Double-element solution type preparation for intravenous injection and intracerebral injection
A binary solution and solvent technology, applied in the directions of non-active ingredient medical preparations, medical preparations containing active ingredients, drug delivery, etc., can solve the problems such as it is difficult to meet the quality stability requirements of temozolomide, and achieve the addition of ingredients. single effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Temozolomide Powder Injection
[0031] Temozolomide was vacuum-dried and dispensed into brown injection vials of 10ml in volumes of 50, 100 and 200mg.
Embodiment 2
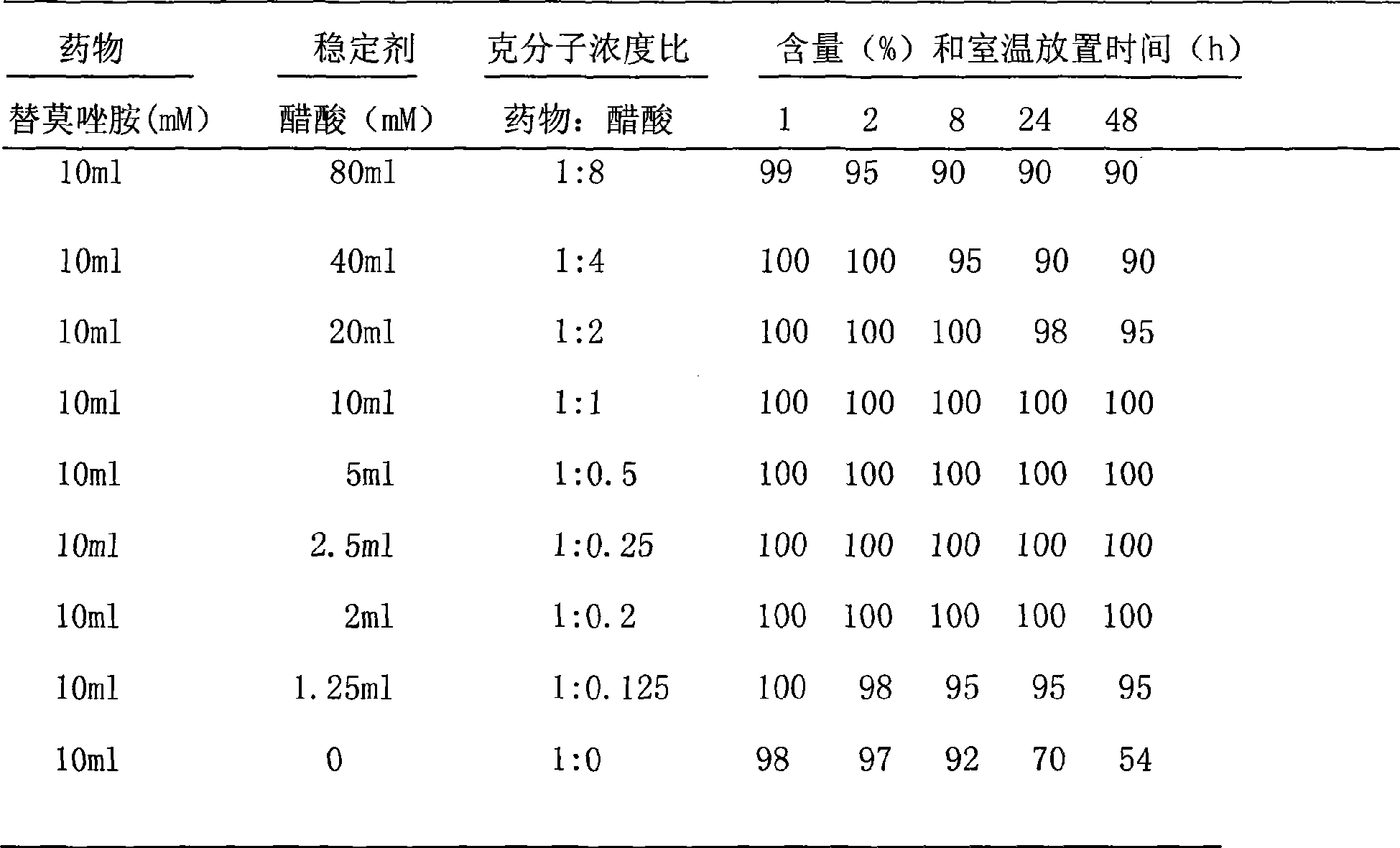
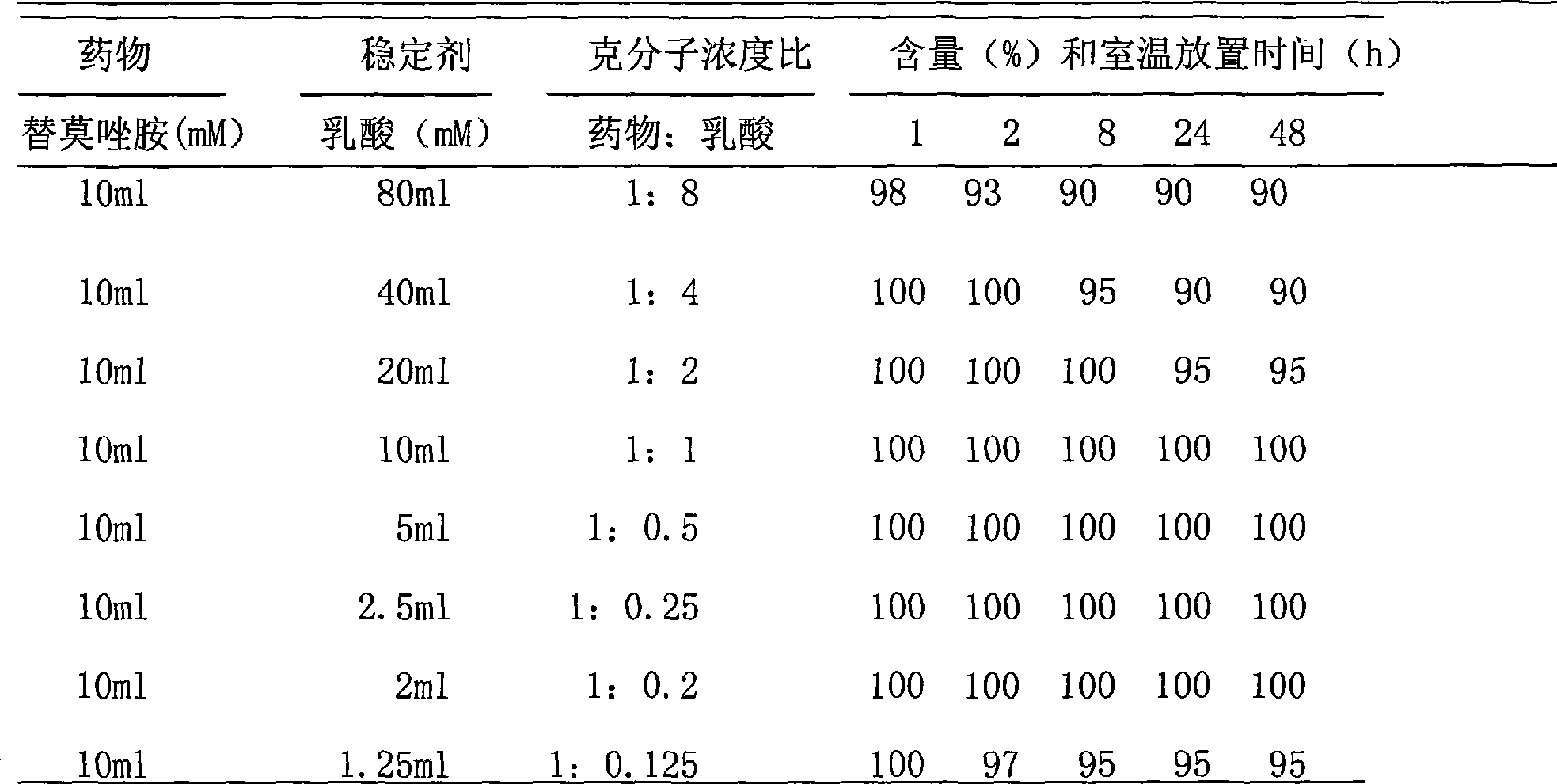
[0032] Embodiment 2 Temozolomide solvent
[0033] Take 3.9ml of glacial acetic acid and add water for injection to make up to 2500ml, and divide it into vials for injection, the filling volume is 2.5, 5 and 10ml.
Embodiment 3
[0034] Example 3 Take 2.5ml / bottle and add to 50mg / bottle Temozolomide solvent to dissolve, and the content is not less than 99% within 48 hours at room temperature.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com