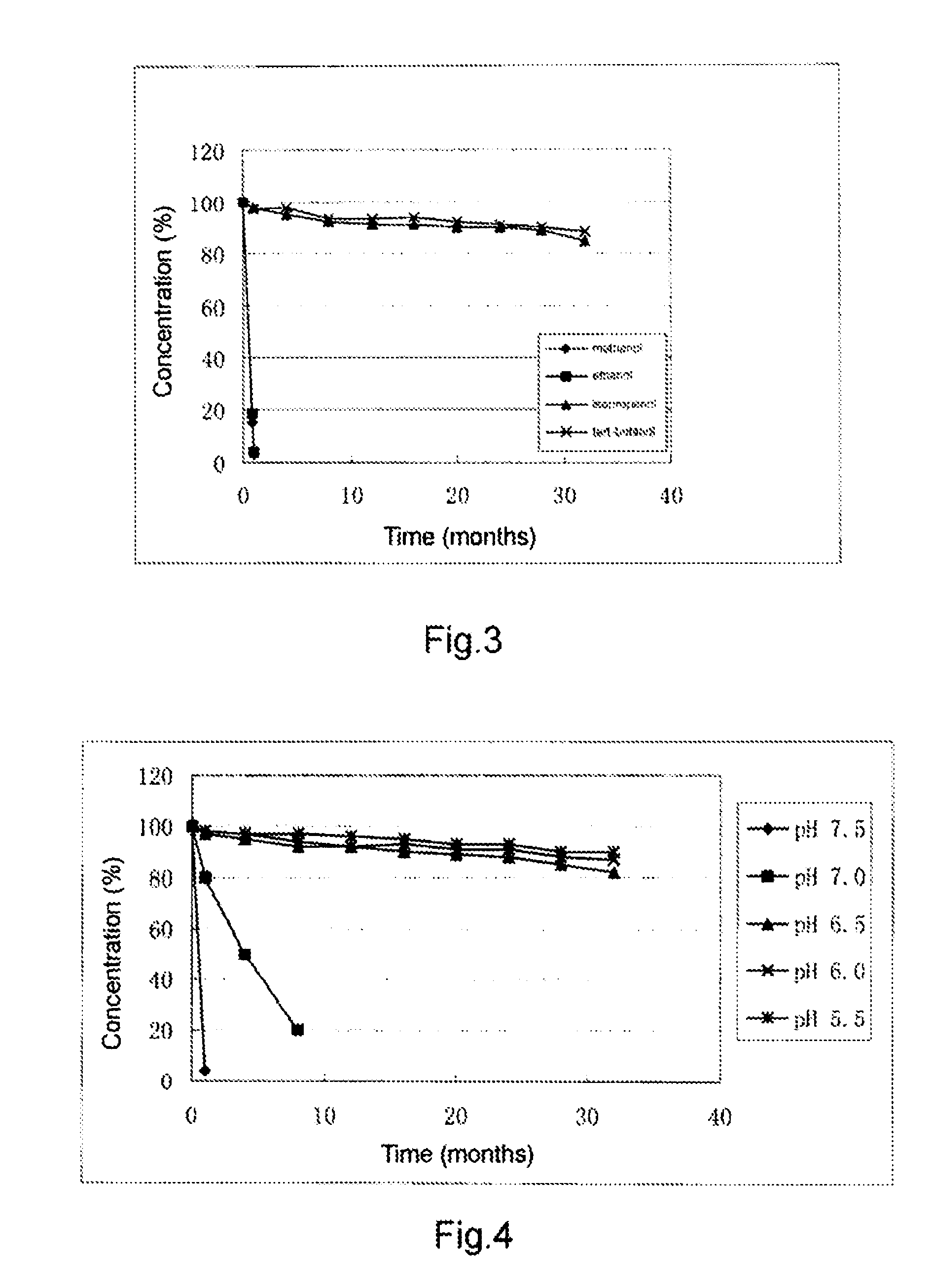
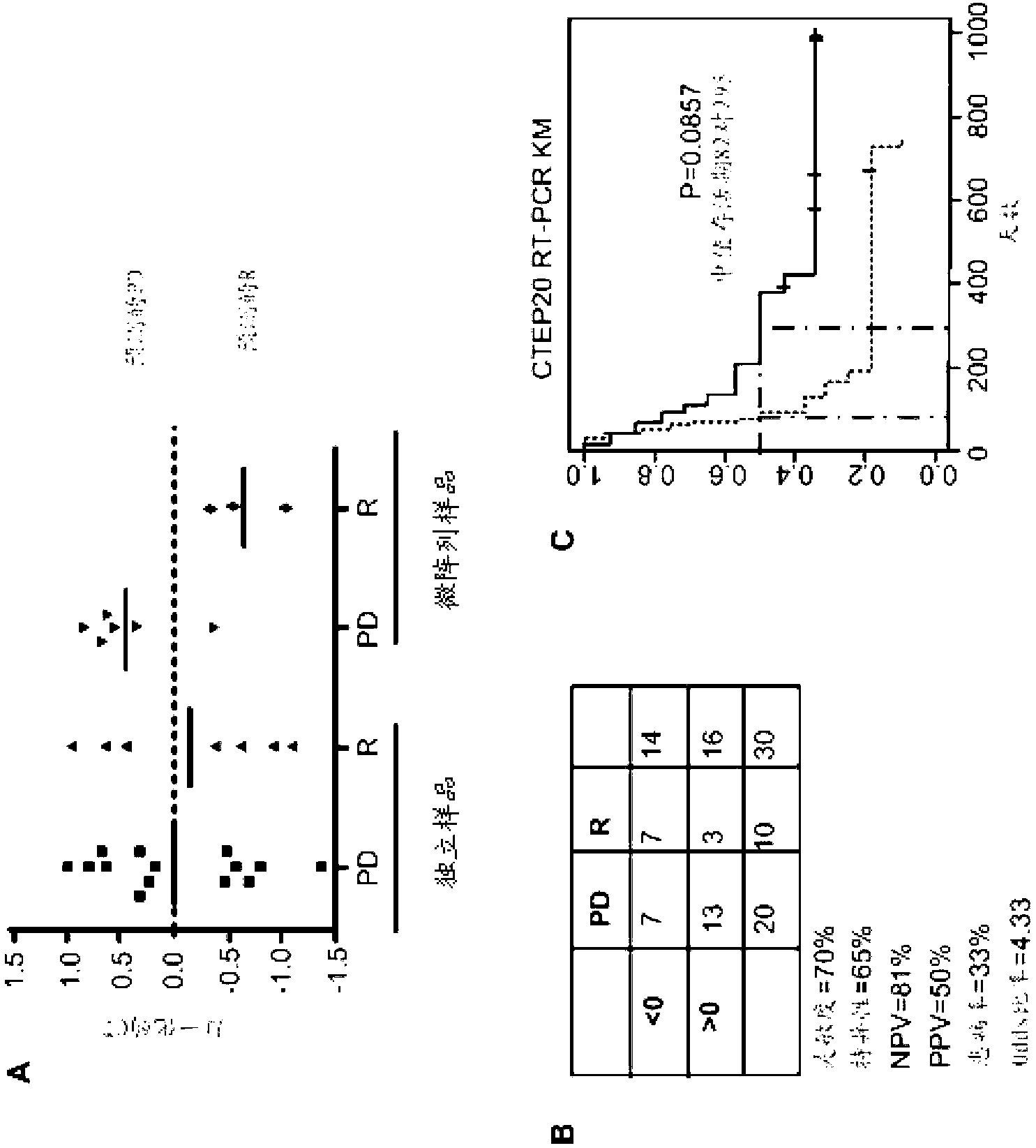
Patents
Literature
76 results about "Temozolomida" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Compositions and methods for the treatment of cancer
InactiveUS20020128228A1Reducing and avoiding adverse effectImprove toleranceBiocideAnimal repellantsIntestinal structureCancer prevention
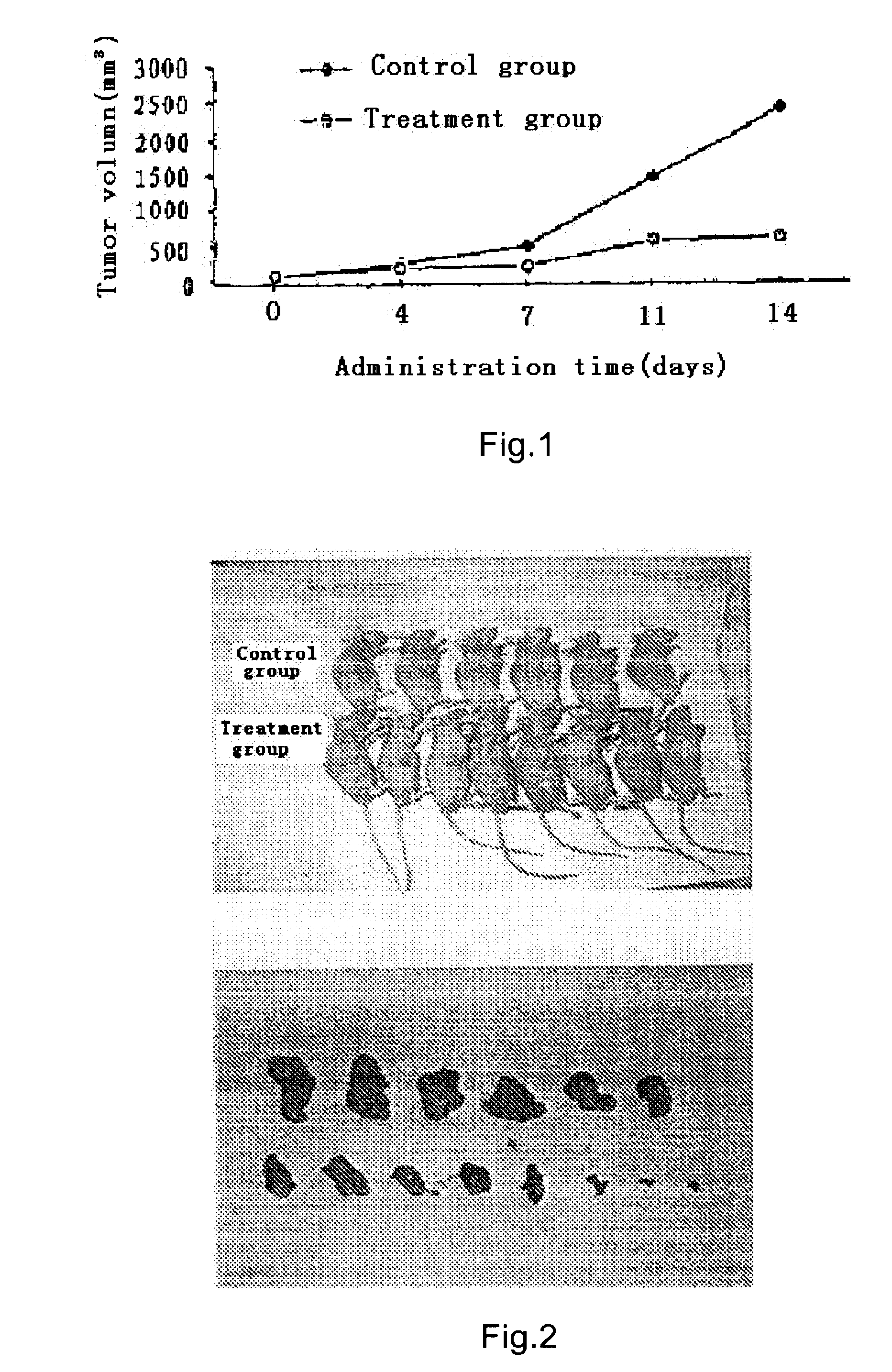
This invention relates to compositions comprising temozolomide and thalidomide which can be used in the treatment or prevention of cancer, in particular malignant melanoma, cancer of the skin, subcutaneous tissue, lymph nodes, brain, lung, liver, bone, intestine, colon, heart, pancreas, adrenals, kidney, prostate, breast, colorectal, or a combination thereof. A particular composition comprises temozolomide, or a pharmaceutically acceptable salt, solvate, or clathrate thereof, and thalidomide, or a pharmaceutically acceptable salt, solvate, or clathrate thereof. The invention also relates to methods of treating or preventing cancer, in particular malignant melanoma, cancer of the skin, subcutaneous tissue, lymph nodes, brain, lung, liver, bone, intestine, colon, heart, pancreas, adrenals, kidney, prostate, breast, colorectal, or a combination thereof, which comprise the administration of temozolomide and thalidomide and another anti-cancer drug to a patient in need of such treatment or prevention. The invention further relates to methods of reducing or avoiding adverse side effects associated with the administration of cancer chemotherapy or radiation therapy which comprise the administration of temozolomide and thalidomide to a patient in need of such reduction or avoidance.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Methoxyamine potentiation of temozolomide anti-cancer activity
InactiveUS6465448B1Prevents APE cleavageDisrupting DNA repairBiocideAnimal repellantsMethoxyamineTemozolomida
This invention generally relates to novel compositions and methods for the treatment of certain cancers. Additionally, this invention relates to novel compositions and methods to screen drugs for the treatment of certain cancers. Specifically, the invention contemplates that temozolomide and methoxyamine, in combination or in sequence, shall be used as a treatment for certain tumors that are resistant to treatment by temozolomide alone.
Owner:CASE WESTERN RESERVE UNIV
Use of dianhydrogalactitol and analogs or derivatives thereof in combination with platinum-containing antineoplastic agents to treat non-small-cell carcinoma of the lung and brain metastases
ActiveUS20160008316A1Increase survivalSuppress growthHeavy metal active ingredientsBiocideTyrosine kinaseCisplatin
The use of dianhydrogalactitol provides a novel therapeutic modality for the treatment of non-small-cell lung carcinoma (NSCLC) and ovarian cancer, as well as other types of malignancy, including brain metastases of NSCLC. Dianhydrogalactitol acts as an alkylating agent on DNA that creates N7 methylation. Dianhydrogalactitol is effective in suppressing the growth of cancer stem cells and is active against tumors that are refractory to temozolomide, cisplatin, and tyrosine kinase inhibitors; the drug acts independently of the MGMT repair mechanism. Dianhydrogalactitol can be used together with other anti-neoplastic agents and can possess additive or super-additive effects.
Owner:DEL MAR PHARMA
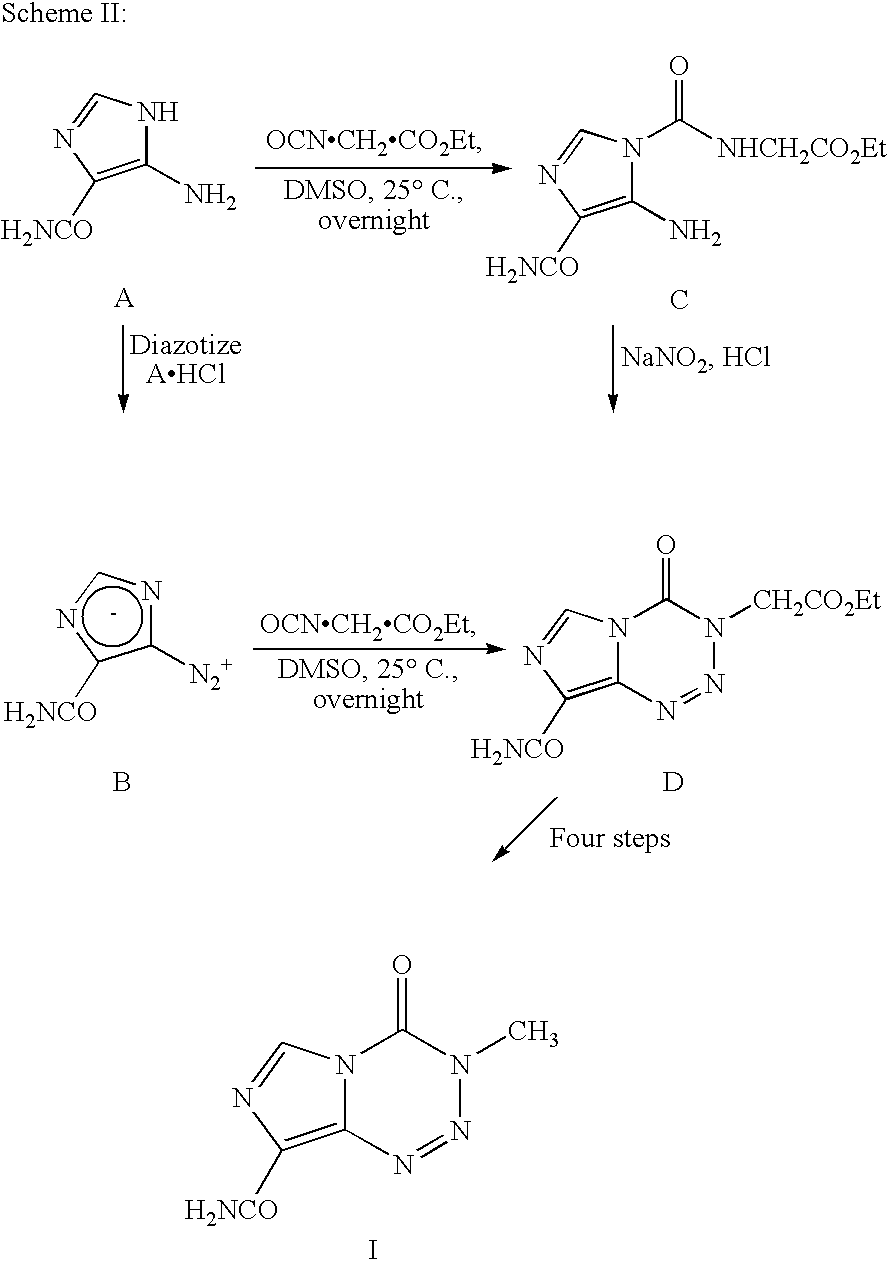
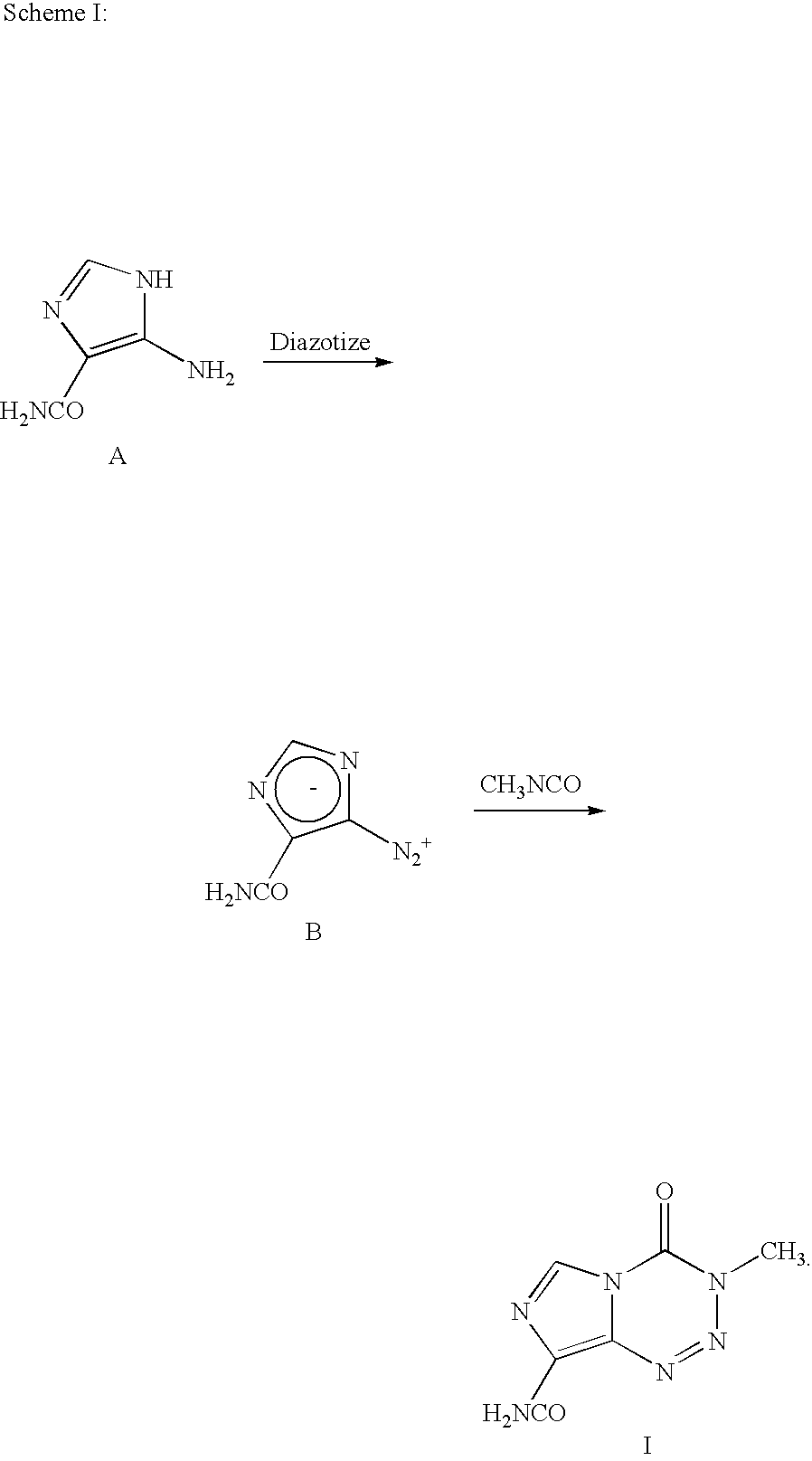
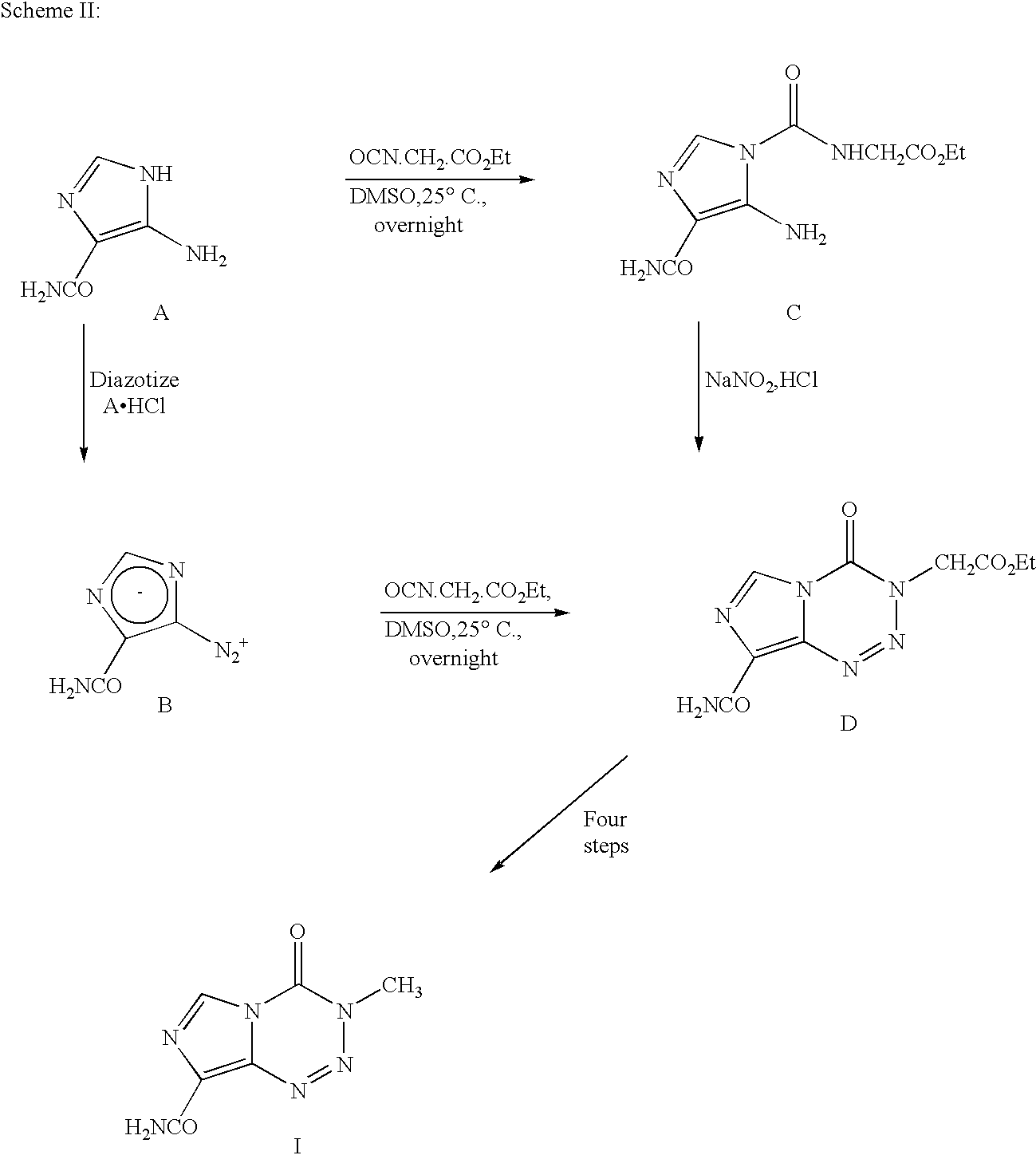
Synthesis of temozolomide and analogs
InactiveUS20050131227A1Promote correct directionOrganic active ingredientsOrganic chemistry methodsStereochemistryTemozolomide
This invention relates to a novel process for the synthesis of Temozolomide, an antitumor compound, and analogs, and to intermediates useful in this novel process.
Owner:SCHERING CORP
Synthesis of temozolomide and analogs
InactiveUS7087751B2Promote correct directionOrganic active ingredientsOrganic chemistry methodsTemozolomidaOrganic chemistry
This invention relates to a novel process for the synthesis of Temozolomide, an antitumor compound, and analogs, and to intermediates useful in this novel process.
Owner:SCHERING CORP
Parp inhibitor compounds, compositions and methods of use
ActiveUS20090098084A1Avoid recoveryImprove survivalHeavy metal active ingredientsBiocideChemotherapy inducedCancer therapy
The present invention relates to tetraaza phenalen-3-one compounds which inhibit poly (ADP-ribose) polymerase (PARP) and are useful in the chemosensitization of cancer therapeutics. The induction of peripheral neuropathy is a common side-effect of many of the conventional and newer chemotherapies. The present invention further provides means to reliably prevent or cure chemotherapy-induced neuropathy. The invention also relates to the use of the disclosed PARP inhibitor compounds in enhancing the efficacy of chemotherapeutic agents such as temozolomide. The invention also relates to the use of the disclosed PARP inhibitor compounds to radiosensitize tumor cells to ionizing radiation. The invention also relates to the use of the disclosed PARP inhibitor compounds for treatment of cancers with DNA repair defects.
Owner:EISAI INC
Controlled releases system containing temozolomide
InactiveUS8821913B2Eliminate inconvenienceBioactivity can be achievedPill deliveryAntineoplastic agentsControl releaseTemozolomida
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
Application of lncRNA TRALR (long-chain non-coding RNA TRALR)
InactiveCN108504737AExtended cycleAccelerated agingMicrobiological testing/measurementBrain gliomaBiology
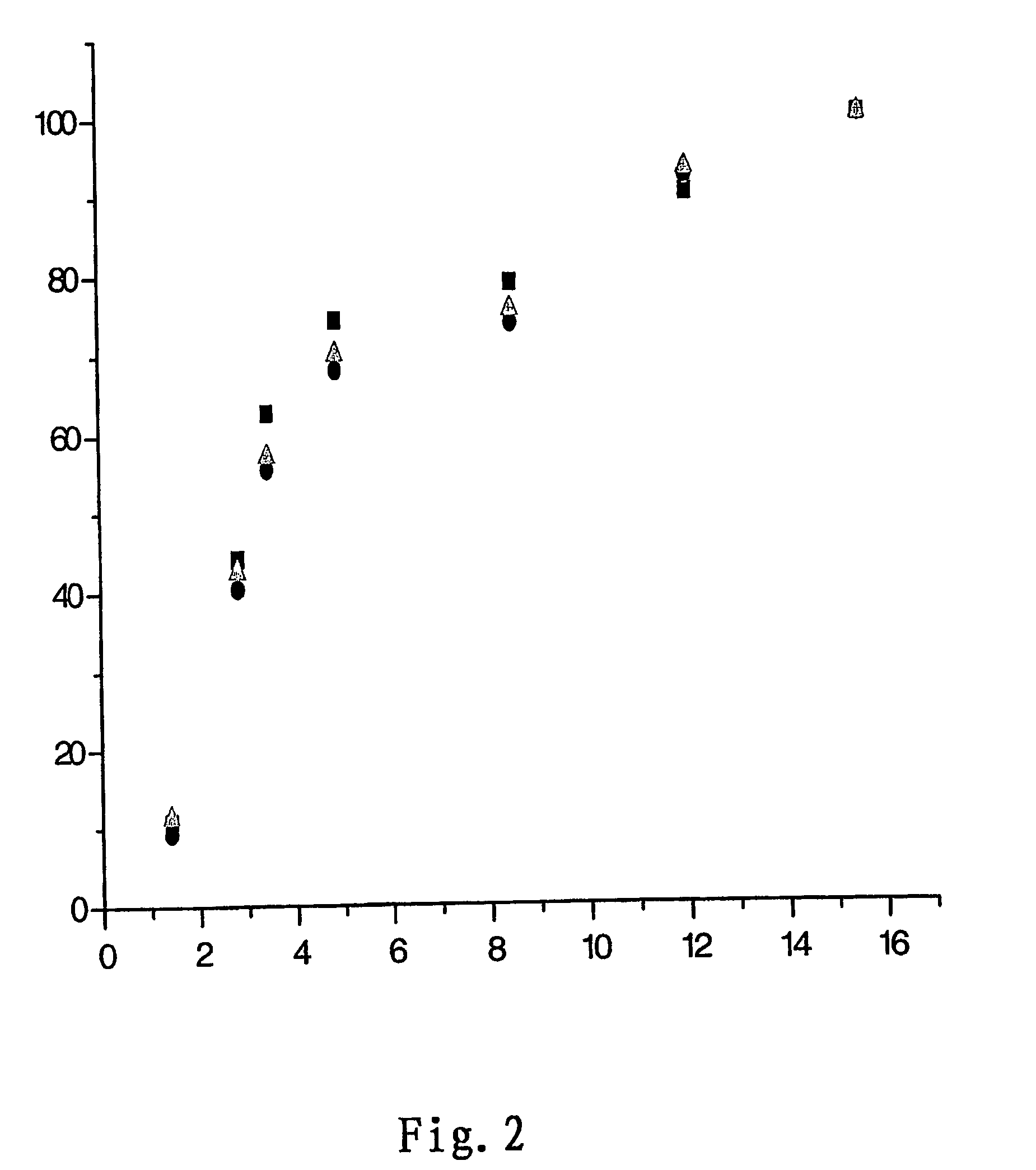
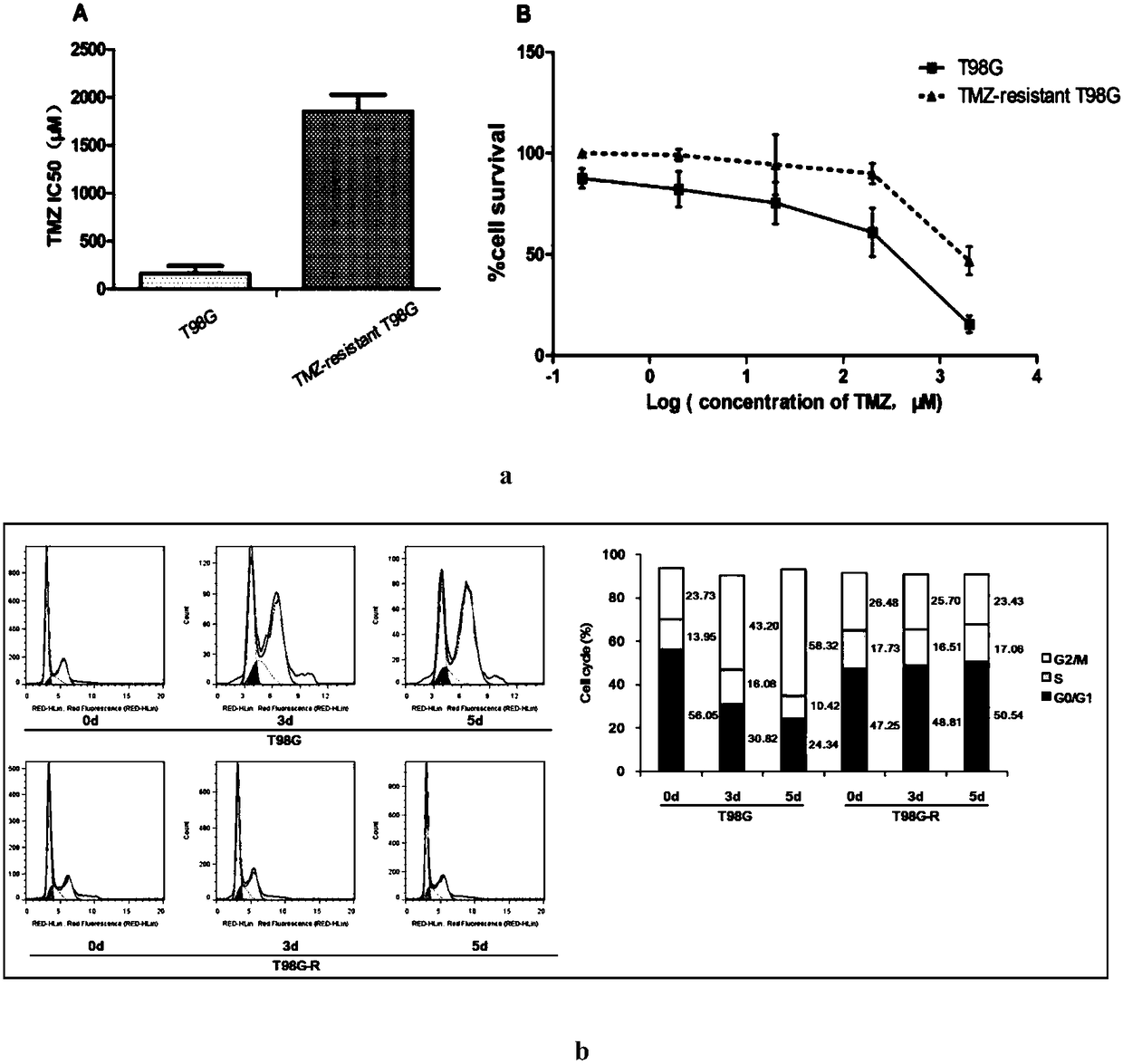
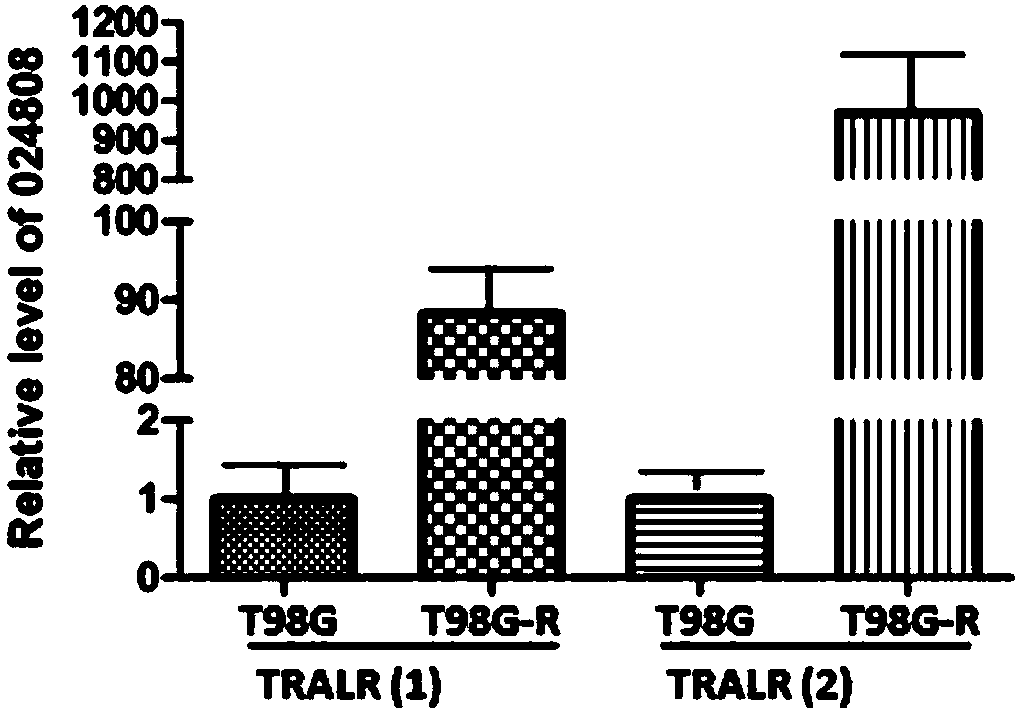
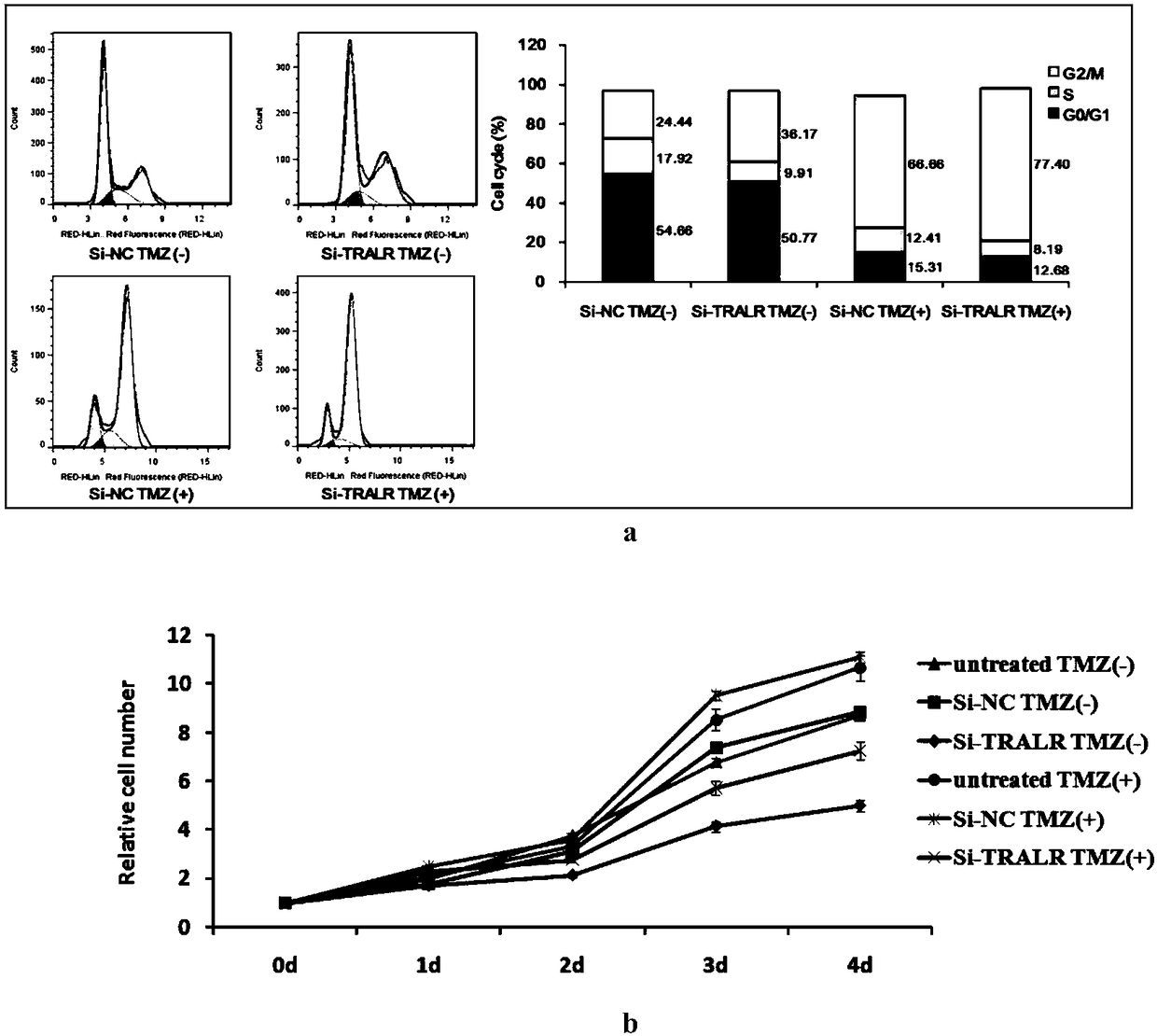
The invention discloses an application of lncRNA TRALR (long-chain non-coding RNA TRALR). According to the research, lncRNA sequencing shows that lncRNA TRALR has abnormal high expression in a temozolomide-resistant brain glioma cell line, by means of specific interference to expression of lncRNA TRALR, BMI1 can be obviously inhibited and p21 level can be remarkably up-regulated, cell cycle arrestand aging are promoted, and sensitivity of brain glioma to temozolomide is increased. Therefore, a reagent for detecting the expression of lncRNA TRALR can be used for preparing preparations for brain glioma diagnosis and prognosis or sensitivity detection of patients with brain glioma, powerful molecular biology basis is provided for diagnosis, prognosis and chemotherapy sensitivity prediction of the patients with brain glioma, and far-reaching clinical significance and great popularization and application prospect are achieved.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
Double-element solution type preparation for intravenous injection and intracerebral injection
InactiveCN101467967AAddress long-term stabilityUse meets the requirementsOrganic active ingredientsPharmaceutical delivery mechanismActive componentRoom temperature
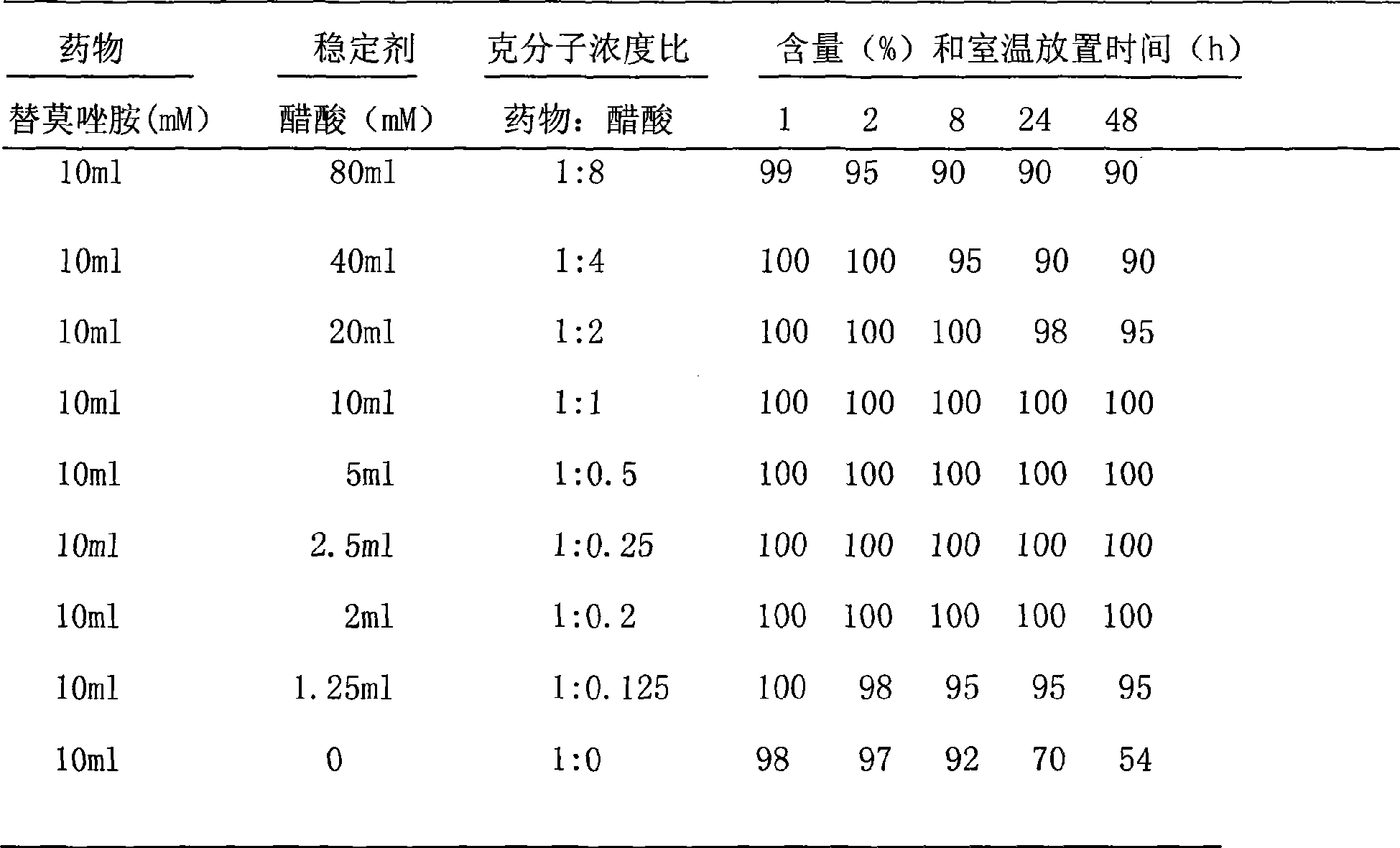
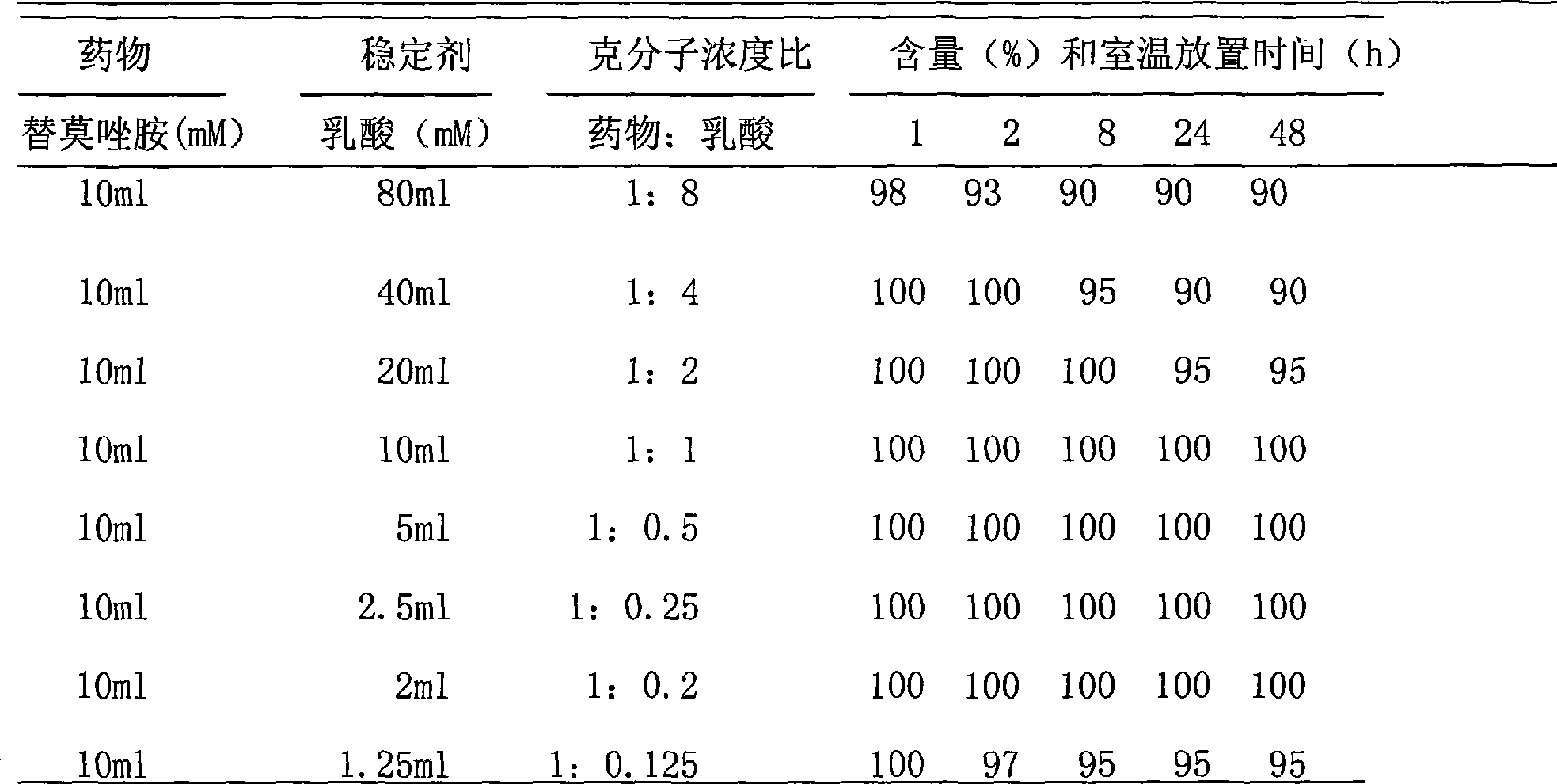
The invention discloses a binary solution type formulation for intravenous and intracerebral injection, shown as right. The main active component thereof is temozolomide with the binary system and having temozolomide sterile powder and solvent for dissolving medicaments. The operating temozolomide solution agent can be obtained through the combination of the solvent. The invention is characterized in that, 1, the binary formulation is stable at the room temperature for over 2 years; 2, the prepared medicaments liquid is stable at the room temperature at least for 48h to meet the operating requirement of injection administration; 3, the solvent is nontoxic and non-irritant, and the prepared medicaments can be used for the intracerebral injection during the operation and stereotaxic intraturmor injection of local chemotherapy.
Owner:北京京卫燕康药物研究所有限公司
Unit dosage forms of temozolomide
InactiveUS20080319039A1Reduced pill burdenPatient compliance is goodOrganic active ingredientsBiocideDiseaseTemozolomida
This invention relates to unit dosage forms of temozolomide. These unit dosage forms are particularly well-suited for decreasing the pill burden and increasing patient compliance. The invention also relates to methods of treating proliferative disorders in a patient with these unit dosage forms. The invention additionally relates to kits comprising these unit dosage forms.
Owner:MERCK SHARP & DOHME CORP
Synthetic method for temozolomide and intermediate
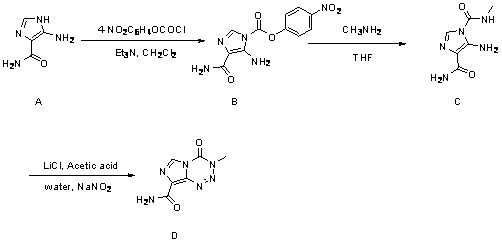
The invention discloses a modified optimized synthetic method for temozolomide and an intermediate thereof. The synthetic method is characterized in that a new oxidation ring-closing reagent is introduced for a reaction with lithium chloride and sodium nitrite in an aqueous solution, and the synthetic method helps to improve the yield of the reaction, increase the controllability on the reaction and avoid usage of methyl isocyanate with relatively high toxicity.
Owner:SINOPHARM A THINK PHARMA
Anti-cancer composition loading both platinum compound and synergist
InactiveCN101011351APharmaceutical delivery mechanismPharmaceutical non-active ingredientsCarboplatinMitozolomide
Disclosed is a slow release injection agent of anticancer composition containing platinum-group compounds and synergistic agent, which comprises slow release microspheres and dissolvent, wherein the slow release microspheres comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being conventional dissolvent or specific dissolvent containing suspension adjuvant. The viscosity of the suspension adjuvant is 100-3000cp (at 20-30 deg C), and is selected from sodium carboxymethylcellulose, the platinum-group compounds are selected from cisplatin, Carboplatin, Nedaplatin or Oxaliplatin, the synergistic agent can be selected from tetrazine drugs such as Mitozolomide or Temozolomide, and / or anticancer antibiotics such as Adriamycin, Aclarubicin, Epirubicin, mitomycin or pidorubicin, the slow release auxiliary materials are selected from polyphosphate ester copolymers such as p(LAEG-EOP), p(DAPG-EOP), copolymer or blend of polyphosphate ester with polylactic acid, Polifeprosan, sebacylic acid and PLGA. The anticancer composition can also be prepared into slow release implanting agent for injection or placement in or around tumor with a period of effective concentration maintenance over 60 days, as well as the treatment effect of appreciably lowering general reaction of the drugs, and improving the treatment effect of the non-operative treatment methods such as chemotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Dual sustained-release anticancer injection
InactiveCN101301264APharmaceutical delivery mechanismPharmaceutical non-active ingredientsMitozolomideMicrosphere
A double sustained release anticancer gel sustained release injection consists of anticancer medicine and amphiphilic block copolymer hydrogel, wherein the anticancer medicine comprises vincristine, vinorelbine, vinblastine, daunomycin, mitoxantrone, mitozolomide and temozolomide, etc., and exists in sustained release preparation injection in the forms of sustained release microsphere, microsphere or micropowder, i.e. the anticancer medicine in anticancer useful quantity is partly or completely wrapped inside the sustained release microsphere. Sustained release gel has temperature-sensitive gelling characteristics and is in the state of fluxible liquid in an environment with the temperature lower than body temperature; moreover, the sustained release gel can be automatically converted into non-flowing water-insoluble gel capable of biodegradation and absorption inside the body of a warm blood so as to slowly release medicine inside part of a tumor; the sustained release microsphere is propitious to release medicine smoothly and slowly, and double sustained release is propitious to control tumor cells entering a dormancy stage; moreover, the medicine which exists in the sustained release gel in the form of micropowder is propitious to release the medicine relatively faster and to control cells in faster proliferation. The double sustained release anticancer gel sustained release injection can used together with radiotherapeutic particle, etc.
Owner:济南基福医药科技有限公司
Pharmaceutical composition for restraining tumors
The invention relates to a pharmaceutical preparation, in particular to a compound pharmaceutical preparation with ginsenoside Rh2 and temozolomide as medicine active ingredients.
Owner:TIANJIN TASLY PHARMA CO LTD
Compounds for use in cancer therapy
ActiveUS20150065531A1Shrink tumorInduce apoptosisOrganic active ingredientsBiocideGeldanamycinApoptosis
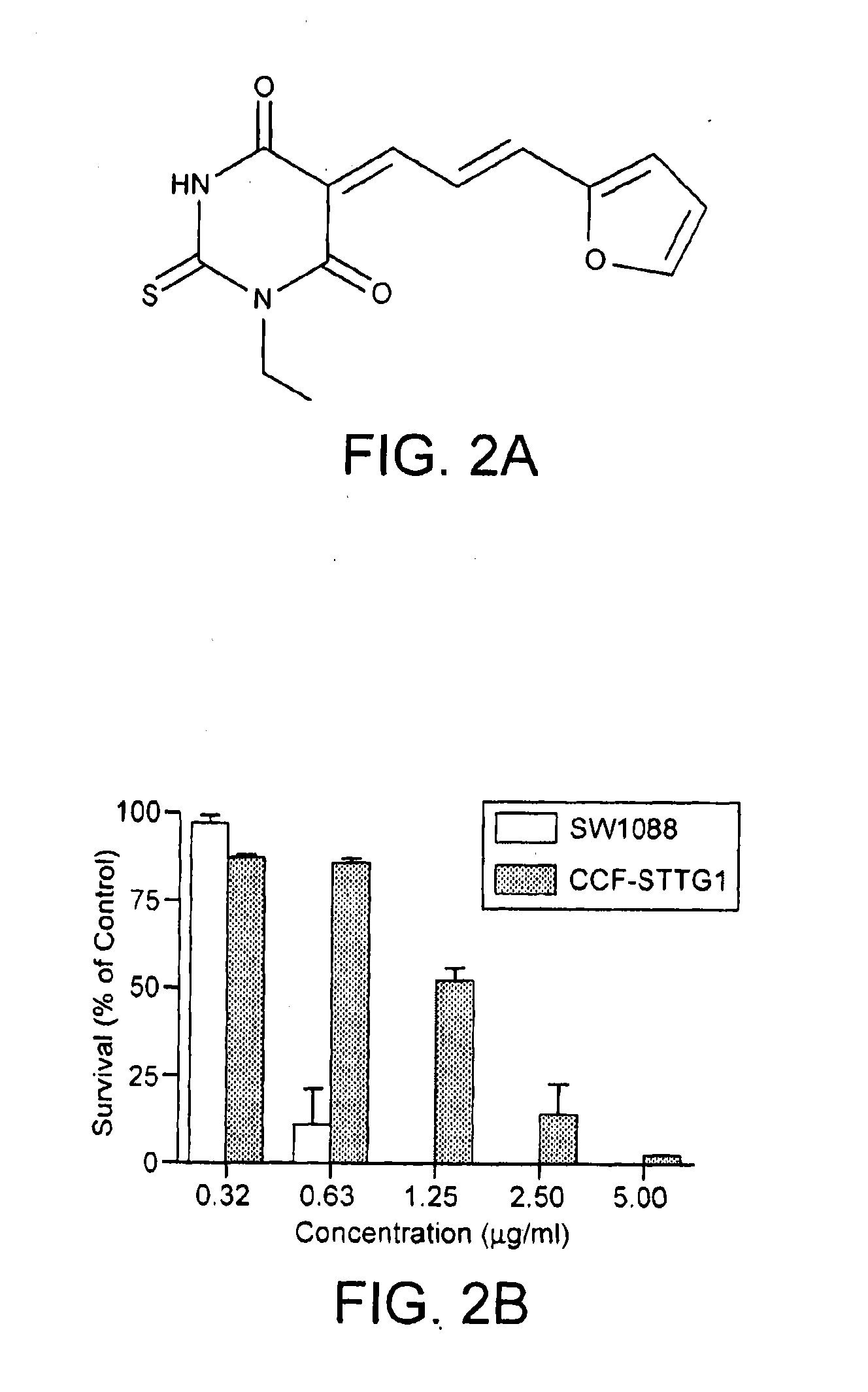
Provided are methods and compositions for use in therapy, and in particular for treating cancer, preferably drug-resistant cancer, and / or radiation resistant cancer. The compounds may be used for reducing tumor size in a mammalian subject and for inducing apoptosis in a tumor cell. The methods are effective on tumor cells that are resistant to drugs such as temozolomide, doxorubicin, and geldanamycin, as well as non-resistant tumor cells. Further provided are barbiturate and thiobarbiturates diene compounds for use in treating cancer, and uses, methods and compositions relating to these compounds.
Owner:NUHOPE
Use of dianhydrogalactitol and analogs and derivatives thereof, together with radiation, to treat non-small-cell carcinoma of the lung and glioblastoma multiforme and suppress proliferation of cancer stem cells
PendingUS20190015379A1Good curative effectReducing sideOrganic active ingredientsNervous disorderDianhydrogalactitolSmall-cell carcinoma
The use of dianhydrogalactitol provides a novel therapeutic modality for the treatment of non-small-cell lung carcinoma (NSCLC) and for the treatment of glioblastoma multiforme (GBM). Dianhydrogalactitol acts as an alkylating agent on DNA that creates N7 methylation. Dianhydrogalactitol is effective in suppressing the growth of cancer stem cells and is active against tumors that are refractory to temozolomide; the drug acts independently of the MGMT repair mechanism.
Owner:DEL MAR PHARMA
Treatment of cancers using a combination comprising parp inhibitors, temozolomide and/or radiation therapy
InactiveUS20200155567A1More responseOrganic active ingredientsPharmaceutical delivery mechanismTemozolomidaPharmaceutical drug
Disclosed herein is a method for the prevention, delay of progression or treatment of cancer in a subject, comprising administering to the subject in need thereof a PARP inhibitor, particularly, (R)-2-fluoro-10a-methyl-7, 8, 9, 10, 10a, 11-hexahydro-5, 6, 7a, 11-tetraazacyclohepta [def] cyclopenta [a] fluoren-4 (5H)-one, a sesqui-hydrate thereof, or a pharmaceutically acceptable salt thereof, in combination with temozolomide and / or radiation therapy. Also, disclosed a pharmaceutical combination comprising a PARP inhibitor, particularly, (R)-2-fluoro-10a-methyl-7, 8, 9, 10, 10a, 11-hexahydro-5, 6, 7a, 11-tetraazacyclohepta [def] cyclopenta [a] fluoren-4 (5H)-one, a sesqui-hydrate thereof, or a pharmaceutically acceptable salt thereof, in combination with temozolomide and the use thereof.
Owner:BEIGENE SWITZERLAND GMBH
Sustained release temozolomide capsules and preparation method thereof
ActiveCN108014097AConstant dissolutionConstant dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsAntioxidantDissolution
The invention relates to sustained release temozolomide capsules and a preparation method thereof, and belongs to the technical field of medicine. Each sustained release temozolomide capsule comprises2-20 sustained release micro-tablets and a capsule shell, wherein each micro-tablet contains 2-20 mg of temozolomide. The sustained release micro-tablets comprise raw and auxiliary materials in percentage by weight as follows: 5%-50% of temozolomide, 5%-40% of a diluent, 10%-70% of a sustained release agent, 0.5%-2% of an antioxidant, 0-5% of an adhesive and 0.5%-2% of a lubricant. The inventionfurther discloses the preparation method of the sustained release temozolomide capsules. The materials are pelletized or mixed and pressed into the micro-tablets directly, and the micro-tablets are loaded to hollow capsules quantitatively. The sustained release temozolomide capsules have the advantages of being good in dissolution, convenient to use, stable in plasma concentration and long in acting time, compliance of patients is improved, the preparation process is simple and lower in cost, and industrial production is easy.
Owner:BEIJING SL PHARMA +1
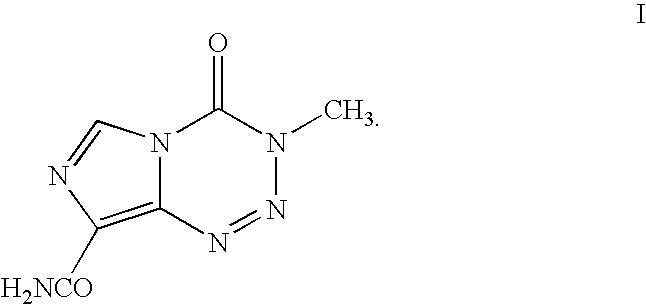
Temozolomide compounds, polymers prepared therefrom, and method of treating a disease
ActiveUS20180079754A1Organic chemistryPharmaceutical non-active ingredientsMedicinal chemistryPolymer
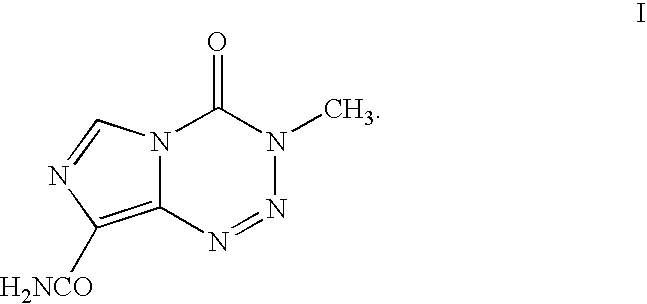
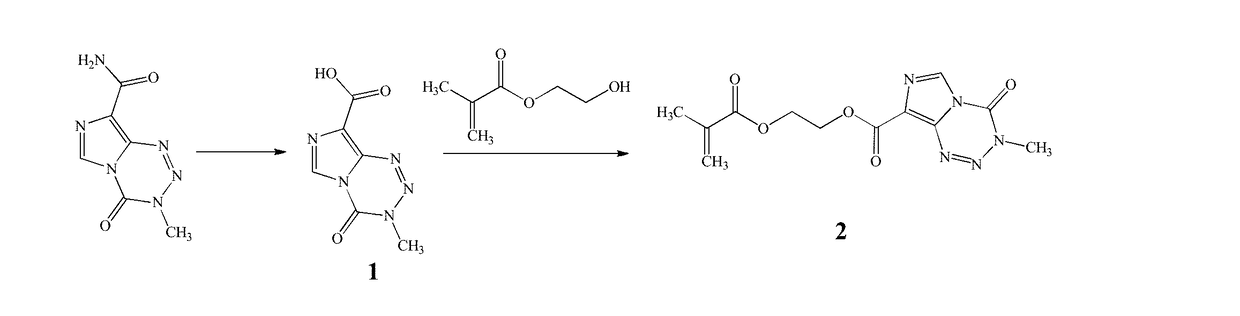
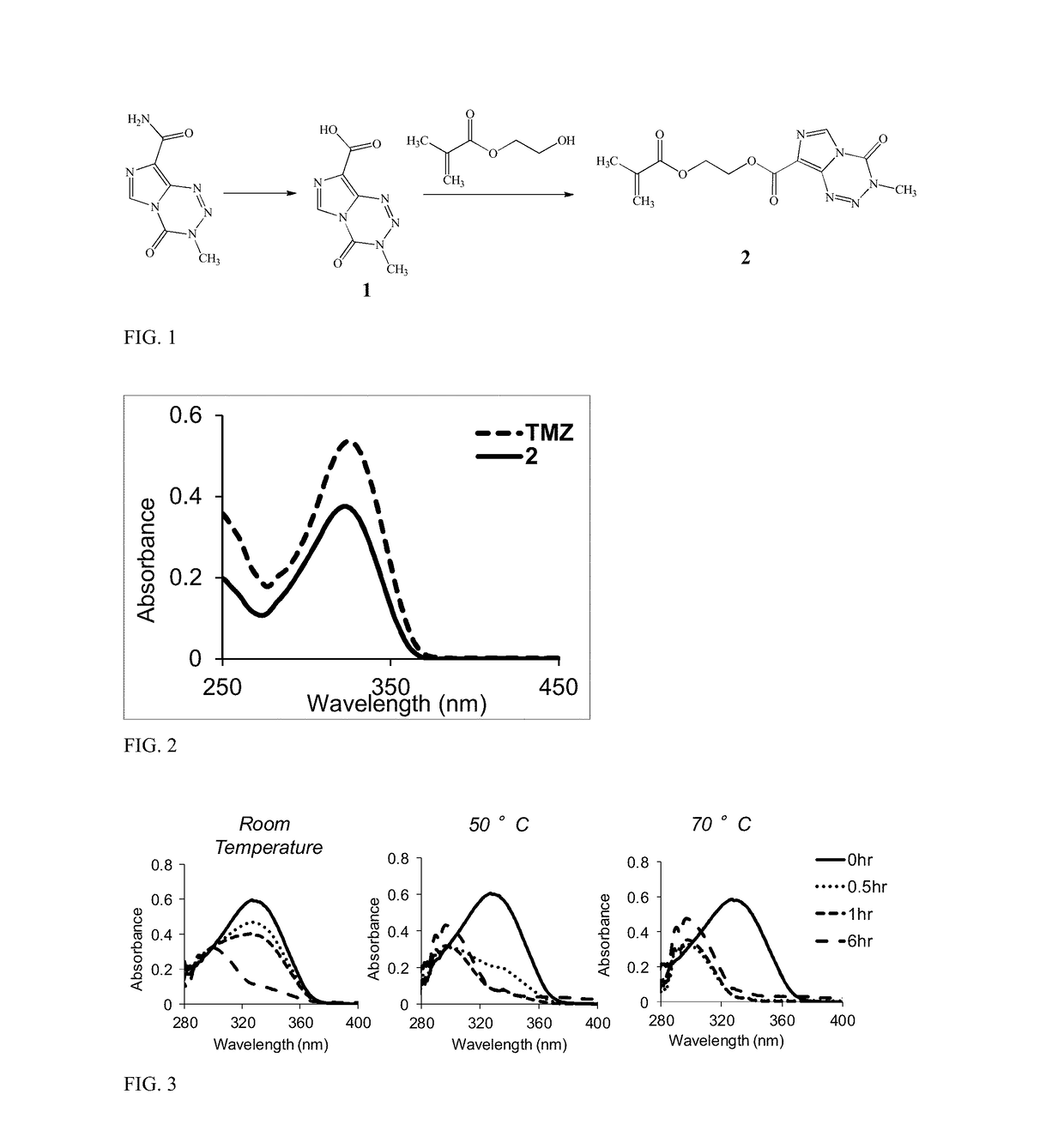
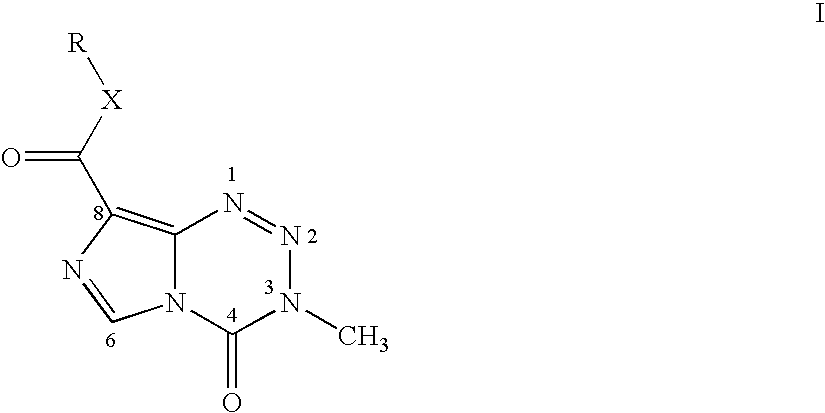
A temozolomide compound according to formula (I)is described, wherein R1, L1, and X are defined herein. The temozolomide compound can be used to prepare polymers comprising temozolomide. Additionally, the polymers comprising temozolomide can be particularly useful in the treatment of certain diseases.
Owner:UNIV OF MASSACHUSETTS
Pharmaceutical Composition Comprising Temozolomide Ester
InactiveUS20080044457A1Inhibition effectGood killing effectBiocideOrganic chemistryTransdermal patchAbnormal tissue growth
The present invention discloses general formula I of Temozolomide-8-carboxylate compounds, the process for preparation, pharmaceutical compositions comprising the compounds and the use of the compounds and pharmaceutical compositions for the manufacture of an antitumor medicament. The said pharmaceutical composition comprises one or more general formula I Temozolomide-8-carboxylate compounds as active ingredient, together with conventional pharmaceutical carriers. The composition also comprises one or more pharmaceutically acceptable acidic material, optionally second or tertiary alcohol or ester or ether derivatives thereof. The said pharmaceutical composition can be made into various common formulations, particularly oral formulations as well as topically transdermal patches. The present invention also discloses the application of the compounds and the compositions to treat tumor.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD +1
Resveratrol-temozolomide eutectic crystal and preparation method and application thereof
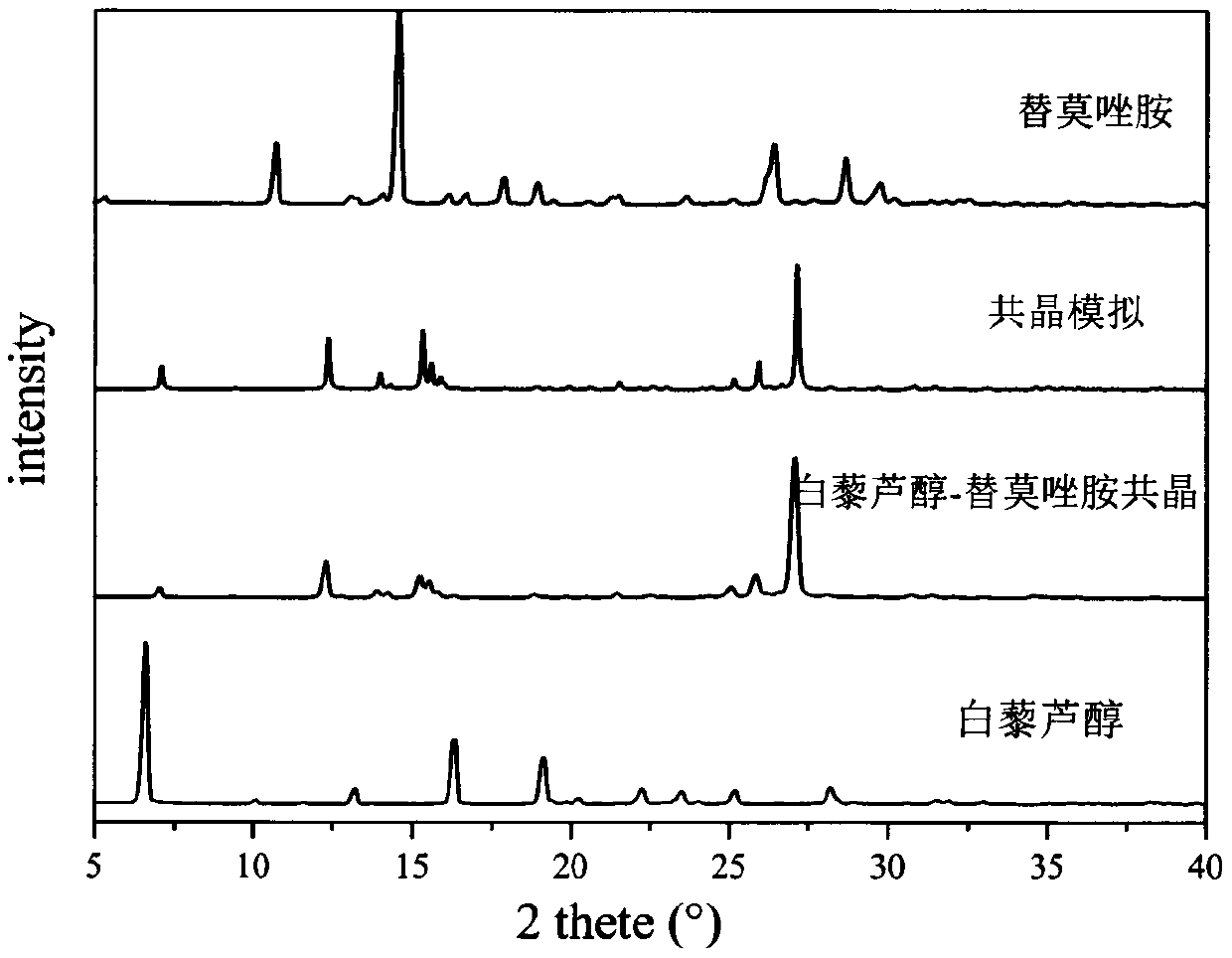
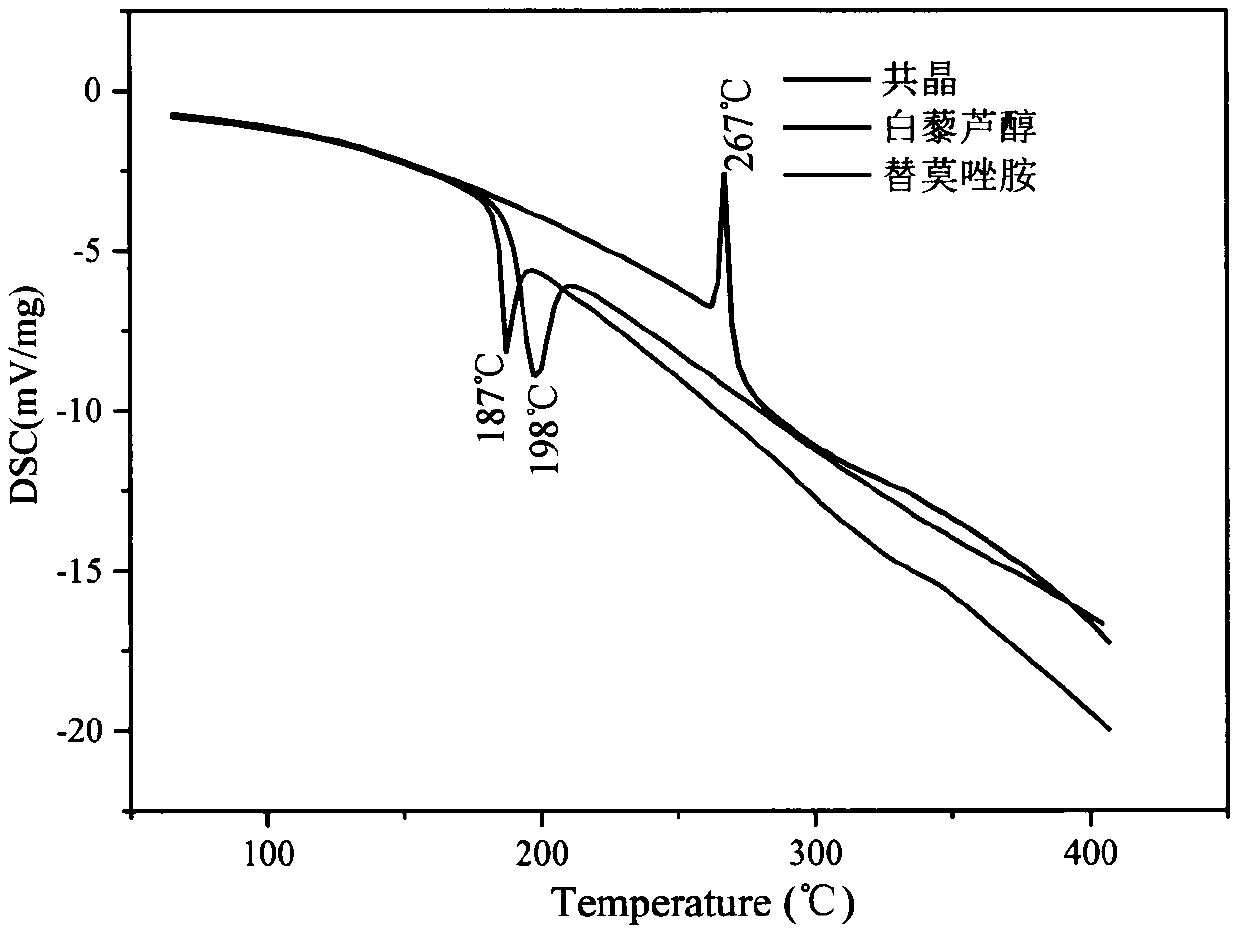
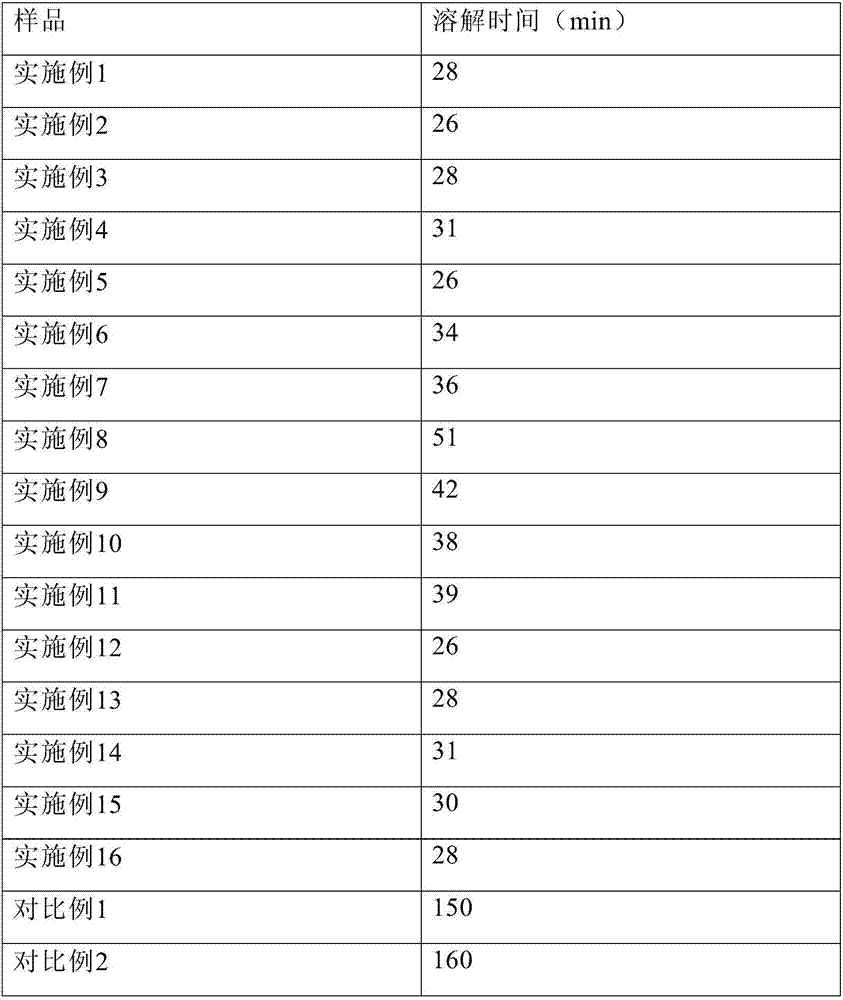
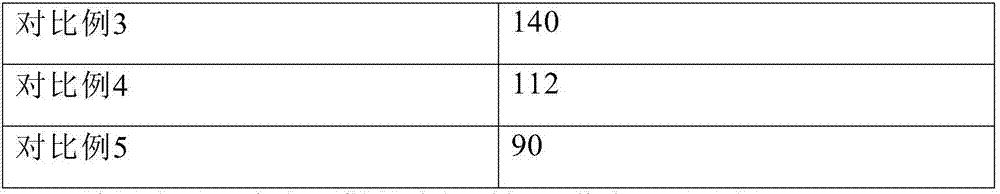
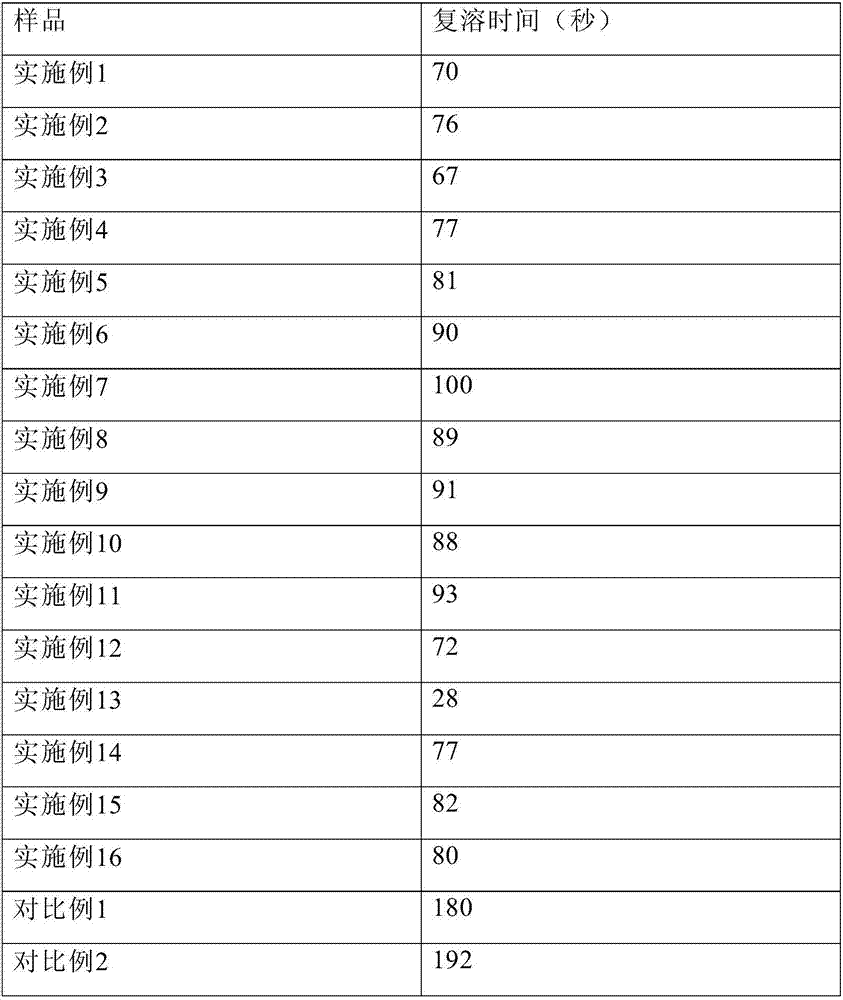
PendingCN111423444AGood curative effectImprove securityOrganic compound preparationOrganic chemistry methodsTemozolomidaPharmaceutical drug
The invention aims to provide a resveratrol-temozolomide eutectic crystal, which can improve the solubility of a medicine, improve the stability and improve the bioavailability, and also provides a preparation method and application of the resveratrol-temozolomide eutectic crystal. The invention relates to the resveratrol-temozolomide eutectic crystal. Resveratrol is used as an active pharmaceutical ingredient and is combined with another drug temozolomide through hydrogen bond weak action to form a resveratrol-temozolomide drug-drug co-crystal, the resveratrol-temozolomide drug-drug co-crystal is crystallized in a monoclinic system, the space group is P21 / c, the axial long axis angle alpha is 90.00 degrees, beta is 95.9755 (1) degrees and gamma is 90.00 degrees.
Owner:GUANGXI UNIV OF CHINESE MEDICINE
Injectable temozolomide powder injection preparation and preparation method thereof
InactiveCN103845294AGood resolubilityQuality is easy to controlPowder deliveryOrganic active ingredientsTemozolomidaEngineering
The invention discloses an injectable anticancer drug temozolomide freeze-dried powder injection preparation and its preparation method. The powder injection preparation is stable, controllable in quality, simple in preparation process and strong in operability, and a prepared product has the characteristics of good solubility, convenient clinical use, a long period of storage and the like.
Owner:杭州容立医药科技有限公司
Temozolomide sustained-release implant for treating solid tumor
InactiveCN101185630AIncreased sensitivityHigh clinical application valueOrganic active ingredientsPharmaceutical delivery mechanismPoly dl lactideTherapeutic effect
A temozolomide sustained release implant for treating solid tumors is characterized in that the sustained release implant includes effective dose of anticancer temozolomide, sustained release excipients and a certain quantity of sustained release moderator. Solid tumors include pancreatic cancer, lung cancer, liver cancer, breast cancer, cerebroma, ovarian cancer, prostatic carcinoma, esophageal carcinoma, lymphadenoma, osteosarcoma and colorectal cancer. Sustained release excipients mainly are one or combination of copolymer of glycolic acid and hydroxyacetic acid, polifeprosan, and poly(L-lactide-co-ethyl phosphate); in the process of decompression, temozolomide can be slowly released to local tumor, thus maintaining effective drug concentration of local tumor as well as significantly reducing overall toxic reaction. Being placed in local tumor, the sustained release implant not only reduces overall toxic reaction of temozolomide, but also selectively improves drug concentration in local tumor, enhancing the therapeutic effects of non-operative therapy such as chemotherapy drugs and radiotherapy.
Owner:SHANDONG LANJIN PHARMA +1
Temozolomide injectable composition and preparation method thereof
ActiveCN107875396AShorten the timeLower levelOrganic active ingredientsMacromolecular non-active ingredientsGlycineTemozolomida
The invention relates to a temozolomide injectable composition and a preparation method thereof. Concretely, the invention discloses a temozolomide composition and a preparation method thereof. The composition is an injectable composition. Glycine and sulfobutylether-beta-cyclodextrin are used as solubilizing agents; organic dissolvants are not added. The temozolomide injectable composition has various advantages of obviously improving the dissolution speed, reducing the solution state existing time of temozolomide during the industrial production, reducing the relevant substance level and thelike.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD +1
Temozolomide powder formulation
ActiveUS9949967B2Good and consistent flowabilityTitrated readily and accuratelyOrganic active ingredientsDispersion deliveryOral medicationTemozolomida
The present invention relates to a solid pharmaceutical composition of temozolomide that has good and consistent flowability as a powder and taste masking and is readily dispersible in an aqueous solution suitable for oral administration, e.g., as a dry sprinkle. This permits patients and healthcare workers to accurately measure doses and safely dispense the drug.
Owner:AMPLIPHARM PHARMA LLC
Methods of determining acute myeloid leukemia response to treatment with farnesyltransferase inhibitors
ActiveCN103328971APredictive validityMicrobiological testing/measurementBiological testingGene expressionPaclitaxel
The disclosed method rapidly identifies with desired accuracy AML patients, including elderly AML patients, likely to respond to treatment with a combination of a farnesyltransferase inhibitor and one or more of etoposide, teniposide, tamoxifen, sorafenib, paclitaxel, temozolomide, topotecan, trastuzumab and cisplatinum. In an embodiment, the improvements include the use of whole blood rather than the customary bone marrow sample, thus making the assay more accurate, rapid, less intrusive, less expensive as well as less painful. The method includes evaluation of a two-gene expression ratio (RASGRP1 :APTX), which with a corresponding threshold, provides sufficient accuracy for predicting the response to the combination treatment. In the preferred embodiment the combination treatment combines tipifarnib (Rl 15777, ZARNESTRA TM ) with etoposide. Further, the elderly AML patients identified as being likely responsive to the combination treatment with tipinifarb and etoposide have a complete recovery rate comparable to the best therapy available for younger patients.
Owner:JANSSEN PHARMA NV
Treatment cancers using combination comprising parp inhibitors, temozolomide and/or radiation therapy
InactiveCN110891576AOrganic active ingredientsOrganic chemistryCancer preventionRadical radiotherapy
Disclosed herein is a method for the prevention, delay of progression or treatment of cancer in a subject, comprising administering to the subject in need thereof a PARP inhibitor, particularly, (R) -2-fluoro-10a-methyl-7, 8, 9, 10, 10a, 11-hexahydro-5, 6, 7a, 11-tetraazacyclohepta [def] cyclopenta [a] fluoren-4 (5H) -one, a sesqui-hydrate thereof, or a pharmaceutically acceptable salt thereof, incombination with temozolomide and / or radiation therapy. Also, disclosed a pharmaceutical combination comprising a PARP inhibitor, particularly, (R) -2-fluoro-10a-methyl-7, 8, 9, 10, 10a, 11-hexahydro-5, 6, 7a, 11-tetraazacyclohepta [def] cyclopenta [a] fluoren-4 (5H) -one, a sesqui-hydrate thereof, or a pharmaceutically acceptable salt thereof, in combination with temozolomide and the use thereof.
Owner:百济神州(苏州)生物科技有限公司
Temozolomide powder formulation
ActiveUS10098874B2Good and consistent flowabilityTitrated readily and accuratelyPowder deliveryOrganic active ingredientsOral medicationTemozolomida
The present invention relates to a solid pharmaceutical composition of temozolomide that has good and consistent flowability as a powder and taste masking and is readily dispersible in an aqueous solution suitable for oral administration, e.g., as a dry sprinkle. This permits patients and healthcare workers to accurately measure doses.
Owner:AMPLIPHARM PHARMA LLC
Anti-cancer composition loading both mtrosourea medicament and synergist
InactiveCN101011346APharmaceutical delivery mechanismPharmaceutical non-active ingredientsMitozolomideTreatment effect
Disclosed is a slow release injection agent of anticancer composition containing nitrosourea drugs and synergistic agent, which comprises slow release microspheres and dissolvent, wherein the slow release microspheres comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being conventional dissolvent or specific dissolvent containing suspension adjuvant. The viscosity of the suspension adjuvant is 100-3000cp (at 20-30 deg C), and is selected from sodium carboxymethylcellulose, the nitrosourea drugs are selected from Carmustine, Nimustine or Fotemustine, the synergistic agent can be selected from tetrazine drugs such as Mitozolomide or temozolomide and / or anticancer antibiotics such as Adriamycin, Aclarubicin, Epirubicin, mitomycin or pidorubicin, the slow release auxiliary materials are selected from polyphosphate ester copolymers such as p(LAEG-EOP), p(DAPG-EOP), copolymer or blend of polyphosphate ester with polylactic acid, Polifeprosan, sebacylic acid and PLGA. The anticancer composition can also be prepared into slow release implanting agent, for injection or placement in or around tumor with a period of effective concentration maintenance over 60 days, as well as the treatment effect of appreciably lowering general reaction of the drugs, and improving the treatment effect of the non-operative treatment methods such as chemotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
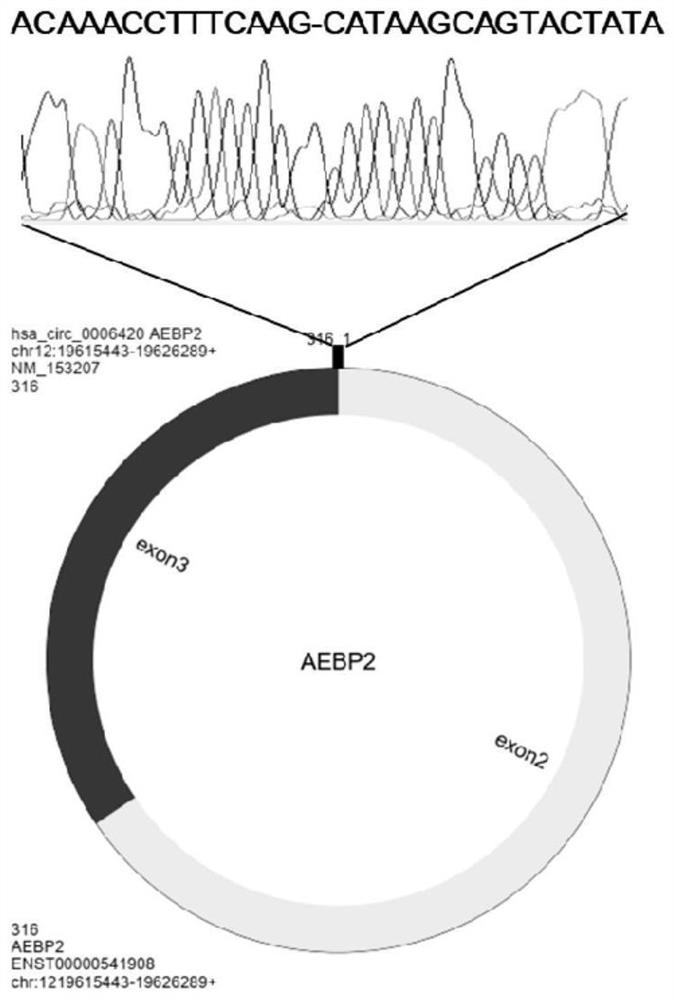
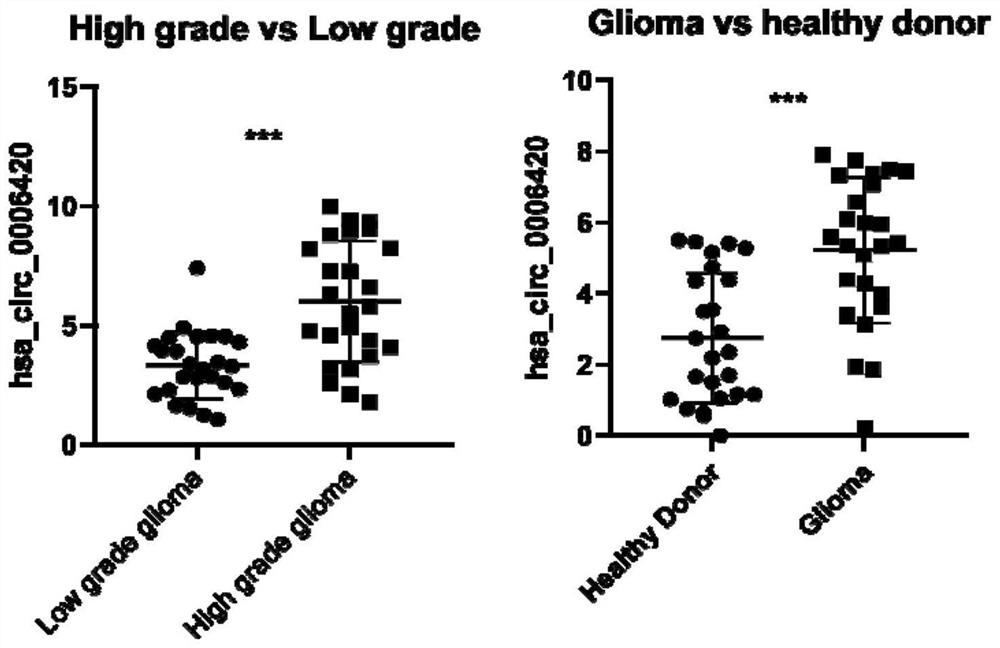
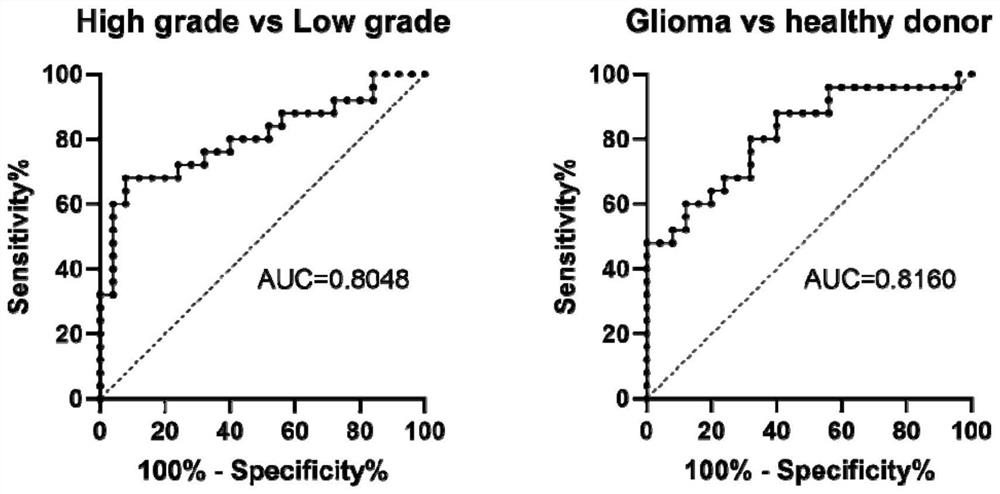
Hsacirc0006420 circular RNA and application thereof in glioma diagnosis and prognosis evaluation
PendingCN114277031AMicrobiological testing/measurementDNA/RNA fragmentationNucleotideCancers diagnosis
The invention discloses an hsacirc0006420 circular RNA (Ribonucleic Acid) and application of the hsacirc0006420 circular RNA in glioma diagnosis and prognosis evaluation, and belongs to the field of cancer diagnosis. The nucleotide sequence of the hsacirc0006420 circular RNA is as shown in SEQ ID NO. 1 (sequence identifier number 1). According to the present invention, the new hsacirc0006420 circular RNA gene is obtained, the expression of the hsacirc0006420 circular RNA in the glioma tumor tissue and the patient blood sample is significantly increased, and the new hsacirc0006420 circular RNA gene can be used for the glioma diagnosis, the response after the temozolomide treatment, and the patient lifetime prediction.
Owner:上海纽仁生物医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com