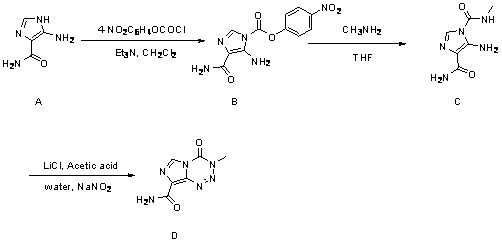
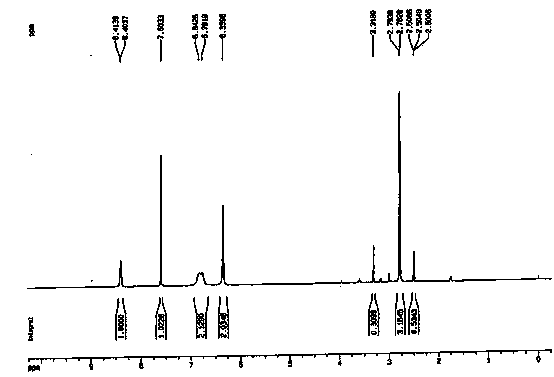
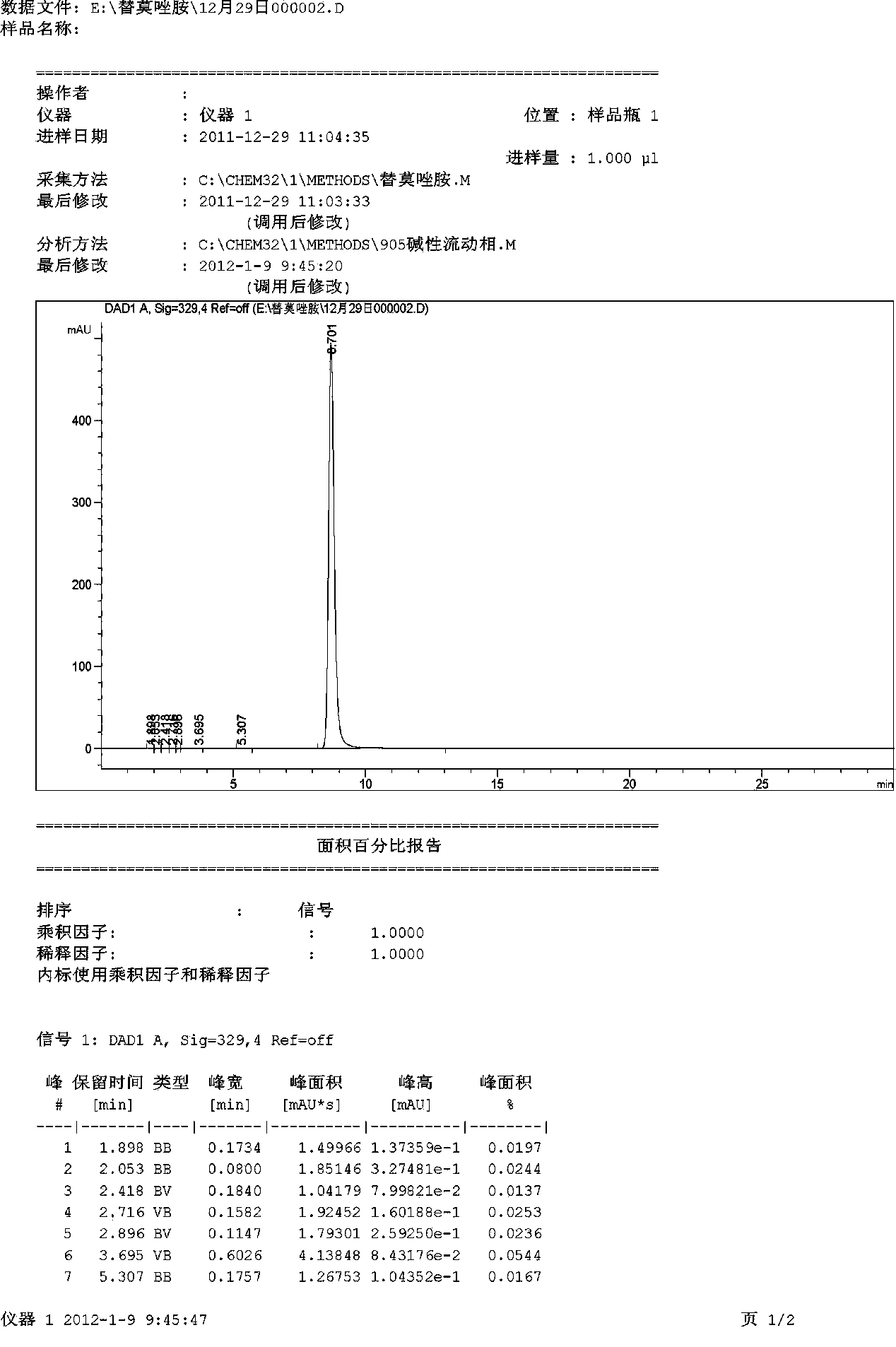
Synthetic method for temozolomide and intermediate
A technology of temozolomide and its synthesis method, which is applied in the field of high-efficiency synthesis, can solve problems such as difficult control of reaction conditions, low reaction yield, and high toxicity of MIC, and achieve the effects of low cost, simple process route, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation of embodiment 1 temozolomide
[0027] Preparation of step A intermediate 2
[0028]
[0029] In a 5 L three-neck round bottom flask equipped with a thermometer, add 5-aminoimidazole-4-carboxamide in sequence A (80g, 634.32 mmol), dichloromethane (1920 mL), triethylamine (176.82 mL, 1268.63 mmol)), stirred at 25°C for 10 minutes, lowered the temperature of the reaction system below 0°C, after 10 minutes, drop Add 4-nitrophenyl chloroformate (255.71 g, 1268.36 mmol) dissolved in 1280 mL of dichloromethane, react at below 0 °C for 4 hours, then control the temperature at 25 °C for 18 hours . The reaction solution was suction-filtered with a Buchner funnel, and the obtained filter cake was beaten and washed with 1000 mL of dichloromethane and 200 ml of water mixture for 1 hour, then suction-filtered again, the filter cake was washed with dichloromethane, and dried at room temperature to obtain a yellow solid product. Yield: 92%, melting point: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com