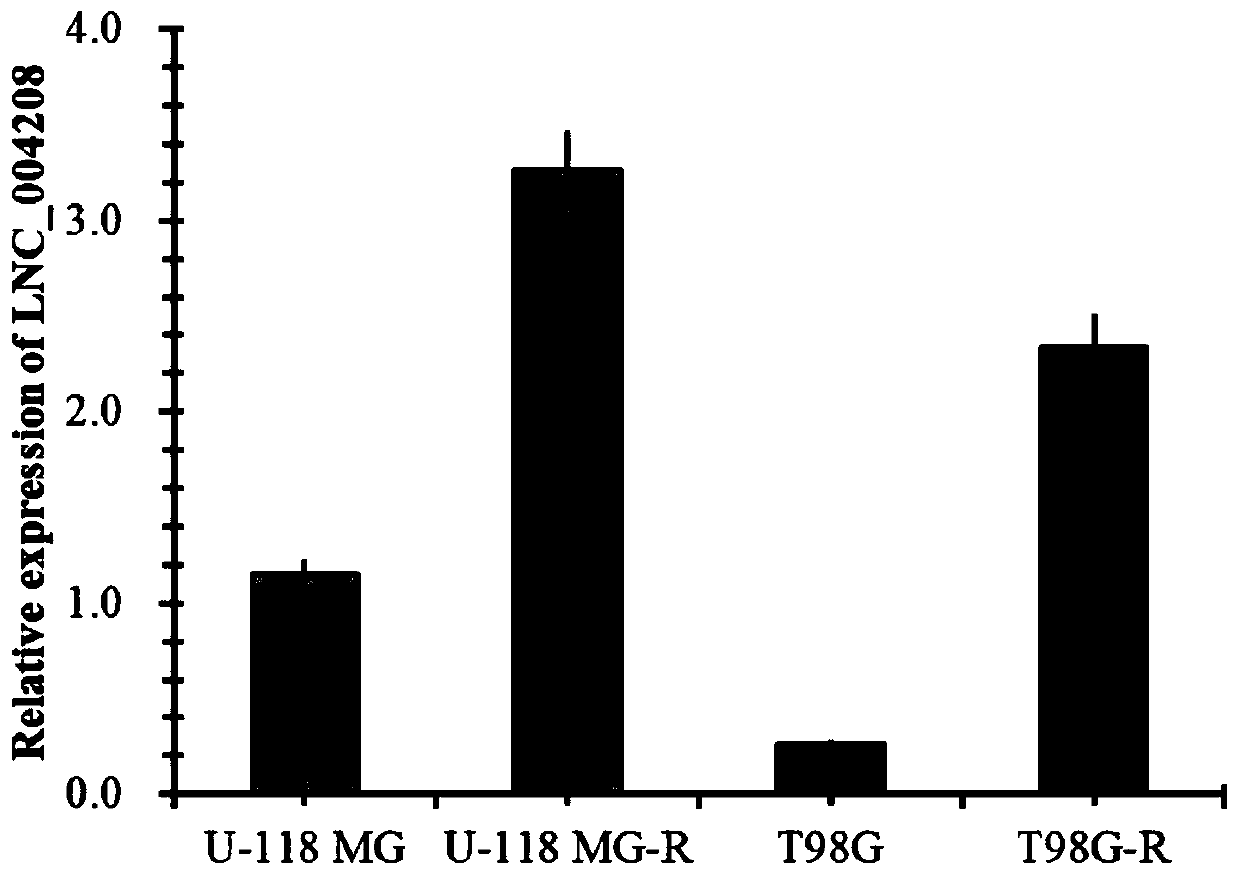
Application of incrnalnc_004208 and its detection reagents in the preparation of glioma prognosis reagents
InactiveCN109679957BMicrobiological testing/measurementDNA/RNA fragmentationDiseaseProgression-free survival
The invention discloses an application of IncRNA LNC_004208 and detection reagent thereof in preparing a glioma prognosis reagent. Through research on and utilization of IncRNA sequencing, the invention finds that IncRNA LNC_004208 has ultrahigh expression in a temozolomide-resisted glioma cell line; through telephone follow-up for 104 glioma patients, detailed inquiry for their first onset time,level, treatment condition, relapse situation, other diseases, other drugs, relapse time, death time, and the like, and registration for survival time and state, a result shows that average survival time and progression free survival of high expression patients are obviously shorter than those of low expression or no expression patients and are related to reactivity of temozolomide; thus, LNC_004208 is a molecular marker related to glioma prognosis; IncRNA LNC_004208 is high in expression; prognosis of patients is poor.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
Novel compound and pharmaceutically acceptable salt thereof
The invention discloses a compound or a pharmaceutically acceptable salt thereof. The compound includes a moiety derived from temozolomide and a moiety derived from elemene, and the moiety derived from temozolomide and the moiety derived from elemene are directly covalently linked or covalently linked via a linker. The invention also discloses a composition comprising the above compound, and a method of treating cancers.
Owner:HANGZHOU PUSH KANG BIOTECH CO LTD
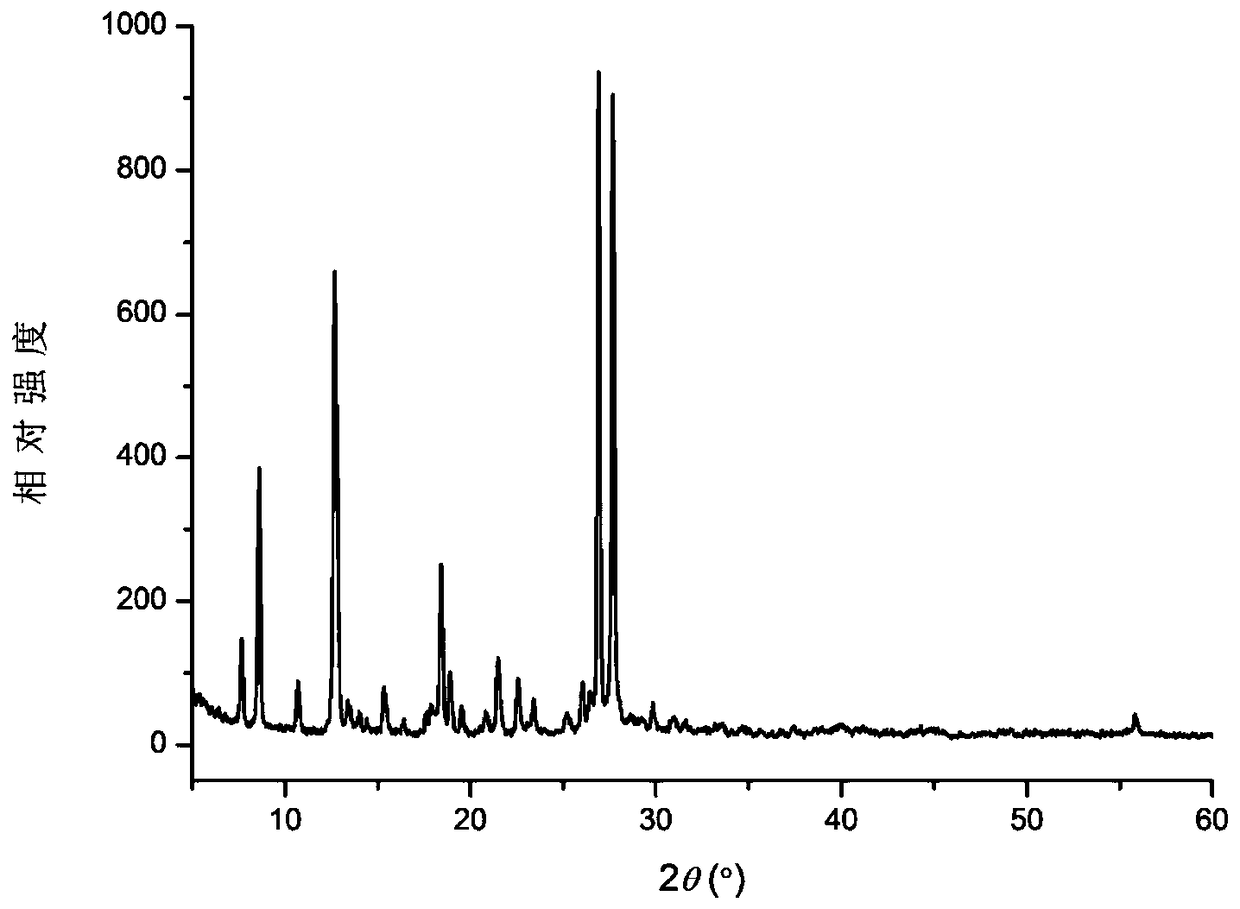
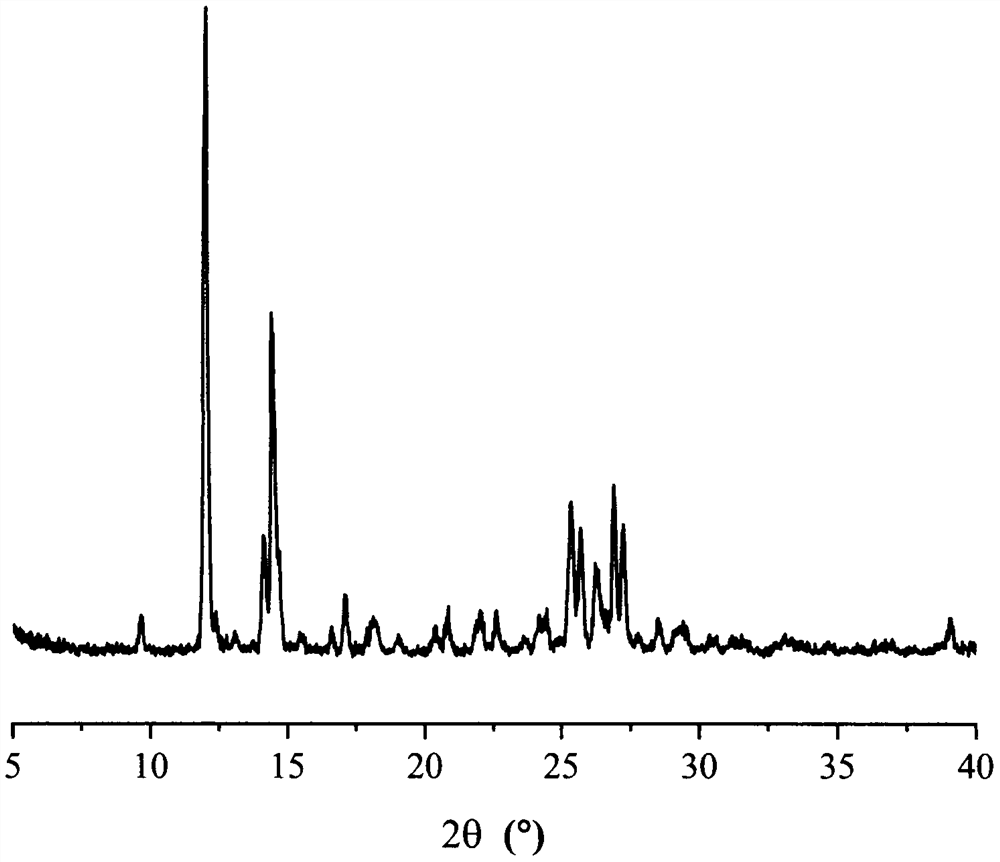
Eutectic crystal of temozolomide and baicalein as well as preparation method for eutectic crystal
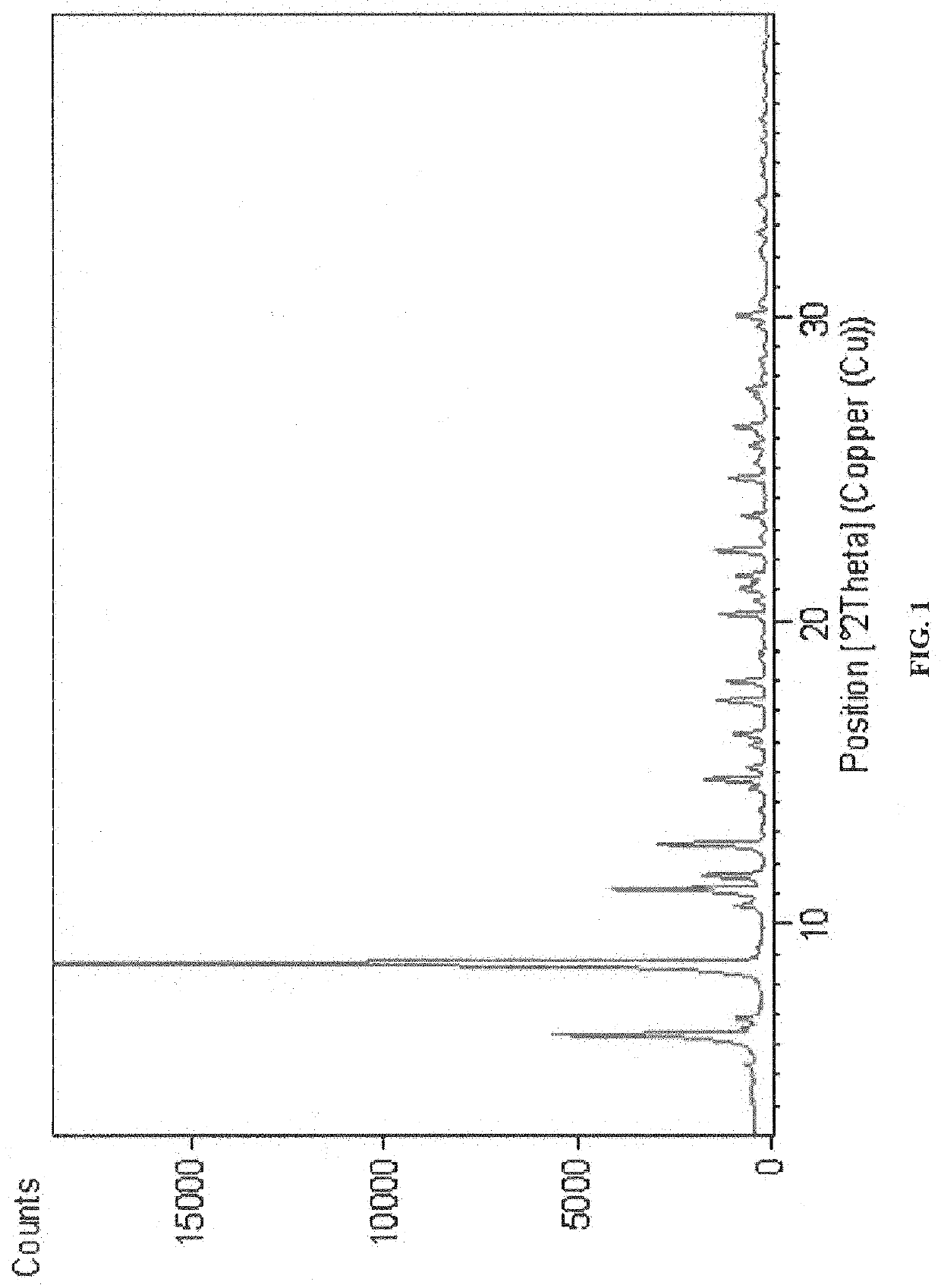
ActiveCN108623601AImprove stabilityImproved Oral Half-LifeOrganic active ingredientsOrganic chemistry methodsSolubilityX-ray
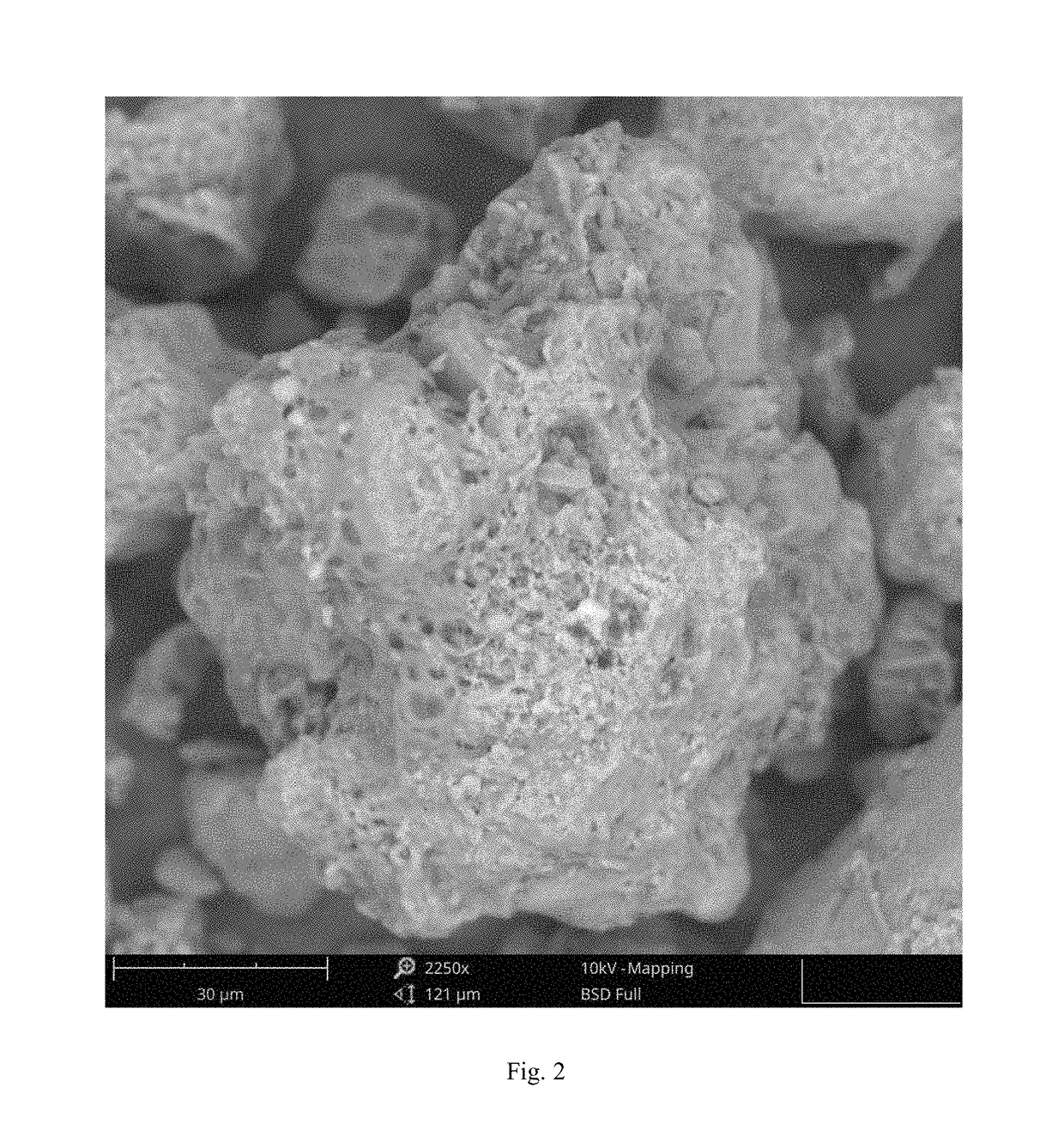
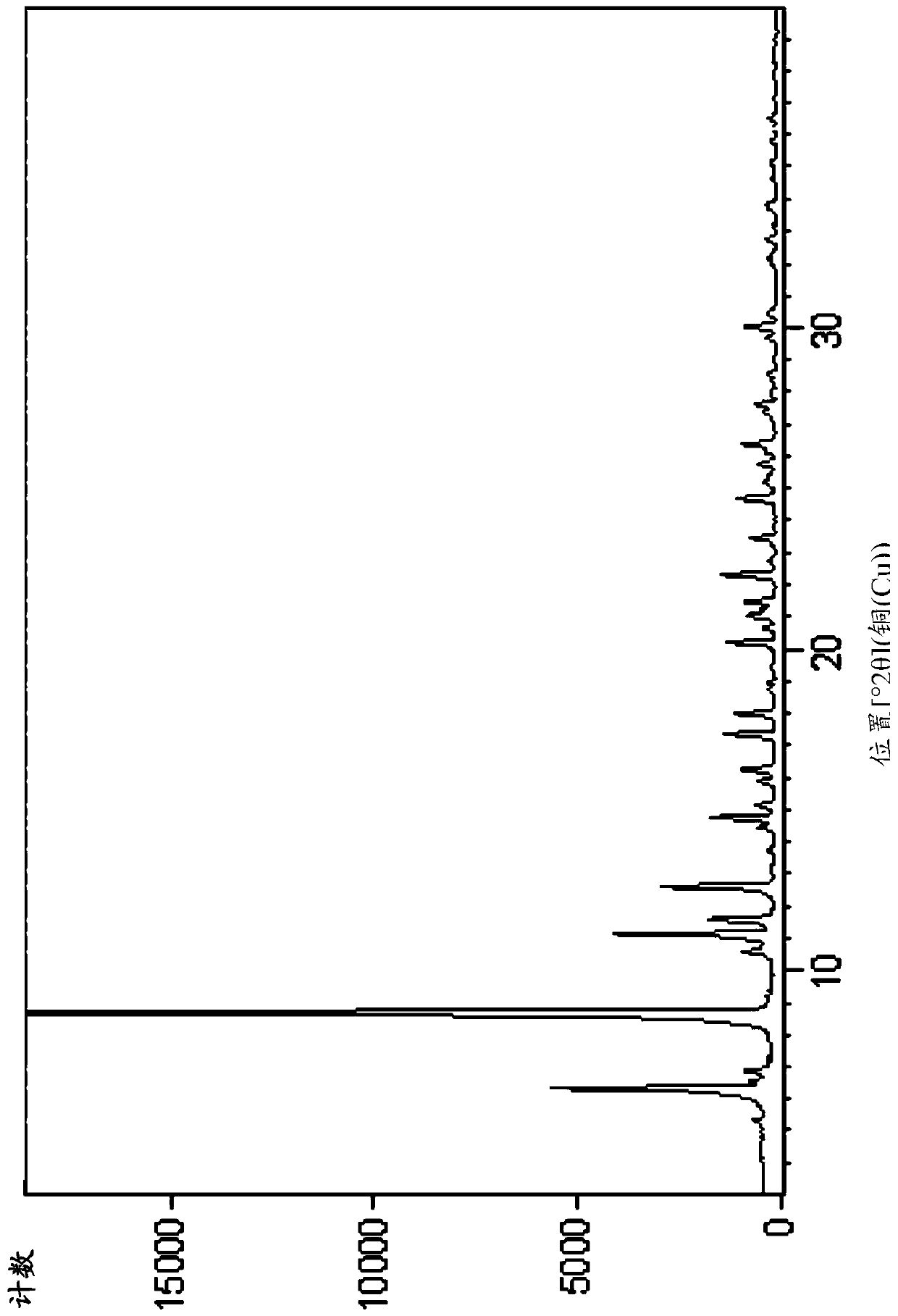
The invention relates to eutectic crystal of temozolomide and baicalein as well as a preparation method for the eutectic crystal. Specifically, an X-ray powder diffraction pattern of the eutectic crystal has characteristic peaks with 2 theta value being 7.7 + / -0.2 degree, 8.6+ / -0.2 degree, and 12.7+ / -0.2 degree. The eutectic crystal provided by the invention has obvious advantages in stability, solubility, pharmacokinetic properties and the like of two pharmaceutical ingredients in comparison with existing temozolomide and baicalein bulk drugs, has an important value in developing drug combination of two drugs.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Anticancer compound, preparation method and use thereof, and composition containing the compound
ActiveCN101190916AAnticancer activity hasHas anti-leukemic activityOrganic active ingredientsOrganic chemistryAbnormal tissue growthTumor cell apoptosis
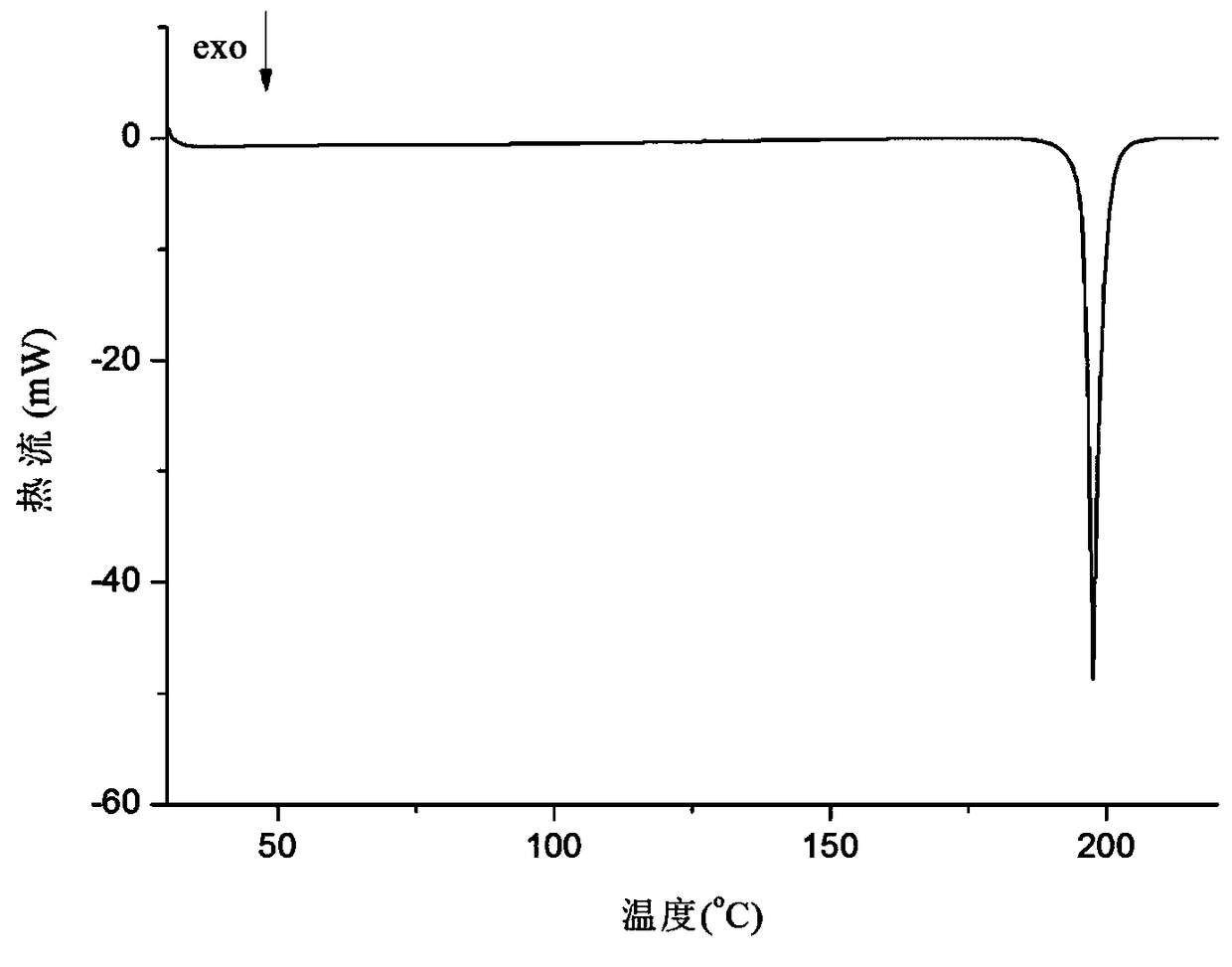
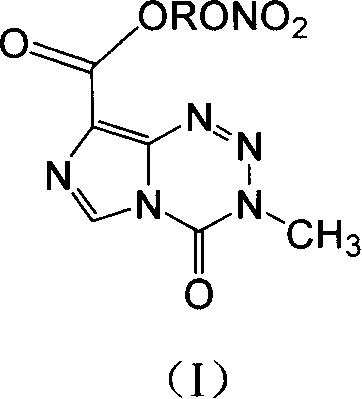
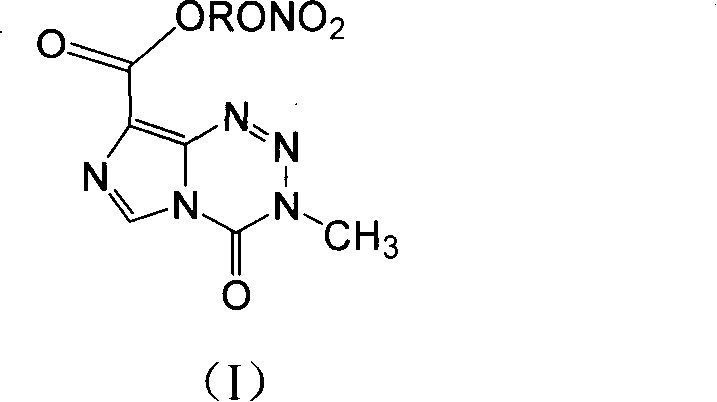
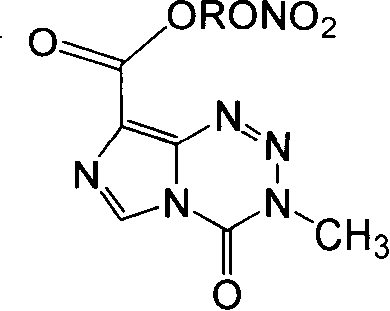
The invention provides compounds of the same class with anticancer activity and purposes thereof. In particular, the invention solves the current defects of temozolomide and can significantly prolong the lives of patients with brain cancer and strengthen the treatment responses of the patients to the temozolomide. The invention combines the effect of inducing apoptosis of tumor cells of NO based on the anti-tumor effect of the temozolomide and applies the basic theories of drug design so as to design and synthesize NO-donating temozolomide nitrate esters derivatives with novel structure and NO releasing activity, namely the synthesis and anti-tumor activity of 3,4-dihydro-3-Methyl-4-oxoimidazo (5,1-d) 1,2,3,5 tetrazine-8-carboxylic carbonate.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
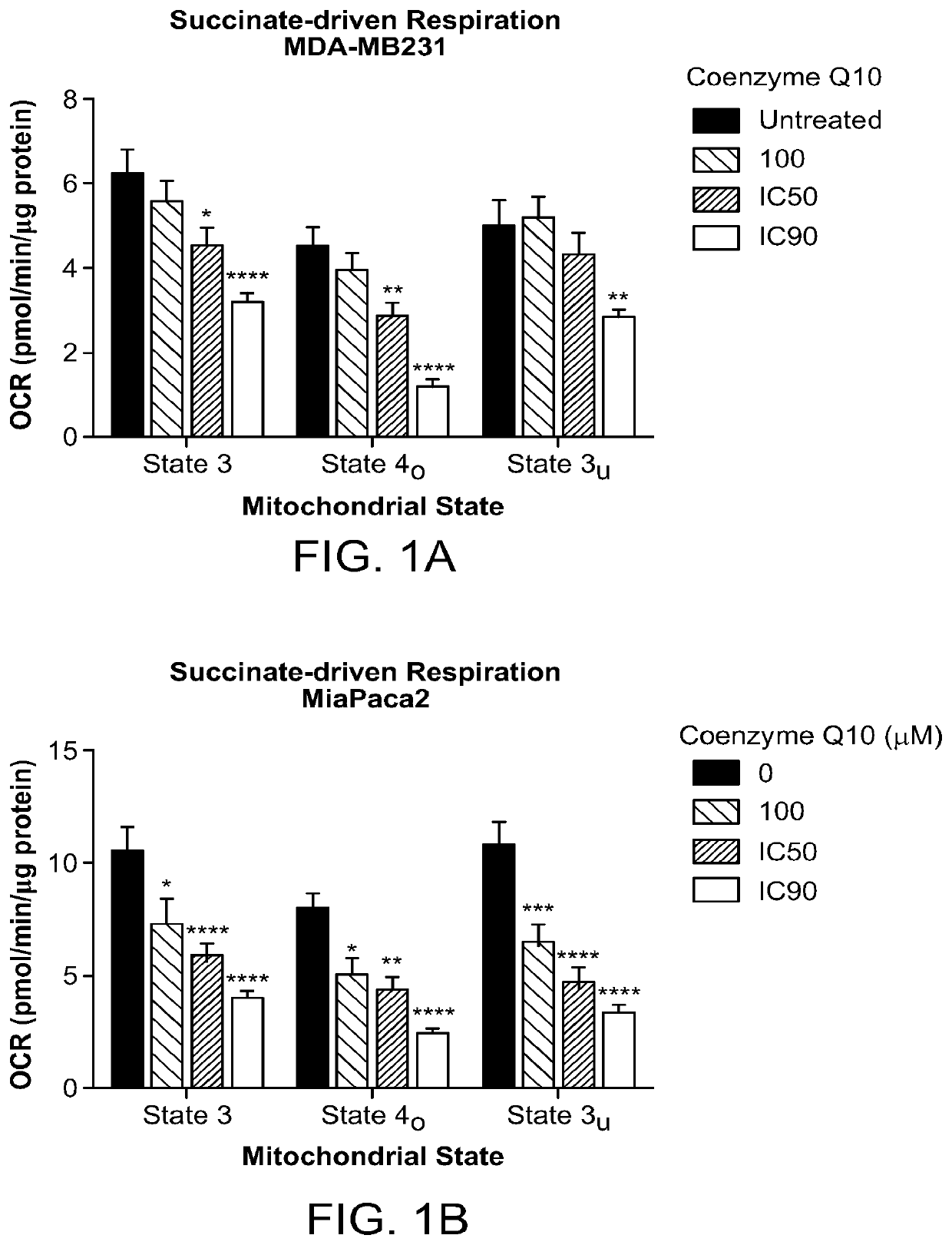
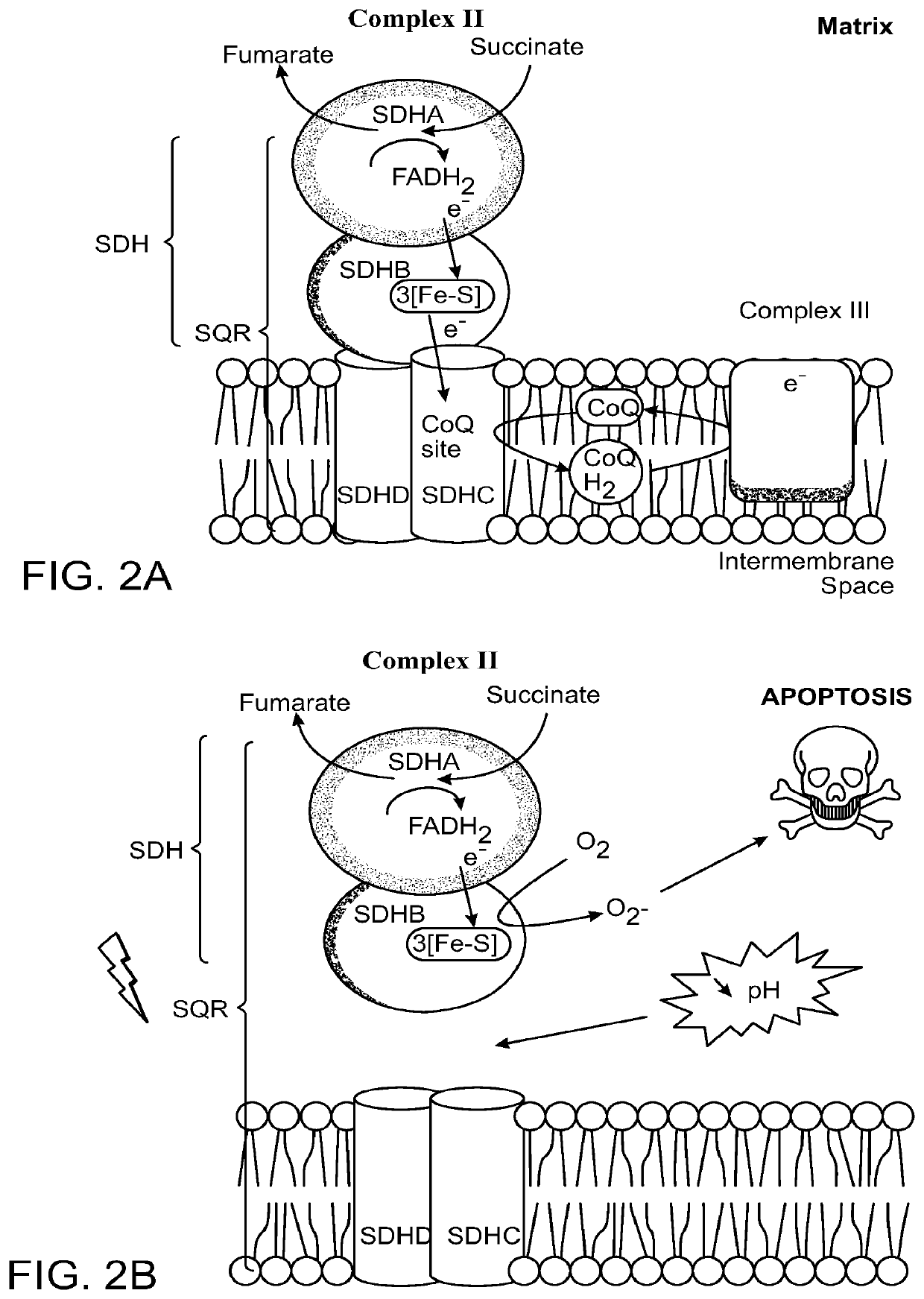
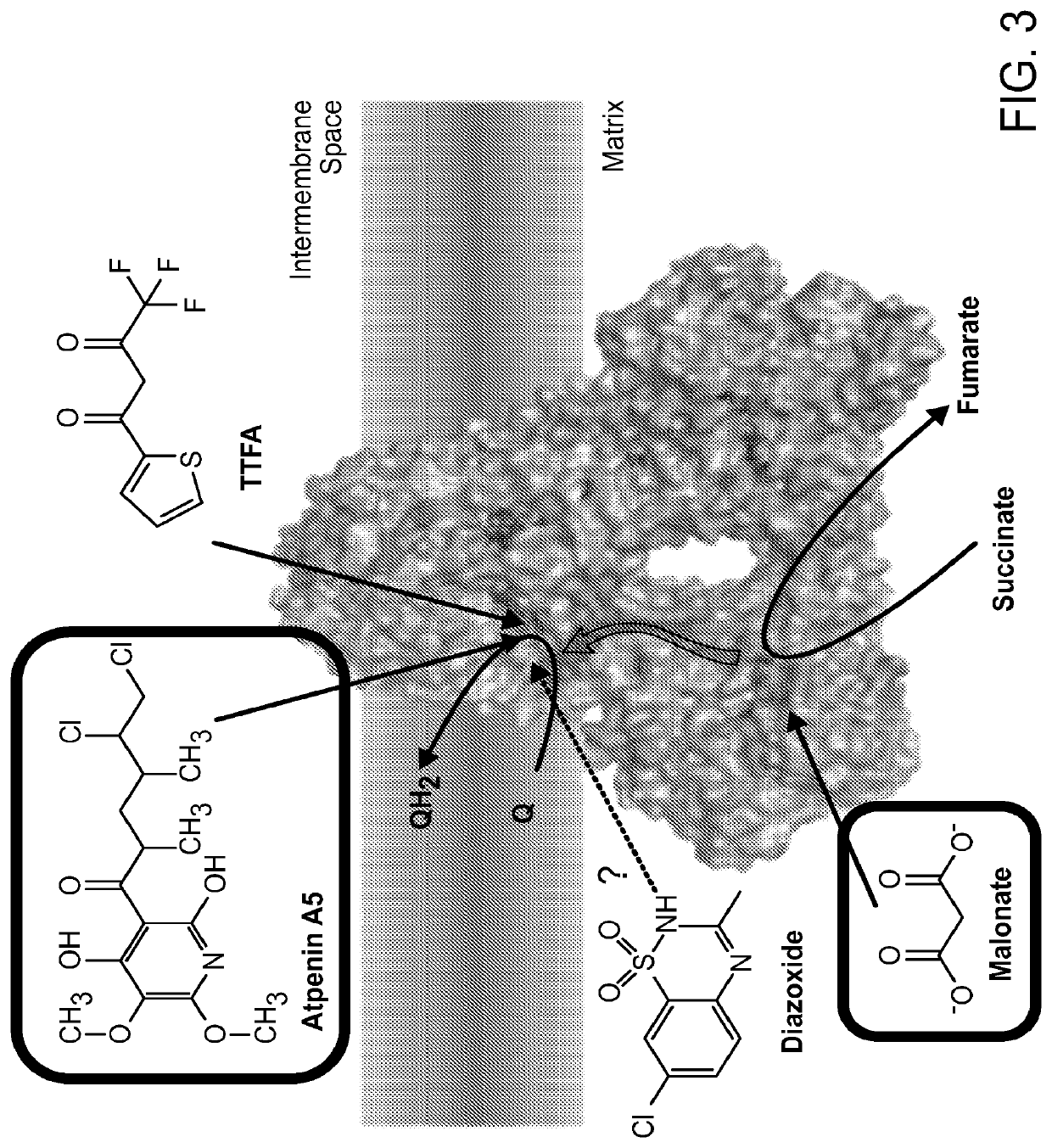
Methods of treatment of temozolomide-resistant glioma using coenzyme q10
PendingUS20220202741A1Organic active ingredientsPharmaceutical delivery mechanismChemical compoundOncology
The invention provides methods and compositions for treatment of a subject with a glioma that has failed treatment with temozolomide (TMZ) comprising administration of a composition comprising a Coenzyme Q10 compound to the subject. The invention also provides a method of treating a cancer that exhibits increased Complex II activity in a subject comprising administration of a composition comprising a Coenzyme Q10 compound to the subject.
Owner:BPGBIO INC
Temozolomide and hesperetin cocrystal and preparation method thereof
PendingCN111689972AImprove stabilityImprove tableting effectOrganic active ingredientsOrganic chemistry methodsTemozolomidaCombinatorial chemistry
The invention discloses a temozolomide and hesperetin cocrystal and a preparation method thereof. The molar ratio of temozolomide to hesperetin in the cocrystal is 1:1, and an X-ray powder diffractionpattern of the cocrystal has characteristic peaks when the 2theta values are 11.9+ / -0.2 degrees, 25.3+ / -0.2 degrees, 26.8+ / -0.2 degrees and 27.1+ / -0.2 degrees. The cocrystal preparation method provided by the invention is simple in process, the crystallization process is easy to control, the reproducibility is good, and the method is suitable for industrial production. The cocrystal can effectively improve the stability and tabletting property of temozolomide, improve the solubility of hesperetin and slow down the dissolution of temozolomide, and provides a research basis for treatment of cancer by combining temozolomide and hesperetin.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
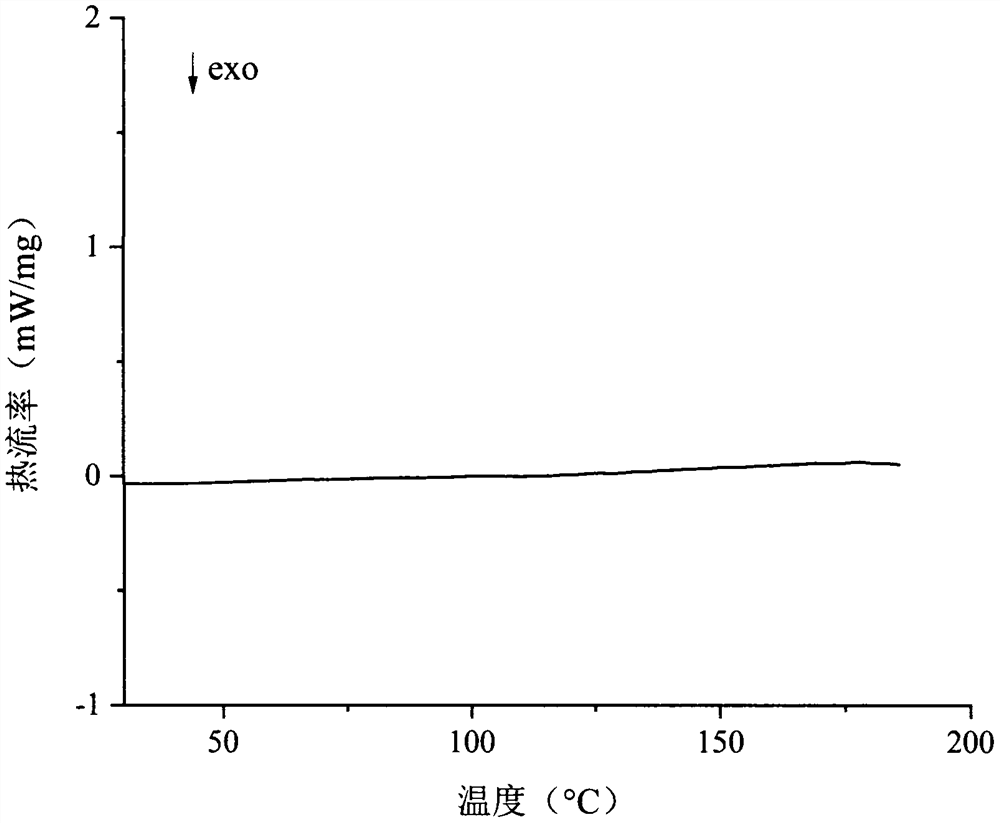
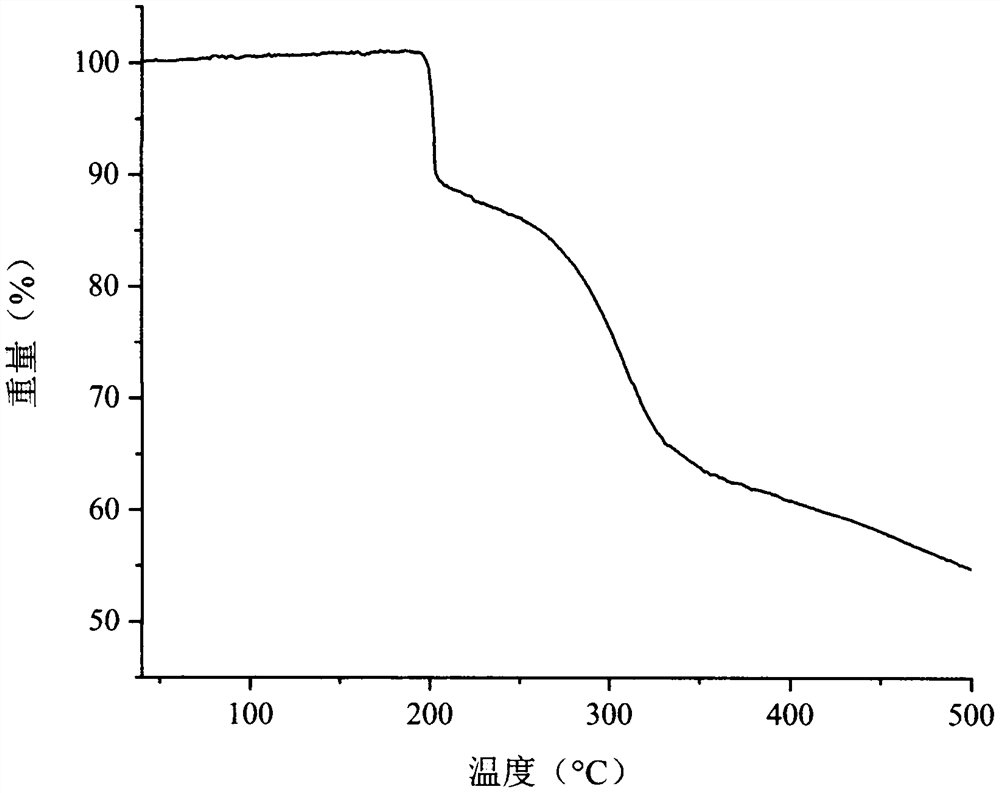
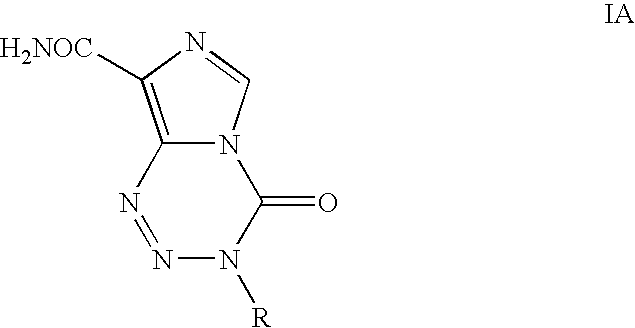
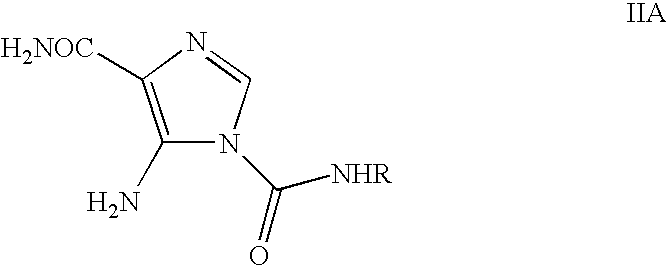
Process for the preparation of temozolomide and analogs
InactiveUS8258294B2Minimize undesired cyclisation productOrganic active ingredientsBiocideSolventMetal halides
A process for the preparation of compounds of formula IA, where R═CH3 (i.e. temozolomide):comprising diazotizing a compound of the formula IIA:where in R is as defined above in the presence of at least one metal halide, an acid and a source of nitrous acid, followed by conversion of acidic solution containing temozolomide. The conversion can be carried out by a liquid-liquid extraction technique in a water immiscible solvent. The temozolomide may be further purified in an acetone-water mixture.
Owner:CIPLA LTD
Methods of treatment using intravenous formulations comprising temozolomide
InactiveCN101678002AOrganic active ingredientsLyophilised deliveryIntravenous therapyTherapeutic treatment
Owner:MERCK & CO INC
Novel application of human CD133 protein 1-108 peptide fragment
Owner:KUNMING INST OF ZOOLOGY CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com