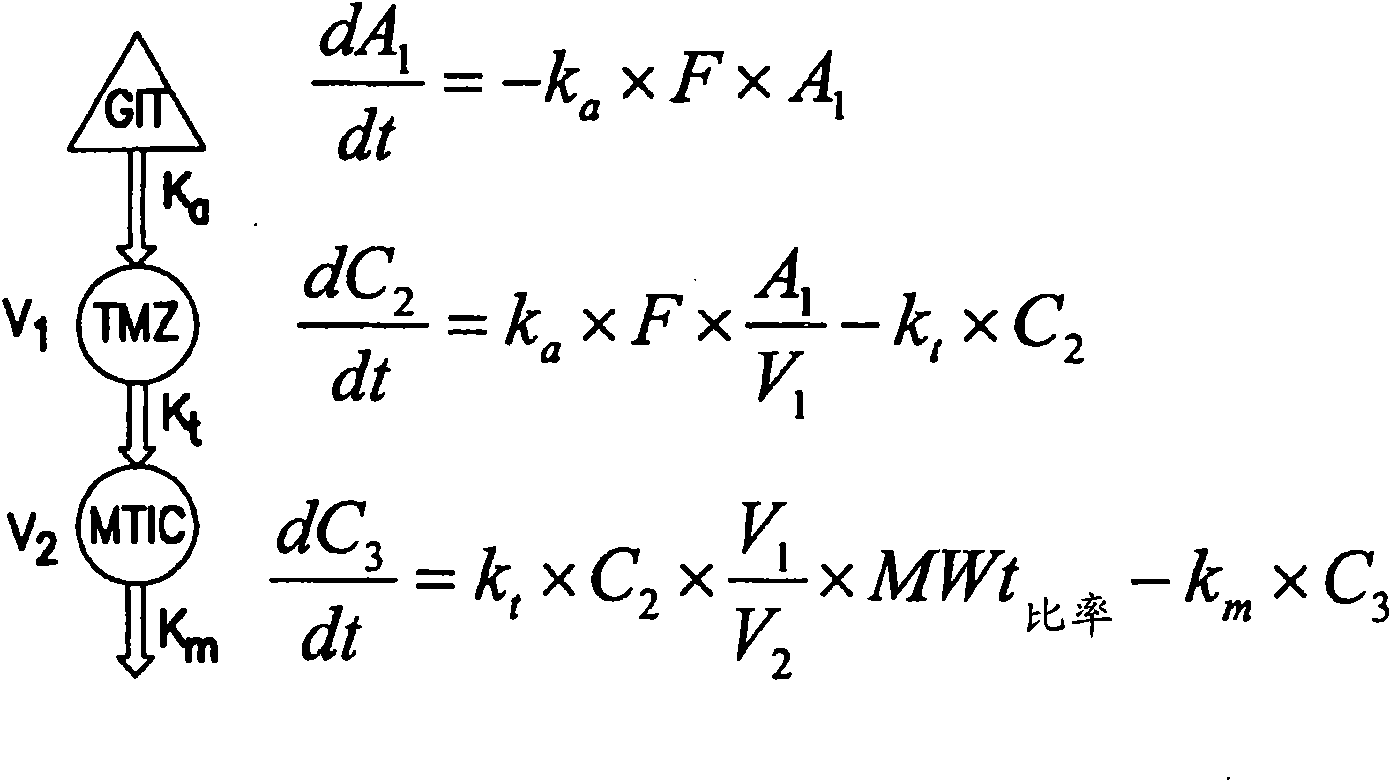Methods of treatment using intravenous formulations comprising temozolomide
A technology for temozolomide and preparations, which is applied in the treatment field of using preparations for intravenous injection containing temozolomide, can solve the problems of reducing the bioavailability of drugs and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
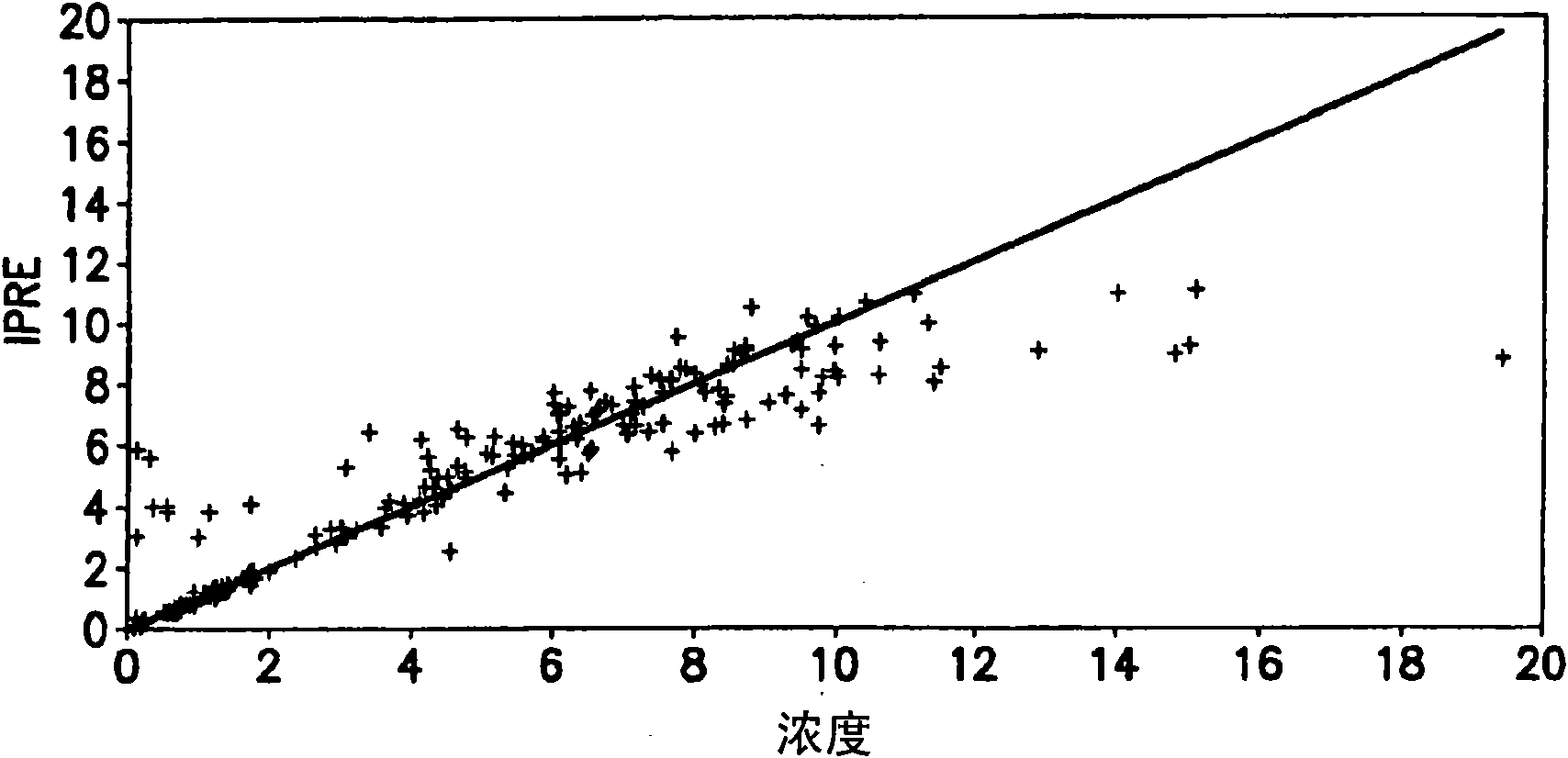
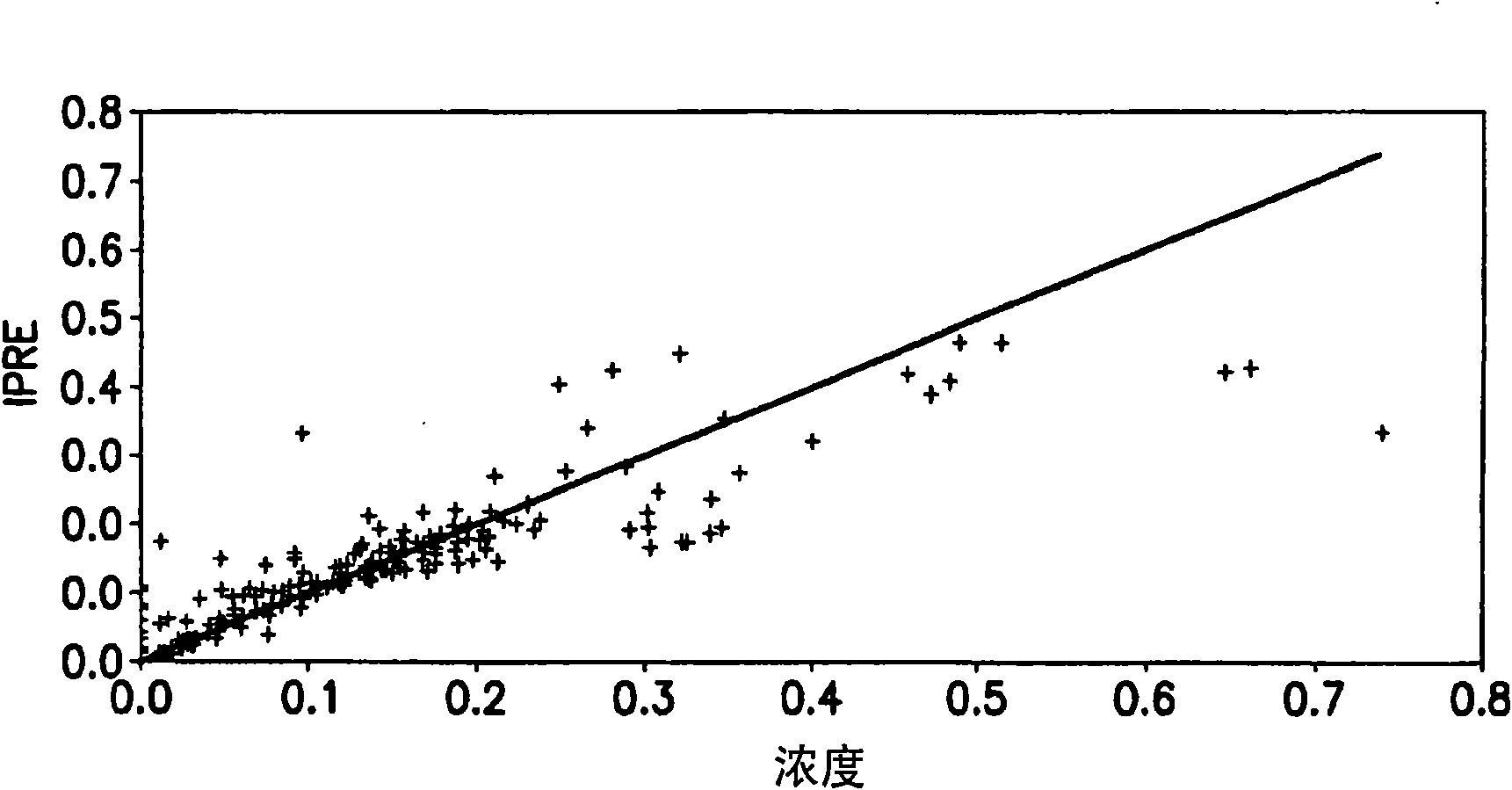
[0152] A population pharmacokinetic model was developed to characterize the distribution of both TMZ and MTIC following PO administration. Pharmacokinetic parameters describing the absorption, distribution, metabolism and excretion of TMZ and MTIC were estimated using oral data obtained from a study (see Middleton, Journal of Clinical Oncology, 15(1): 158-166 (2000)). A PK model with first order absorption, unicompartmental distribution of TMZ, first order elimination of TMZ, unicompartmental distribution of MTIC and first order elimination of MTIC was used. Drugs and Pharmaceutical Sciences, Volume 15: Pharmacokinetics, Gibaldi and Perrier, Second Edition, 1982. The mathematical equations describing the model are shown in figure 1 middle.
[0153] In the equation shown in the figure, k a is the absorption rate constant, F is the bioavailability of TMZ following oral administration of the drug, k t To describe the rate constant for the conversion of TMZ to MTIC, V 1 is th...
Embodiment 2
[0174] Clinical trial simulations were performed using the IV PK model and the Pharsight trial simulator. Multiple simulation protocols were performed to evaluate different oral bioavailability and the total time that TMZ would need to be infused into the systemic blood circulation to match the Cmax and AUC obtained following oral administration. Each simulation was repeated 100 times and the probability of success was defined as the number of times the study passed the four bioequivalence criteria (Cmax TMZ, CmaxMTIC, AUC TMZ and AUC MTIC). Confidence intervals for Cmax and AUC for IV administration were within 80-125% of those for oral administration. Table 4 shows the results of these simulations.
[0175] Table 4
[0176]
[0177] * The four standards are: Cmax TMZ, Cmax MTIC, AUC TMZ and AUC MTIC.
[0178] Simulation results using an IV PK model show that if the total oral TMZ bioavailability (F) is greater than or equal to 90% (ie F = 0.9-1.0), no IV dose adjustme...
Embodiment 3
[0180] Formulations for intravenous injection according to the invention are provided in Table 5. The formulation was prepared according to the method of US Patent 6,987,108 and had a pH of about 4.
[0181] table 5
[0182] Component
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com