Treatment cancers using combination comprising parp inhibitors, temozolomide and/or radiation therapy
An inhibitor, cancer technology, applied in the directions of X-ray/γ-ray/particle irradiation therapy, drug combination, medical preparations containing active ingredients, etc., can solve the problem of insufficiently obtained, undisclosed clinical benefits, and aggravate the adverse effects of myelosuppression and other issues to achieve an effective anti-tumor response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
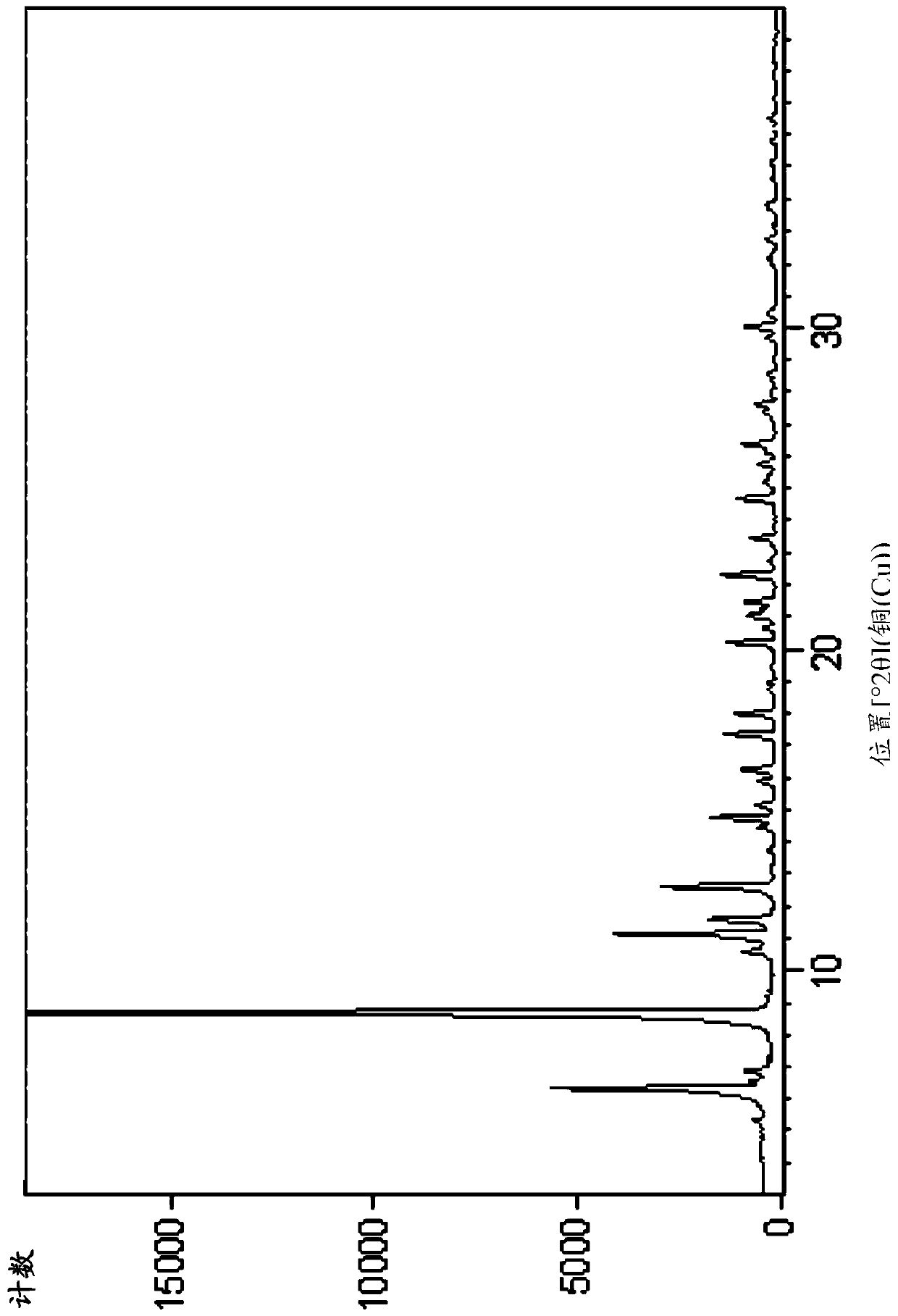
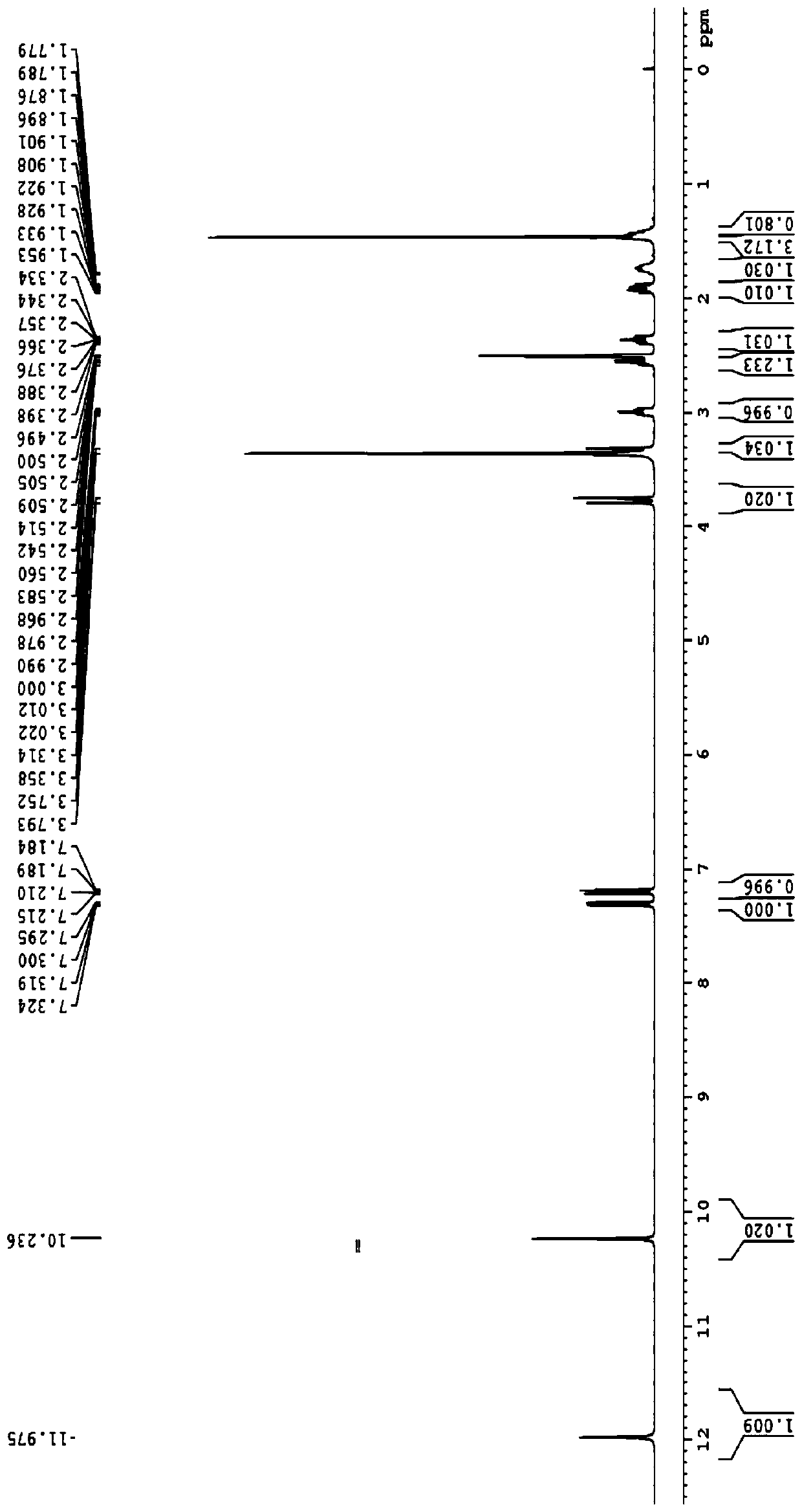
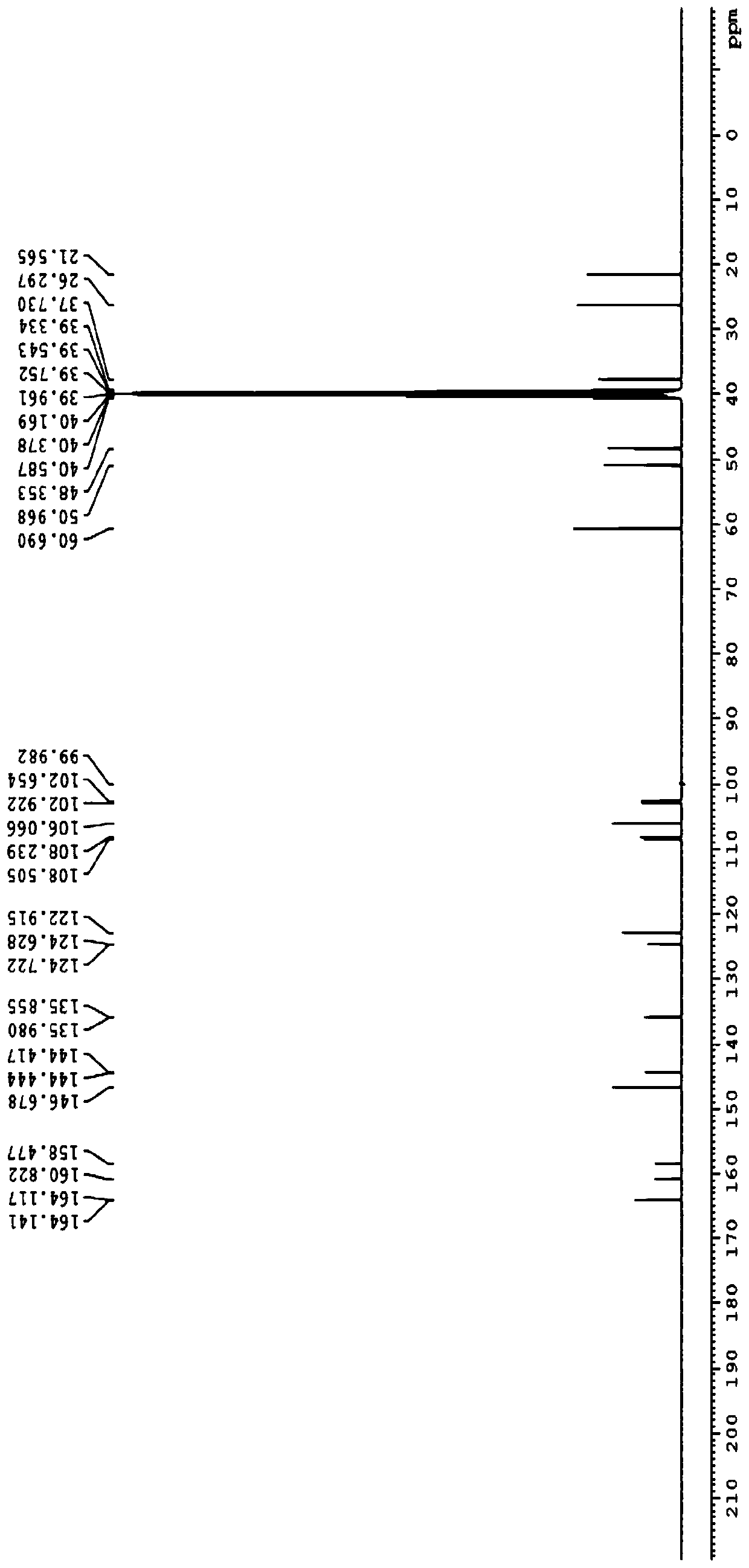
[0174] Embodiment 1. Preparation of compound A and compound B
[0175] Step 1: Synthesis of Compound-2
[0176]
[0177] Tert-butyl bromoacetate (51.7Kg) was dissolved in anhydrous acetonitrile (72Kg). The temperature was raised to 65°C-75°C, then methylpyrroline (22Kg) was added. After the reaction was complete, the reaction mixture was concentrated and residual acetonitrile was removed by adding THF and then concentrating. After GC showed complete removal of acetonitrile, more THF was added and stirred. The resulting solid was collected by filtration. 44.1 Kg of off-white solid Compound-2 was obtained. 1 H NMR (400MHz, DMSO-d6) δ4.91(s,2H),4.15(m,2H),3.29(m,2H),2.46(s,3H),),2.14(m,2H),1.46( s, 9H) ppm.
[0178] Step 2: Synthesis of Compound-3
[0179]
[0180] To a THF-cooled (-60°C) solution of trimethylsilylacetyne (12.4 Kg) was added n-butyllithium in hexane (43.4 Kg). After complete addition of n-BuLi solution, the resulting mixture was stirred for anot...
Embodiment 2
[0203] The effect of the combination of embodiment 2 PARP inhibitor and temozolomide (TMZ)
[0204] Compound B as a single agent has demonstrated excellent in vitro activity against tumor cell lines with defects in the HR pathway. In vivo, compound B showed potent antitumor activity against BRCA1 mutant mouse xenograft model (MDA-MB-436 breast cancer) and was 16-fold more potent than olaparib. In a pharmacokinetic (PK) / pharmacodynamic (PD) study, oral administration of Compound B produced a time- and dose-dependent inhibition of PARylation in MDA-MB-436 breast cancer xenografts in mice. Inhibition of PARylation in tumor tissue correlates with compound B tumor drug concentrations.
[0205] The antiproliferative effect of Compound B in combination with TMZ was evaluated in eight human GB cell lines resistant to single agent TMZ (EC50 of 32 μM or greater). Compound B exhibited synergy with TMZ in 7 of 8 cell lines, with 5-fold or greater changes in EC50 for TMZ. This synergy w...
Embodiment 3
[0207] Embodiment 3: clinical trial
[0208] An open-label, multicenter, multidose, dose-escalation phase 1b / 2 study of compound B in combination with radiation therapy (RT) and / or temozolomide (TMZ).
[0209] (1) In patients with first-line glioblastoma (GB) with unmethylated MGMT promoter ("unmethylated GB") Compound B in combination with RT in patients.
[0210] Compound B (60 mg BID) was administered to patients in combination with RT at increasing exposures of 2, 4 and 6 weeks for 6 to 7 weeks. After completion of RT, the patient received no further treatment.
[0211] (2) Compound B in combination with both TMZ and RT in subjects with first-line unmethylated GB.
[0212] Compound B (60 mg BID) was administered to patients in combination with RT for 6 to 7 weeks and increasing doses of TMZ. After completion of RT, the patient received no further treatment.
[0213] (3) In subjects with relapsed / refractory GB with methylated or unmethylated MGMT promoter Comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com