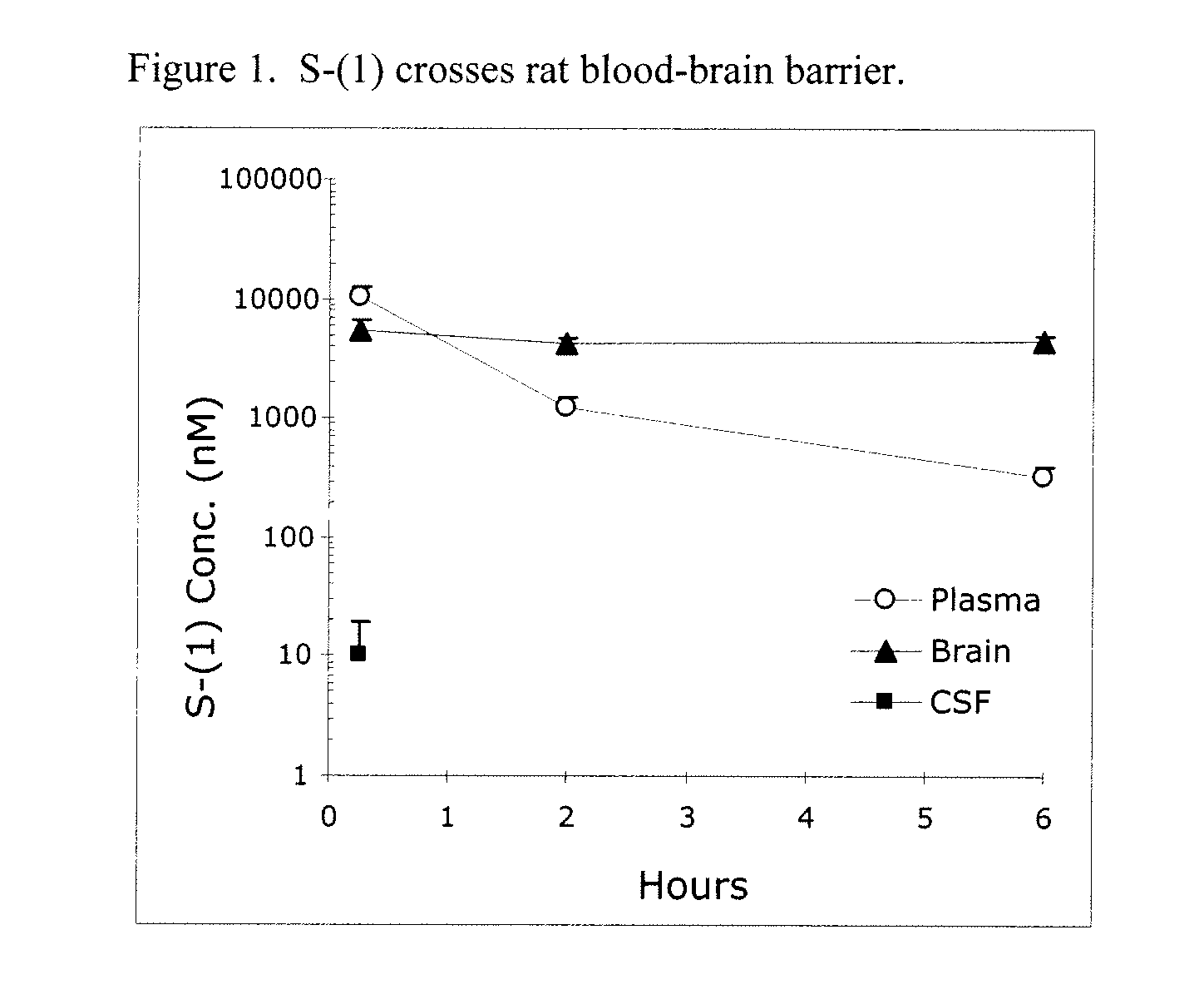
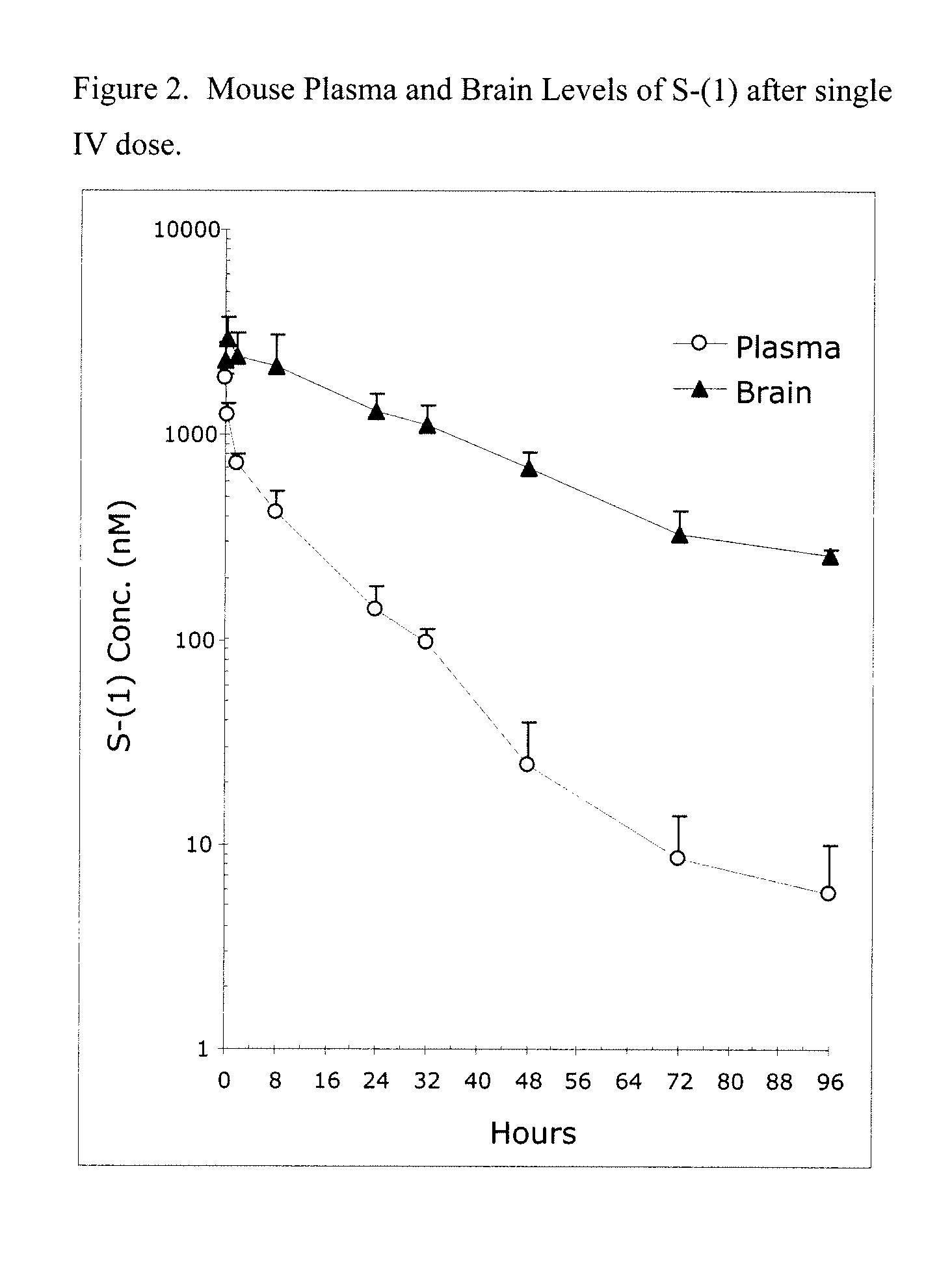
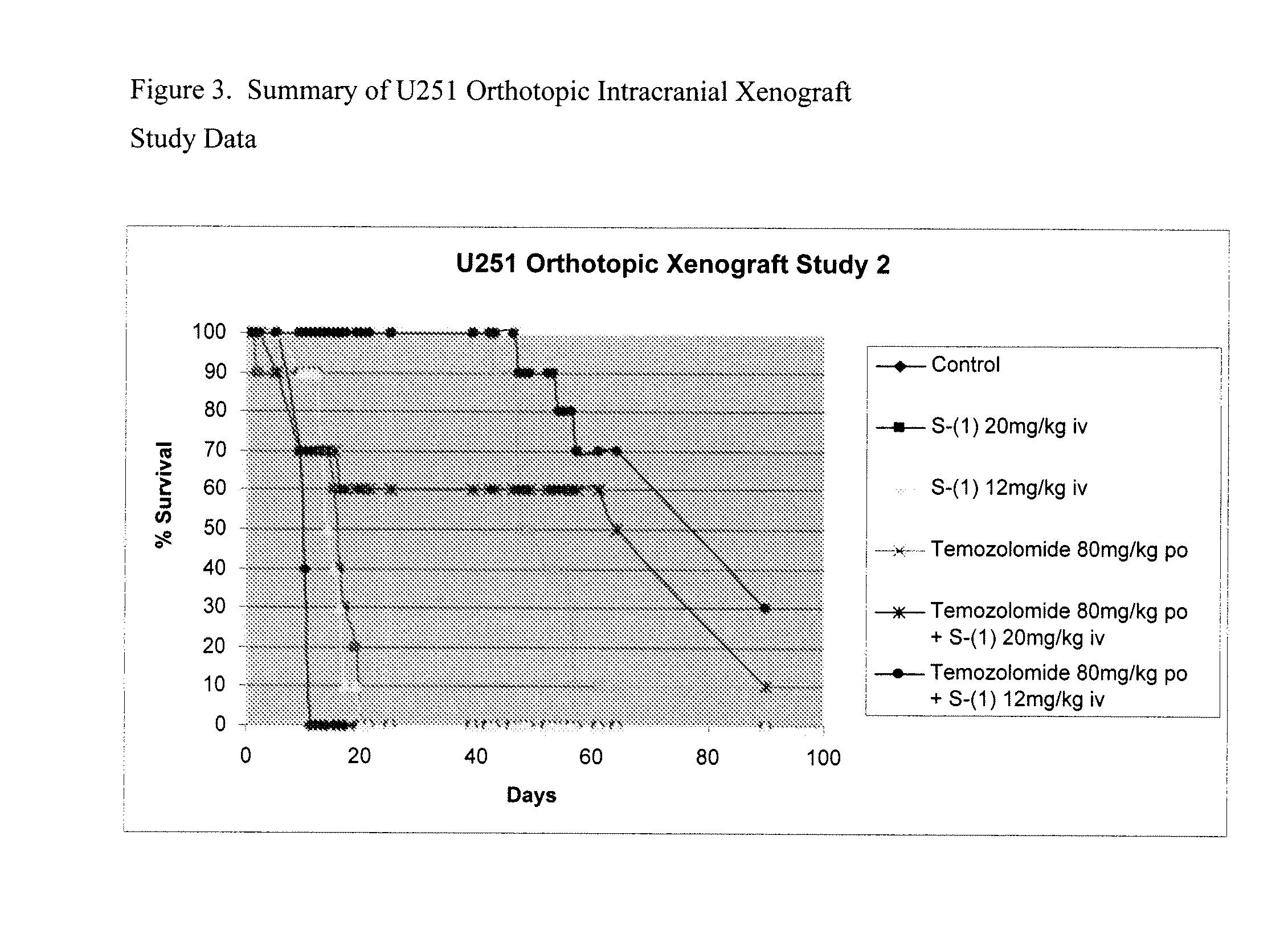
Taxane analogs for the treatment of brain cancer
a technology of brain cancer and analogs, applied in the direction of biocide, antibody medical ingredients, drug compositions, etc., can solve the problems of limiting the usefulness of the drug class, cell dividing, protein loss of flexibility,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Chemical Example 1
Separation of diastereoisomers of Formula (3) by Normal Phase Chromatography
[0090]A solution of the compound of formula (3), which comprises a mixture of diasteroisomers of formula (1) and formula (2) (570 mg) was concentrated to light yellow oil, dried in the vacuum oven for 15 min and re-dissolved in 35:65 MTBE / n-heptane. The solution was loaded onto a flash chromatography column packed with spherical silica (YMC-1701, 56 g), which had been conditioned with 35:65 MTBE / n-heptane. The solution flask was rinsed (2×) with ˜2 mL of MTBE onto the column. The column was eluted with 35:65 MTBE / n-heptane and fractions (25 mL) were collected. Fractions containing the pure product (fractions 23-25) as indicated by visual spotting (to identify the elution of UV active material) and by TLC analysis (50:50 MTBE / n-heptane) were collected, pooled and concentrated to give 305 mg of the diastereoisomer of formula S-(1) as a white solid.
[0091]The compound S-(1) was characterized by...
examples
[0125]In Vitro ED50 MT Polymerization Study
[0126]In this tubulin binding assay, microtubule protein (MTP) is used as a substrate. The assay contains bovine tubulin plus microtubule associated proteins (MAP). MTP is polymerized into microtubules in the presence of DAPI (4′,6′-diamidino-2-phenylindole), a fluorescent compound. DAPI binds to tubulin; when microtubules are formed and there is an enhancement of fluorescence. The microtubule formation is measured as a function of time, using a fluorescence plate reader. The ED50 values obtained with this method are in good agreement with older sedimentation techniques. The more current assay, using DAPI, is faster and uses less protein. The method used is based on the procedure published by Donna M. Barron, et al, “Fluorescence-based high-throughput assay for antimicrotuble drugs” Analytical Biochemistry, 315: 49-56, 2003, which is incorporated by reference in its entirety. The excitation wavelength, in that assay, was set at 370 nm and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| reaction time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com