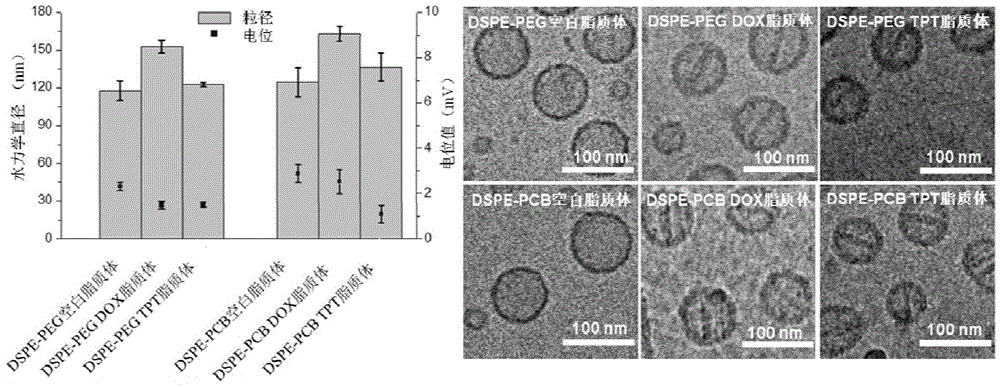
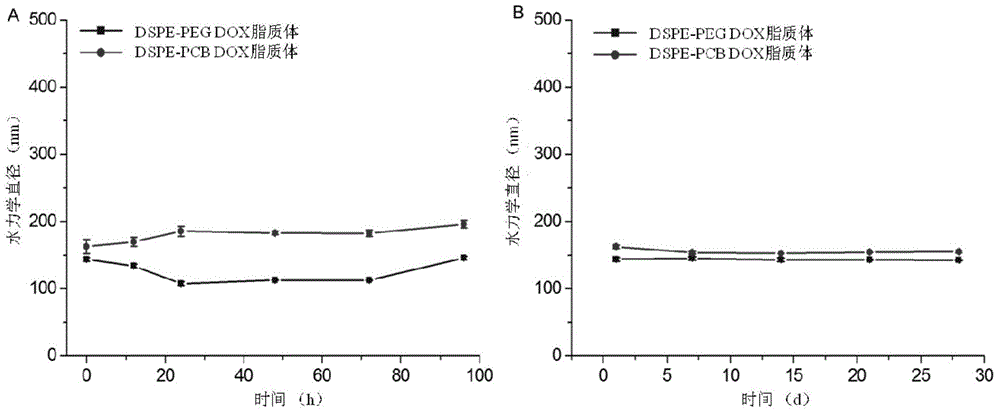
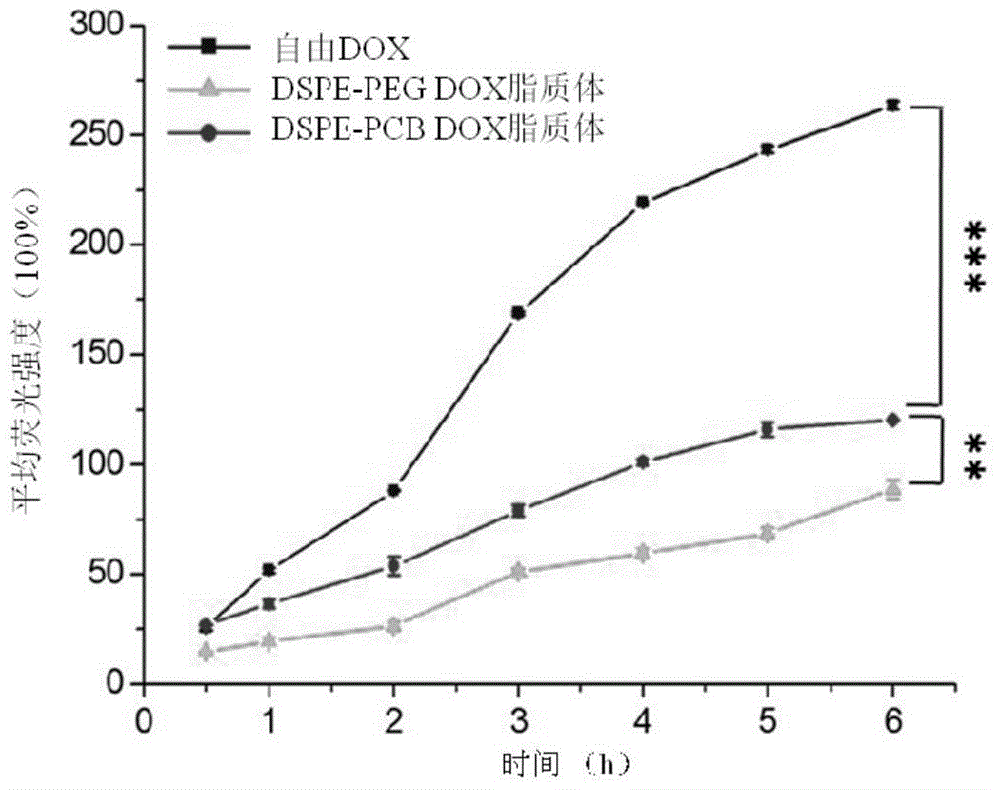
Long-circulating liposome capable of avoiding accelerated blood clearance (ABC) phenomenon, and preparation method and application thereof
A long-circulation liposome technology that accelerates blood clearance, applied in liposome delivery, blood diseases, medical preparations of non-active ingredients, etc., can solve liposome long-circulation function reduction, toxic side effects, and poor therapeutic effect and other problems to achieve the effect of increasing serum stability and simplifying treatment operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The preparation of embodiment 1 liposome pharmaceutical preparation
[0057] 1) Prepare blank liposomes: weigh phospholipid POPC 60.00mg, cholesterol 24.74mg and DSPE-PCB 20 40.86mg in a 100mL round bottom flask, add 30mL of chloroform to fully dissolve the solid, shake well. Using a rotary evaporator at a rotational speed of 140 rpm and a temperature of 55° C., the solvent chloroform was removed by rotary evaporation under reduced pressure to form a thin oil film, which was dried with a vacuum pump for 12 hours to ensure that the chloroform was completely removed. Add 30 mL of 200 mM ammonium sulfate solution into the flask, and use an ultrasonic cleaner to ultrasonicate for 30 min at a frequency of 90% to form a translucent emulsion. The emulsion is added to a high-pressure homogenizer, and under the condition of a pressure of 100 MPa, overpressure is performed 5 times. Add the emulsion into the liposome extruder, under the pressure of 150MPa, overpressure 10 times ...
Embodiment 2
[0059] The preparation of embodiment 2 liposome pharmaceutical preparations
[0060] 1) Prepare blank liposomes: weigh 60 mg of phospholipid POPC, 24.74 mg of cholesterol and DSPE-PCB 10 50.88mg in a 100mL round bottom flask, add 30mL of chloroform to fully dissolve the solid, shake well. Using a rotary evaporator at a rotational speed of 100 rpm and a temperature of 40°C, the solvent chloroform was removed by rotary evaporation under reduced pressure to form a thin oil film, which was dried with a vacuum pump for 6 hours to ensure that the chloroform was completely removed. Add 10 mL of 200 mM ammonium sulfate solution into the flask, and use an ultrasonic cleaner to ultrasonicate for 15 min at a frequency of 50% to form a translucent emulsion. Add the emulsion into a high-pressure homogenizer, and under the condition of a pressure of 50 MPa, overpressure 3 times. Add the emulsion into the liposome extruder, and under the pressure of 100MPa, overpressure 3 times to prepare ...
Embodiment 3
[0062] The preparation of embodiment 3 liposome pharmaceutical preparations
[0063] 1) Prepare blank liposomes: weigh 60.00 mg of phospholipid POPC, 24.74 mg of cholesterol and DSPE-PCB 5016.43mg in a 100mL round bottom flask, add 30mL of chloroform to fully dissolve the solid, shake well. Using a rotary evaporator at a rotational speed of 200 rpm and a temperature of 80°C, the solvent chloroform was removed by rotary evaporation under reduced pressure to form a thin oil film, which was dried with a vacuum pump for 36 hours to ensure that all chloroform was removed. Add 15mL of 200mM ammonium sulfate solution into the flask, and use an ultrasonic cleaner to sonicate for 1 hour at a frequency of 150%, to form a translucent emulsion. Add the emulsion into a high-pressure homogenizer, and under the pressure of 150MPa, overpressurize 10 times. Add the emulsion into the liposome extruder, under the pressure of 200MPa, overpressure 15 times to prepare DSPE-PCB 20 Modified blank ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com