Patents
Literature
216 results about "Vein injection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vein injection is also called sclerotherapy. This is a procedure that is performed to improve the appearance of varicose veins. The therapy involves the injection of a solution, which is called a sclerosant, directly into the affecting vein to damage its internal lining and promote blood clotting from within.
Drug delivery and monitoring system
InactiveUS20060144942A1Easy to fillDrug and medicationsMedical devicesInjection portMonitoring system
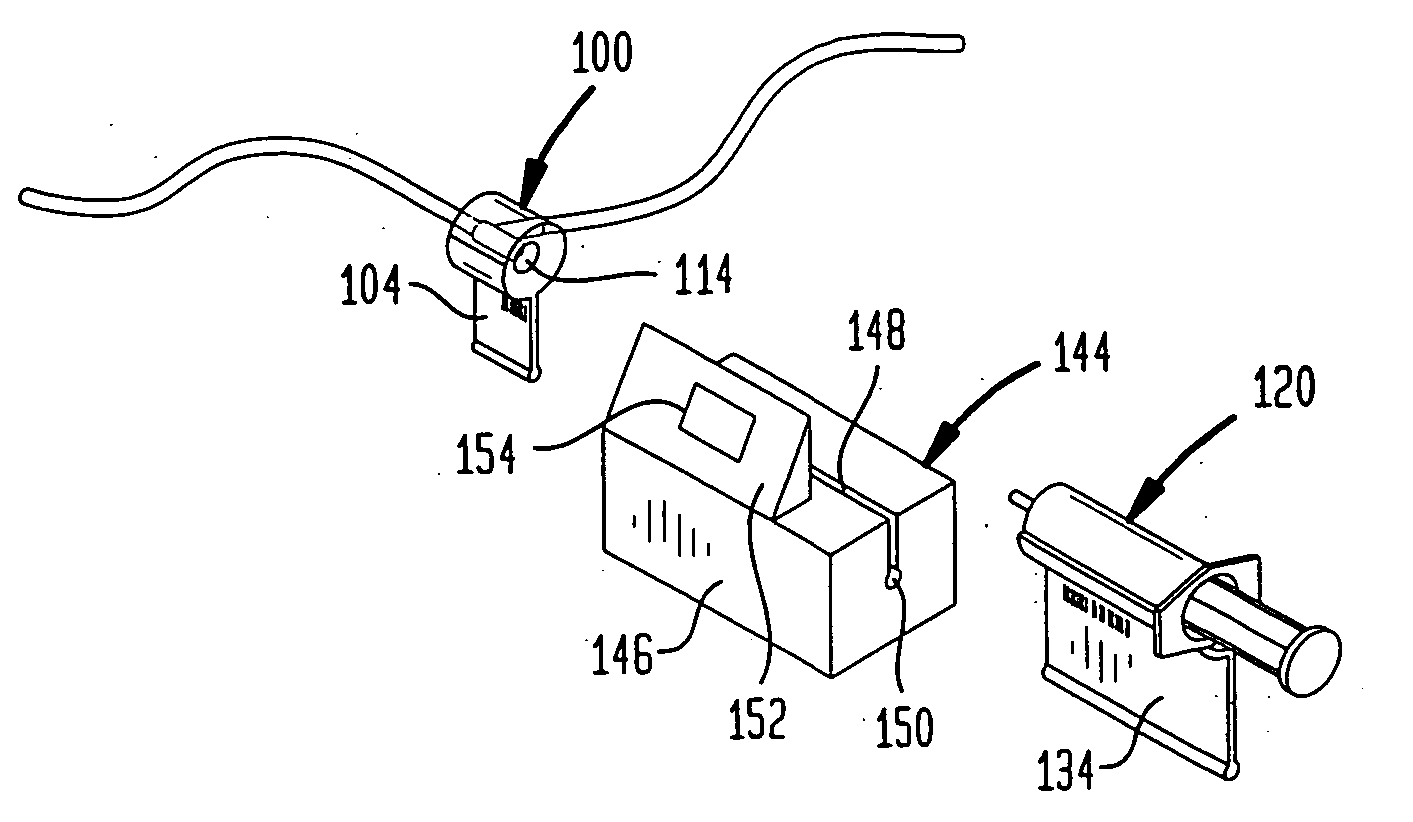
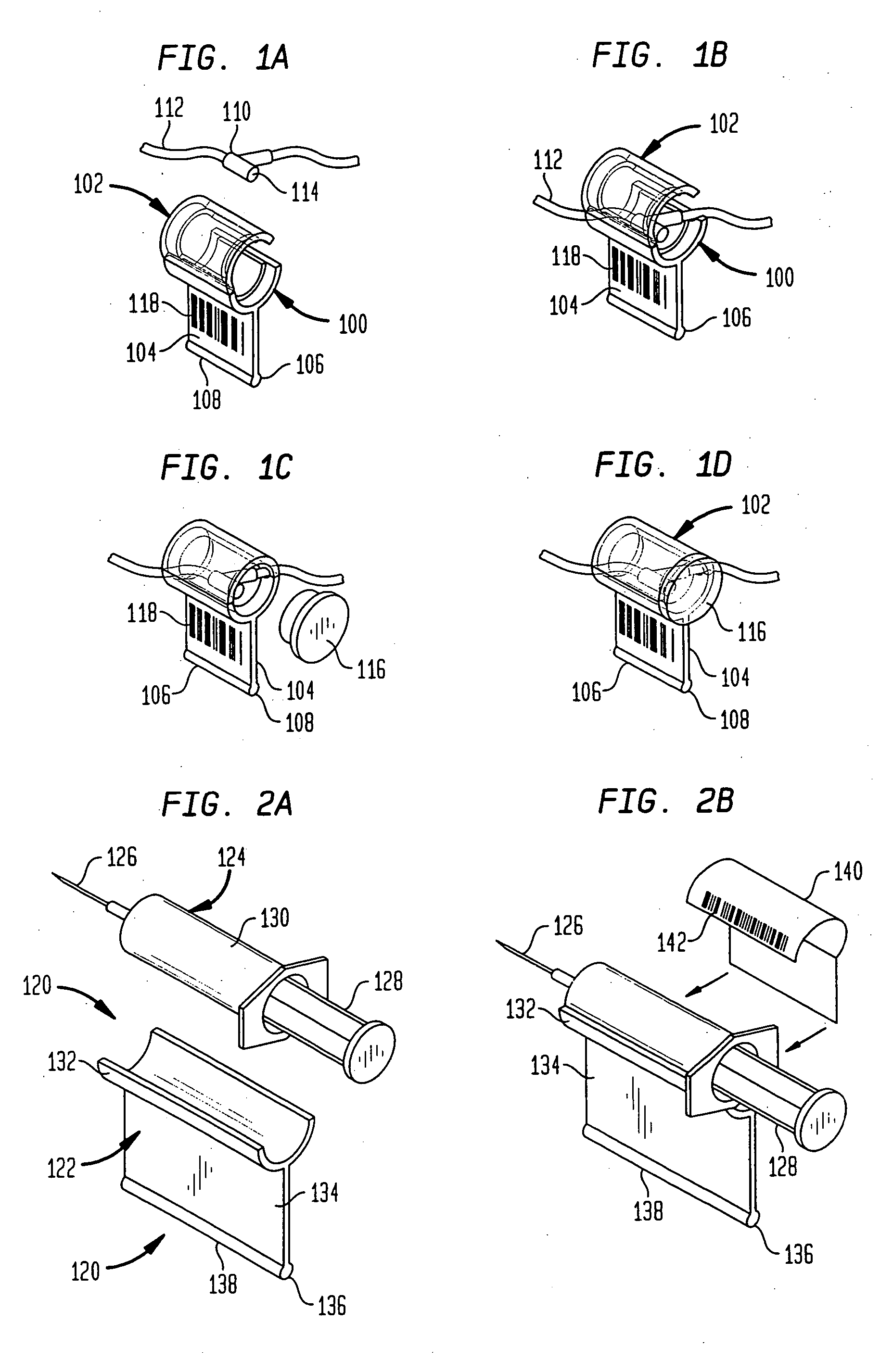
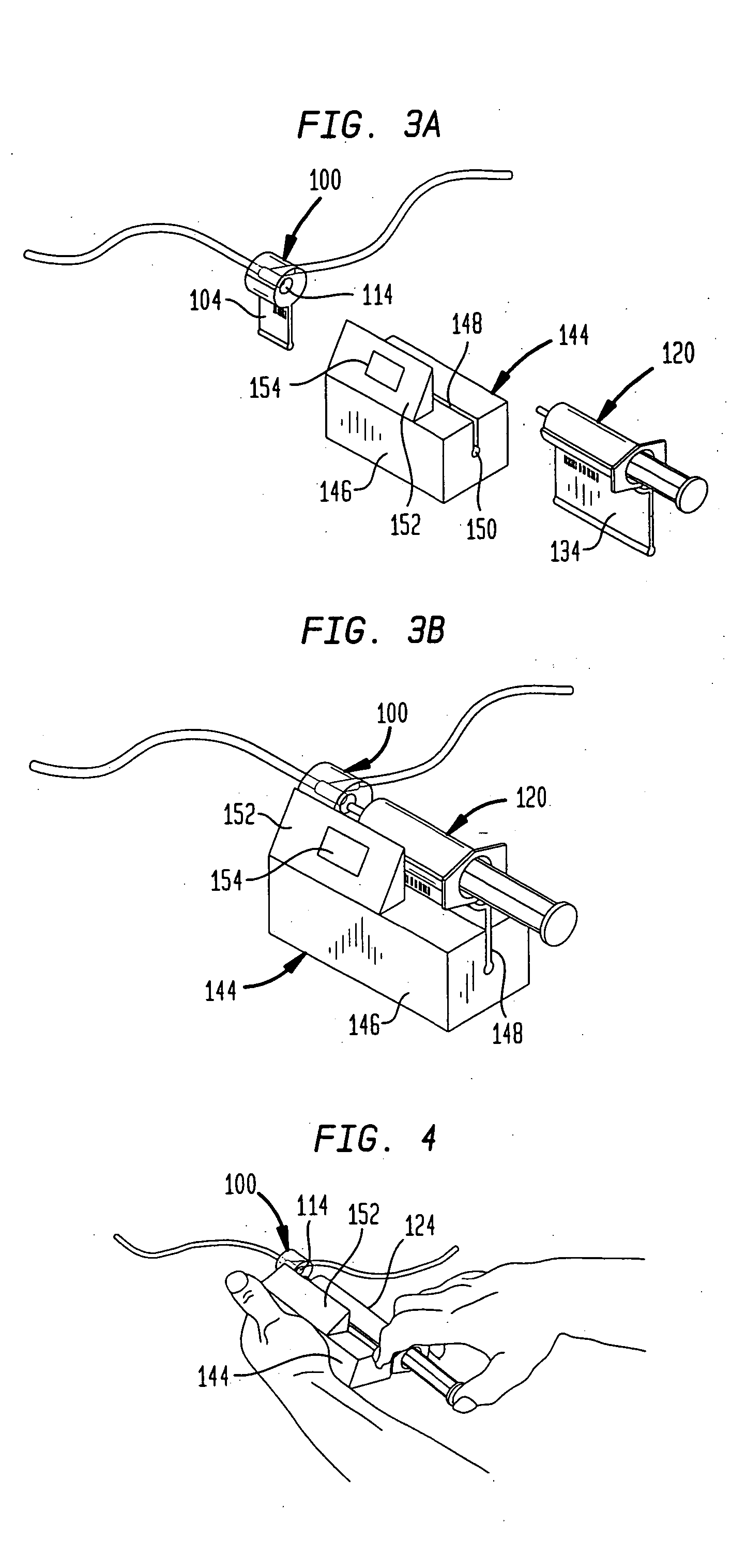
A drug administration system includes a cradle attached about an intravenous injection port having a flange extending therefrom. The cradle supports first drug administration information in the nature of machine and human readable code, for example, barcode. A syringe including a needle includes a flange extending from the syringe. The syringe supports second drug administration information in machine and / or human readable form. A scanner module is constructed to slidably receive the flange of the cradle and syringe whereby the syringe needle is aligned with the intravenous injection port. The module may be provided with an electronic scanning system for identifying the first and second drug administration information, as well as determining the amount of the drug being administered from the syringe to the injection port by monitoring movement of the syringe plunger. The information and data may be stored within the module for uploading to a remote location.
Owner:IBM CORP
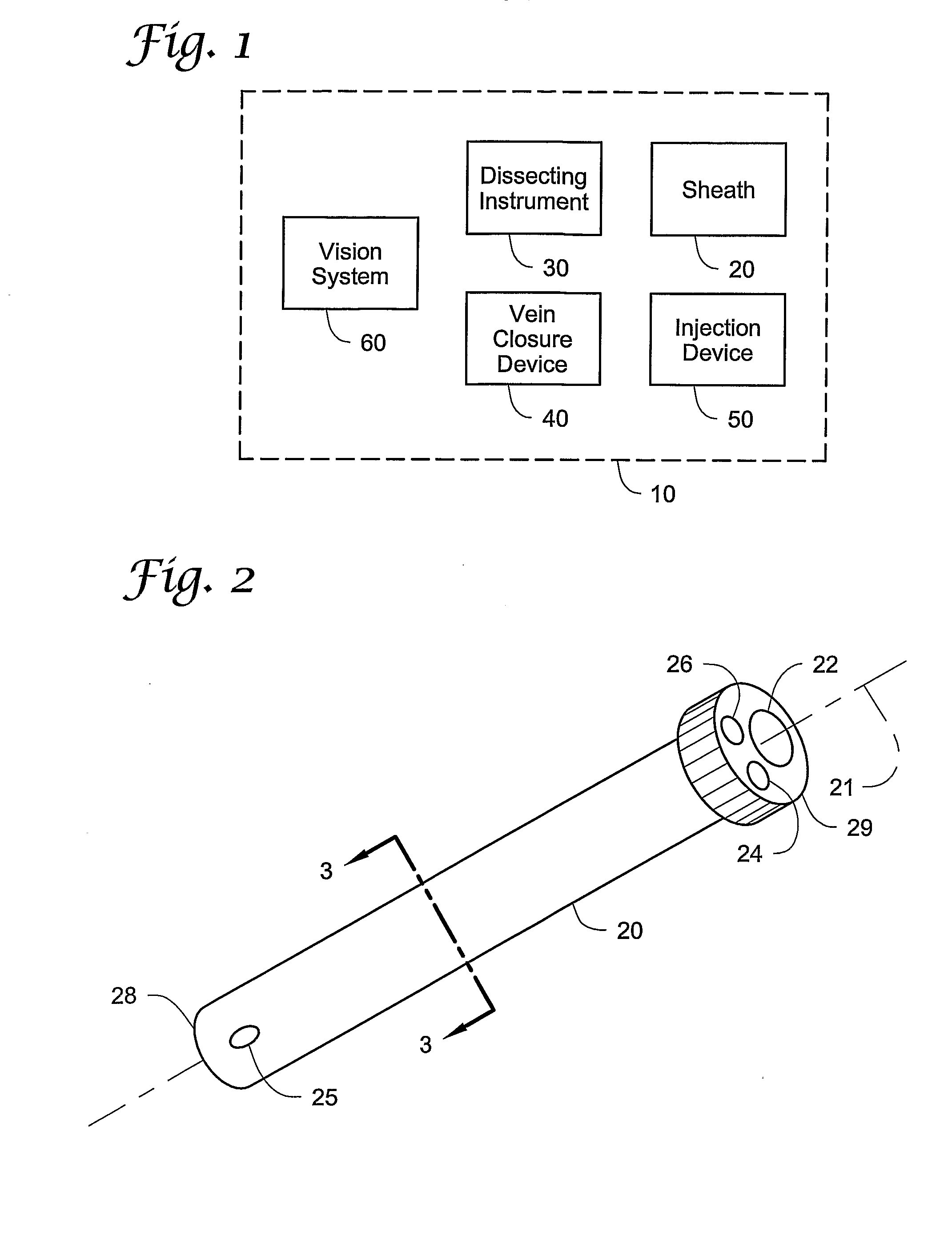
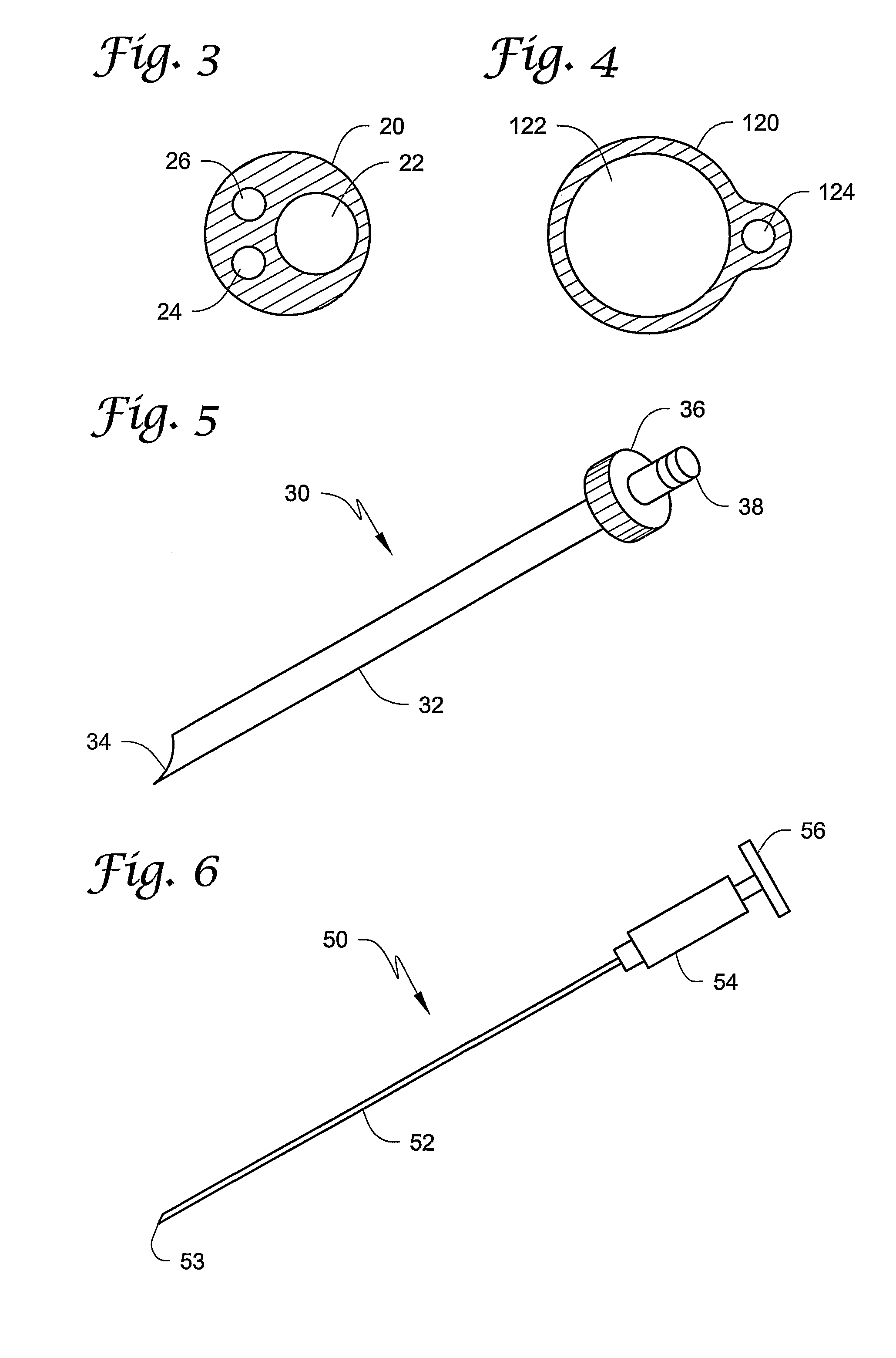
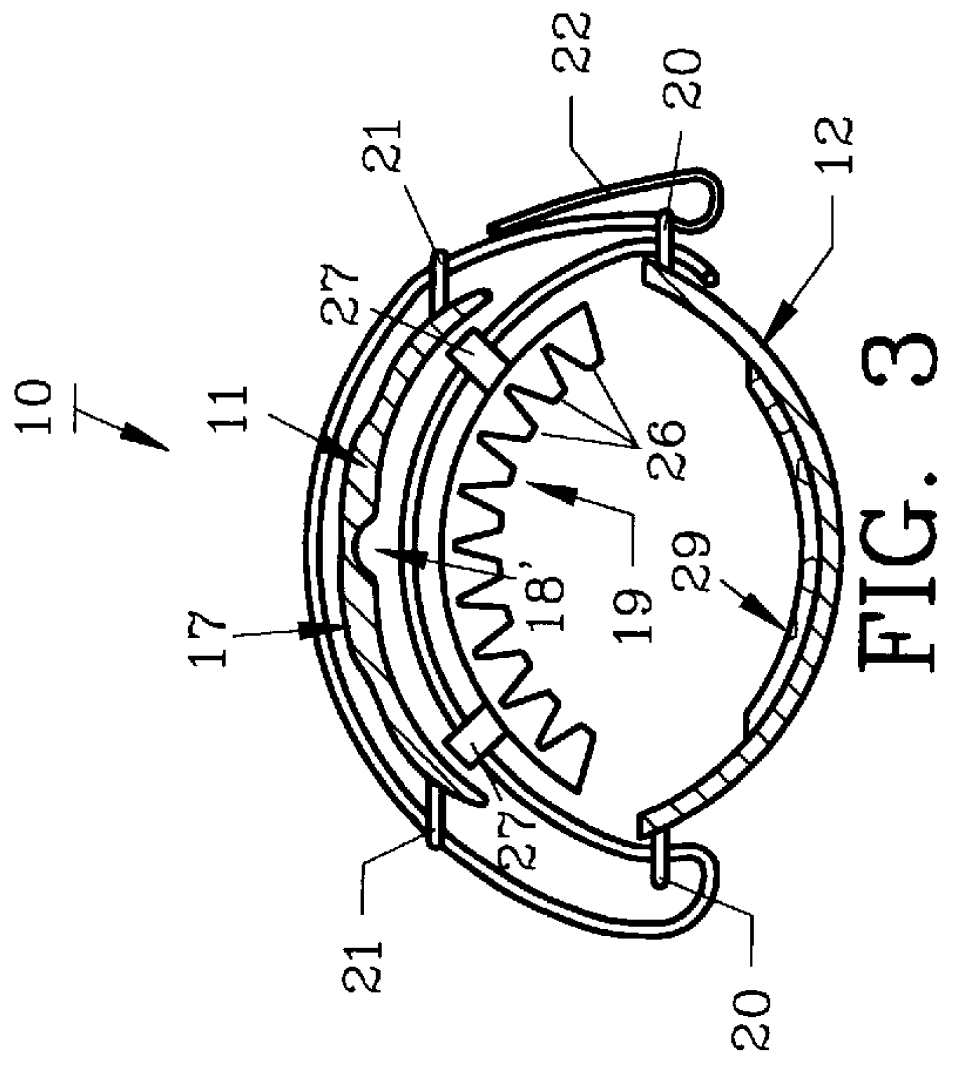
Vein closure and injection kits and methods
ActiveUS20090222003A1Low costReduce riskSuture equipmentsBalloon catheterSaphenopopliteal junctionVein closure
Kits, apparatus, devices and methods for treating varicose veins caused by an incompetent venous junction (e.g., the saphenofemoral and / or saphenopopliteal junctions) are disclosed. The kits may include a sheath, a vein closure device, and an injection device. Alternatively, a vein closure device may include an integral injection channel and injection device. Methods may include injecting a vein with a sclerosing agent through a needle that extends in a direction that is not aligned with a longitudinal axis of a sheath or shaft.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
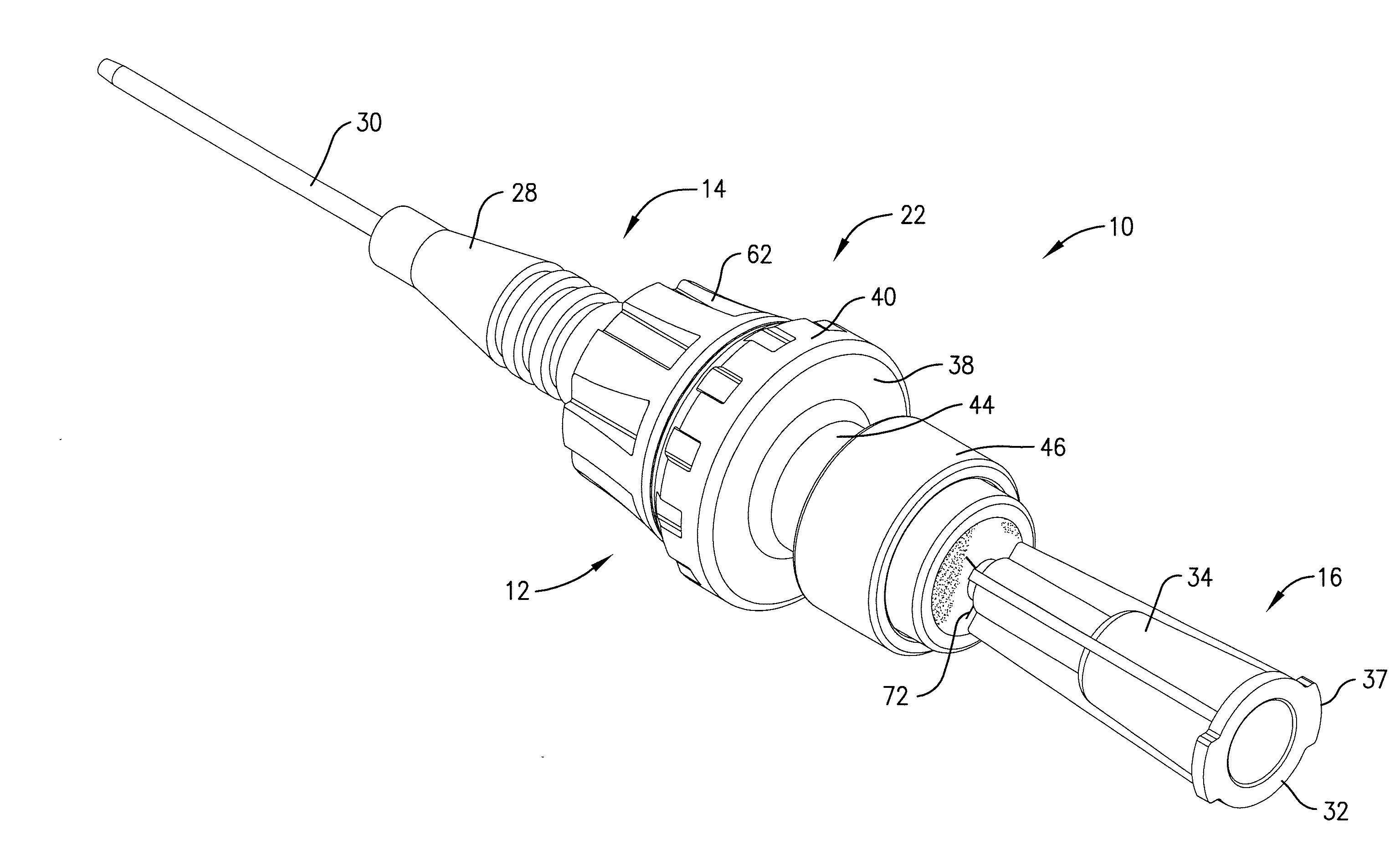
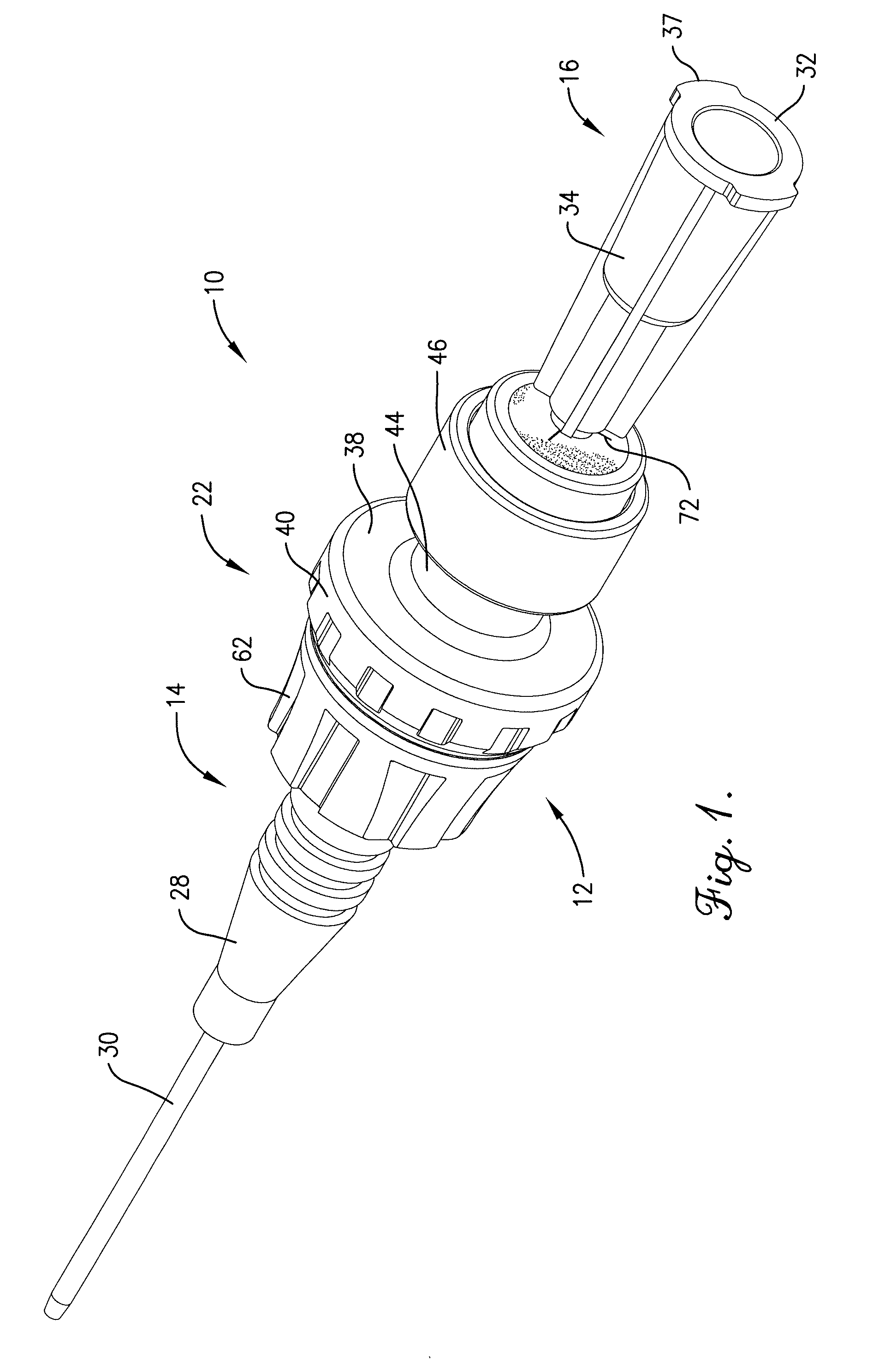
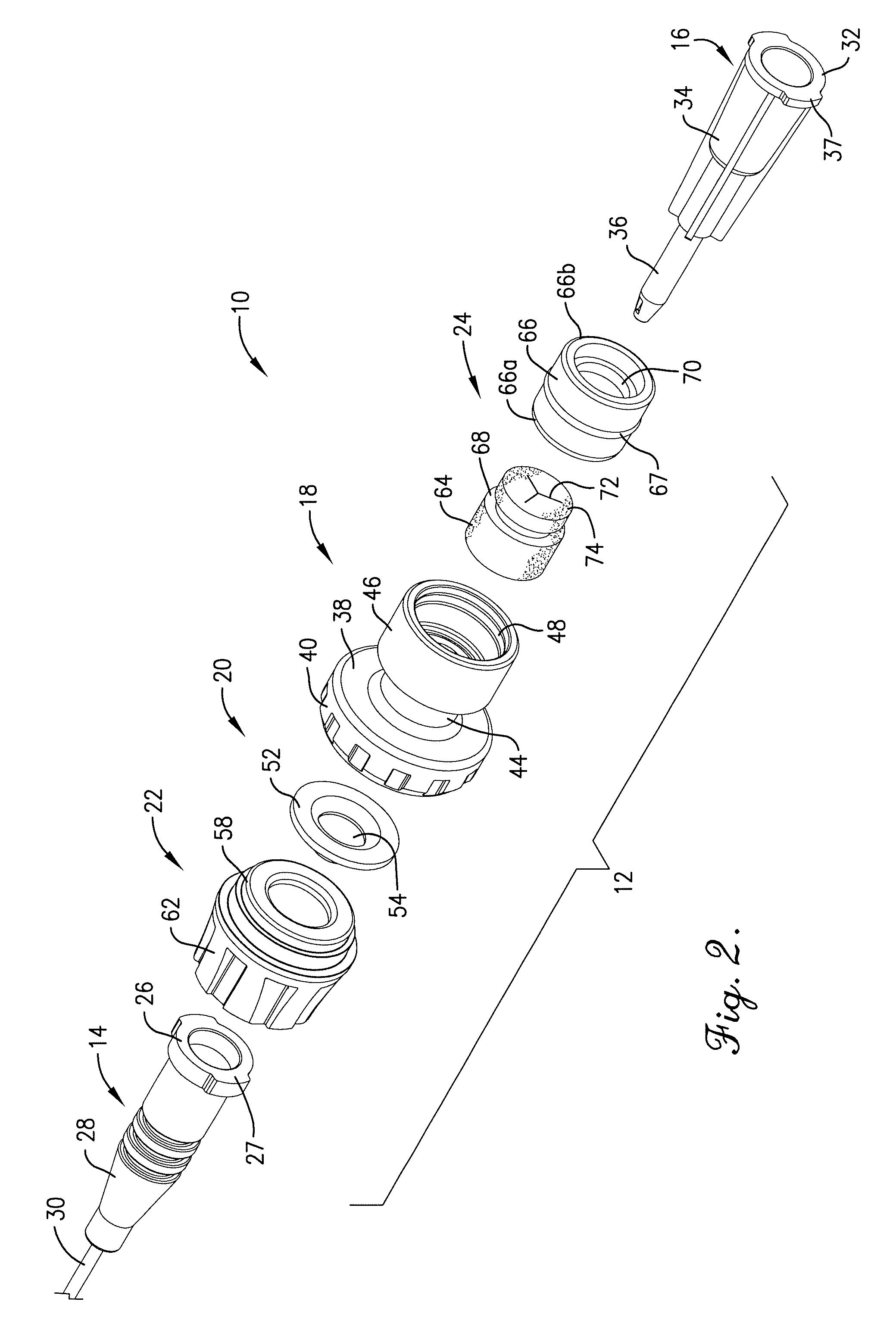
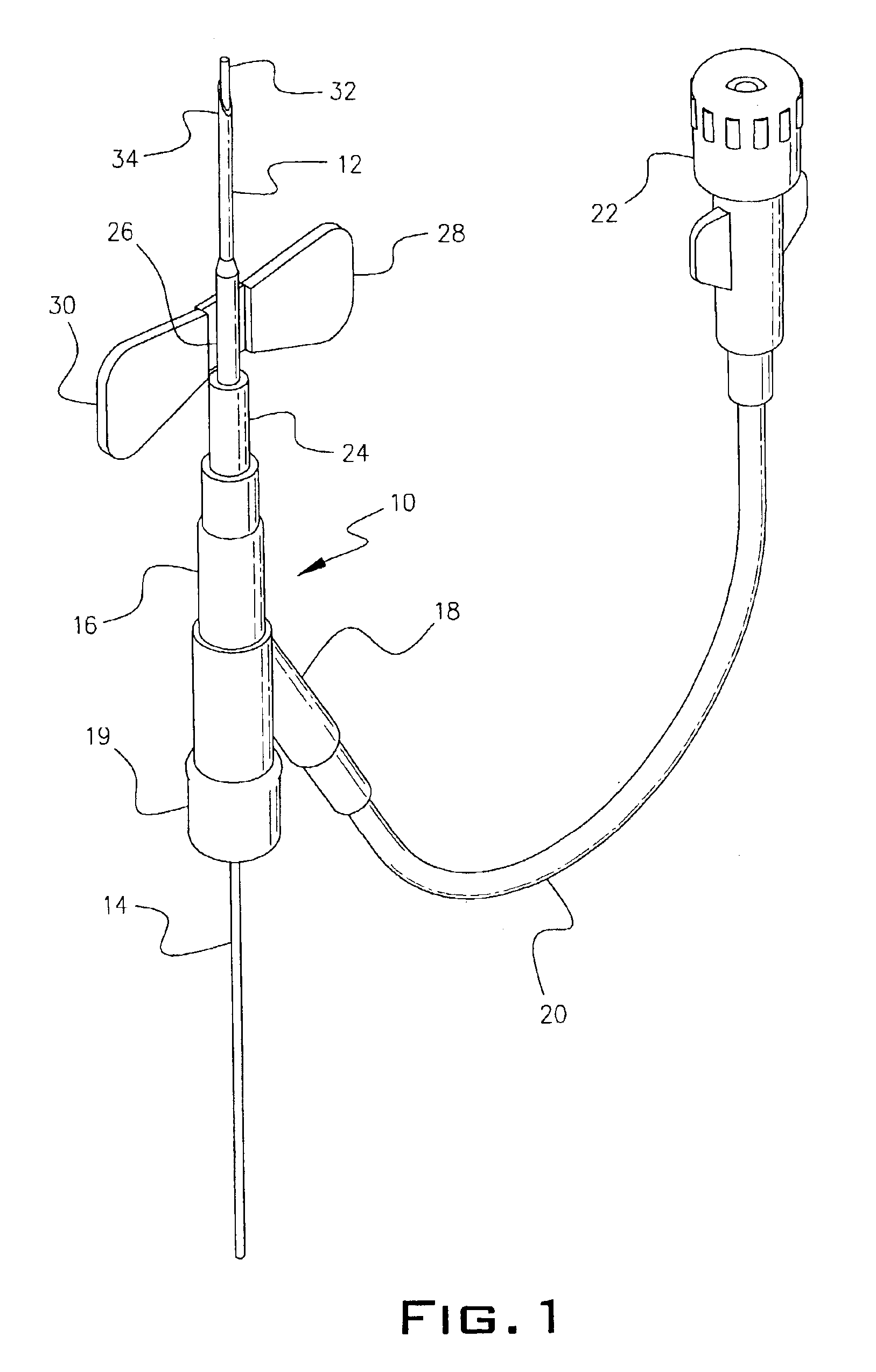
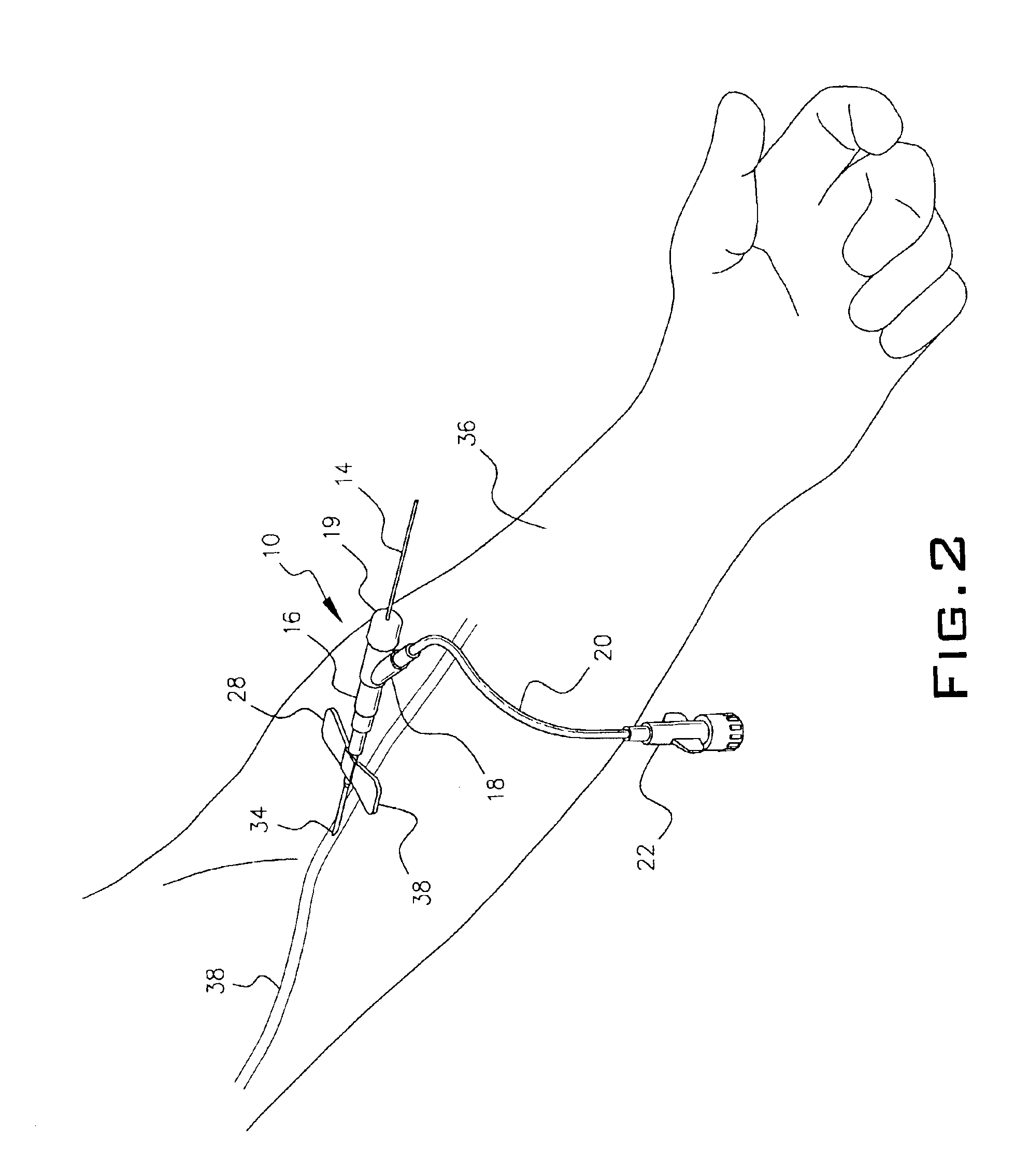
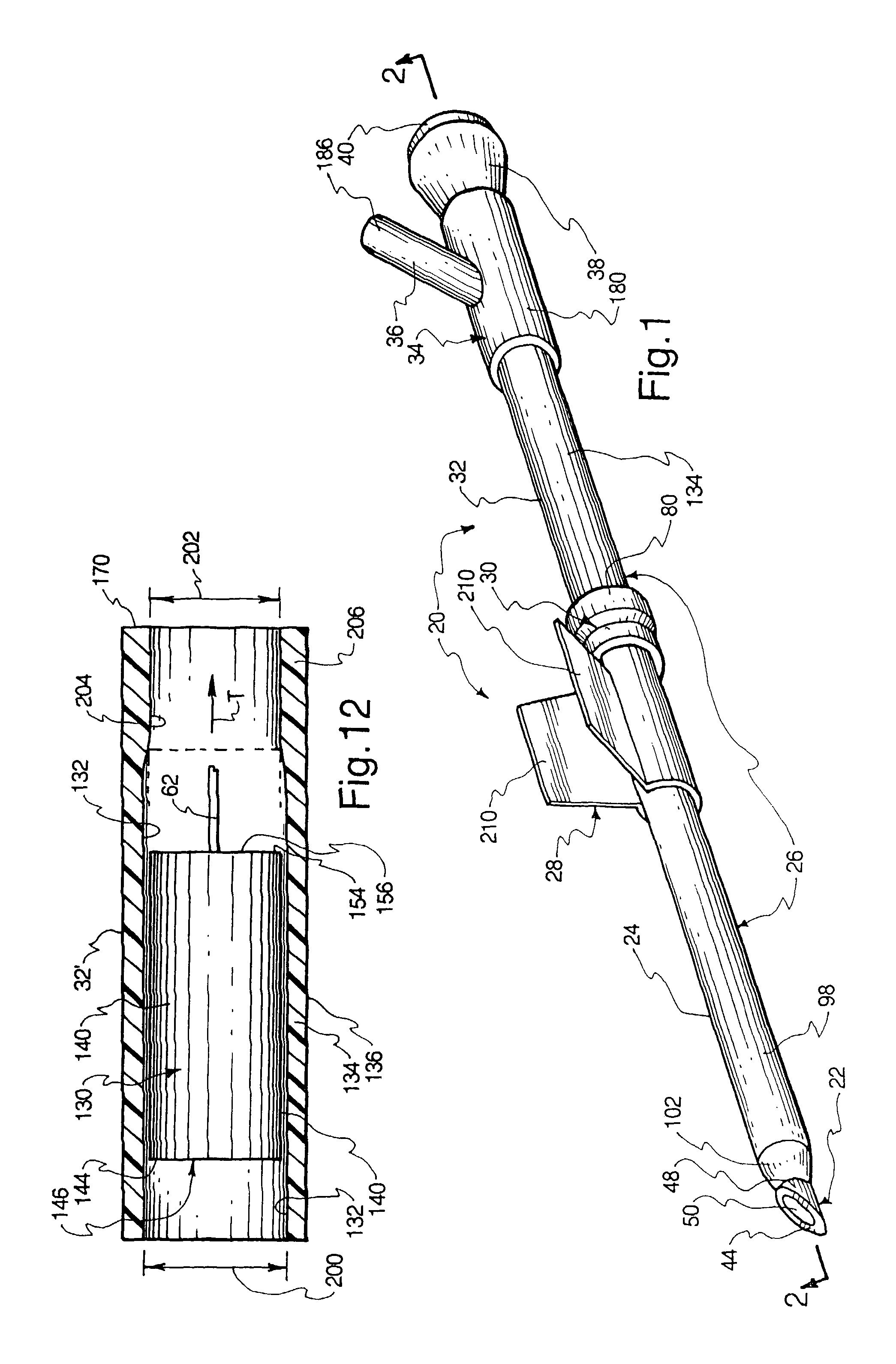
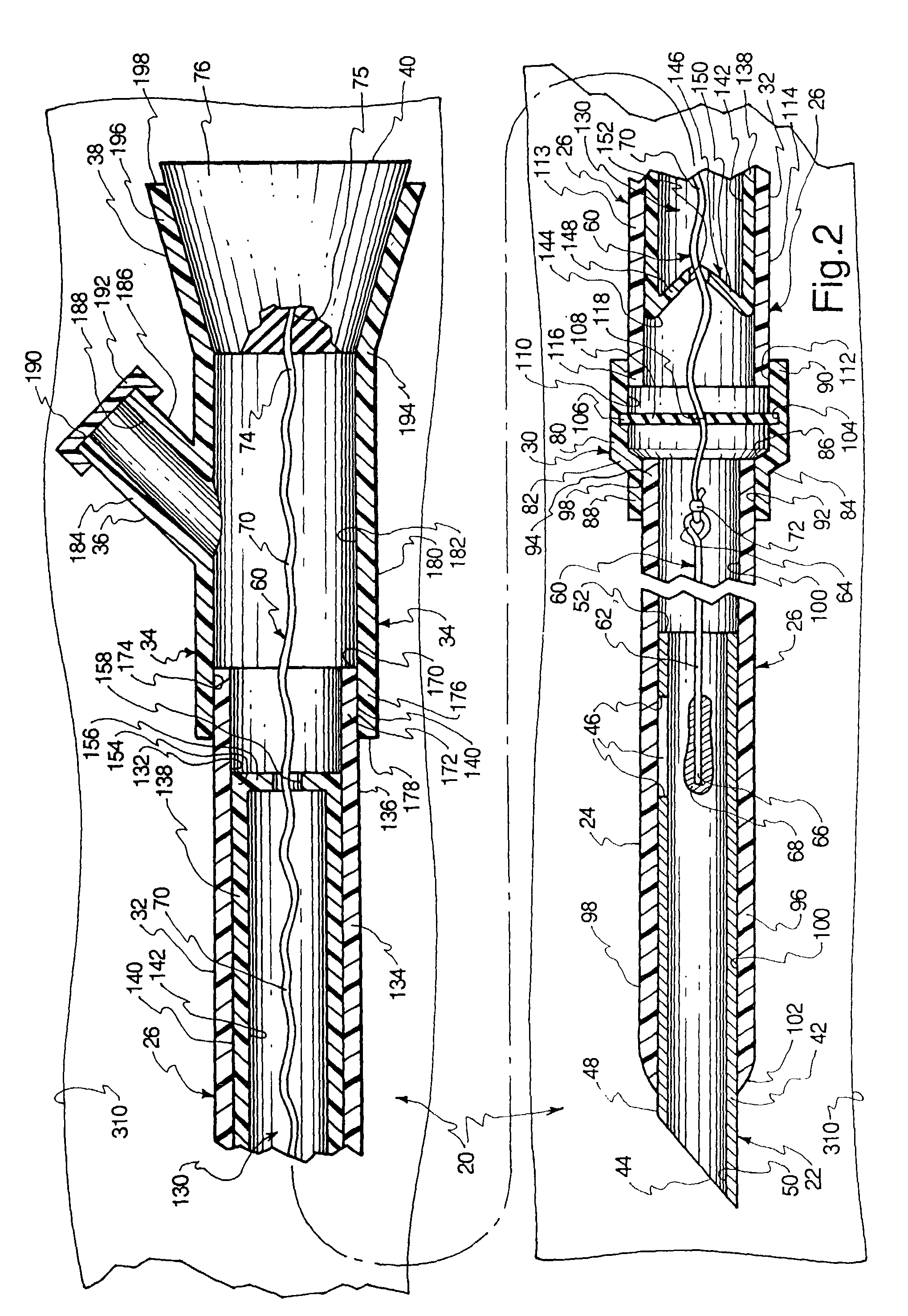
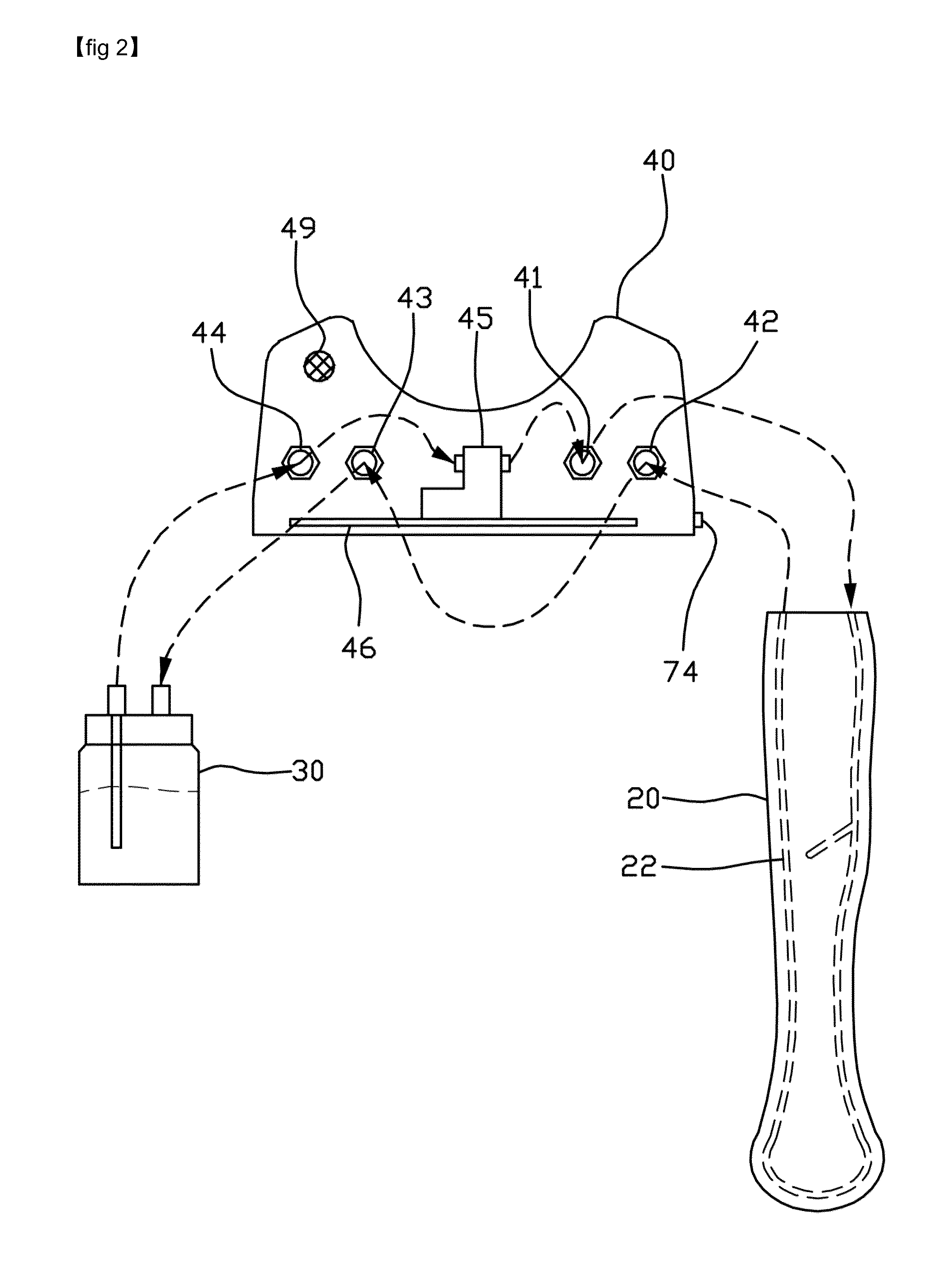
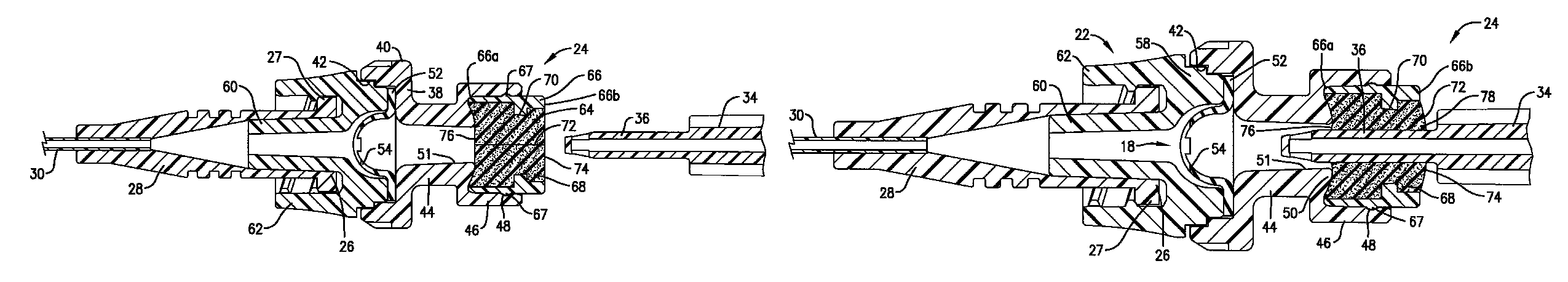
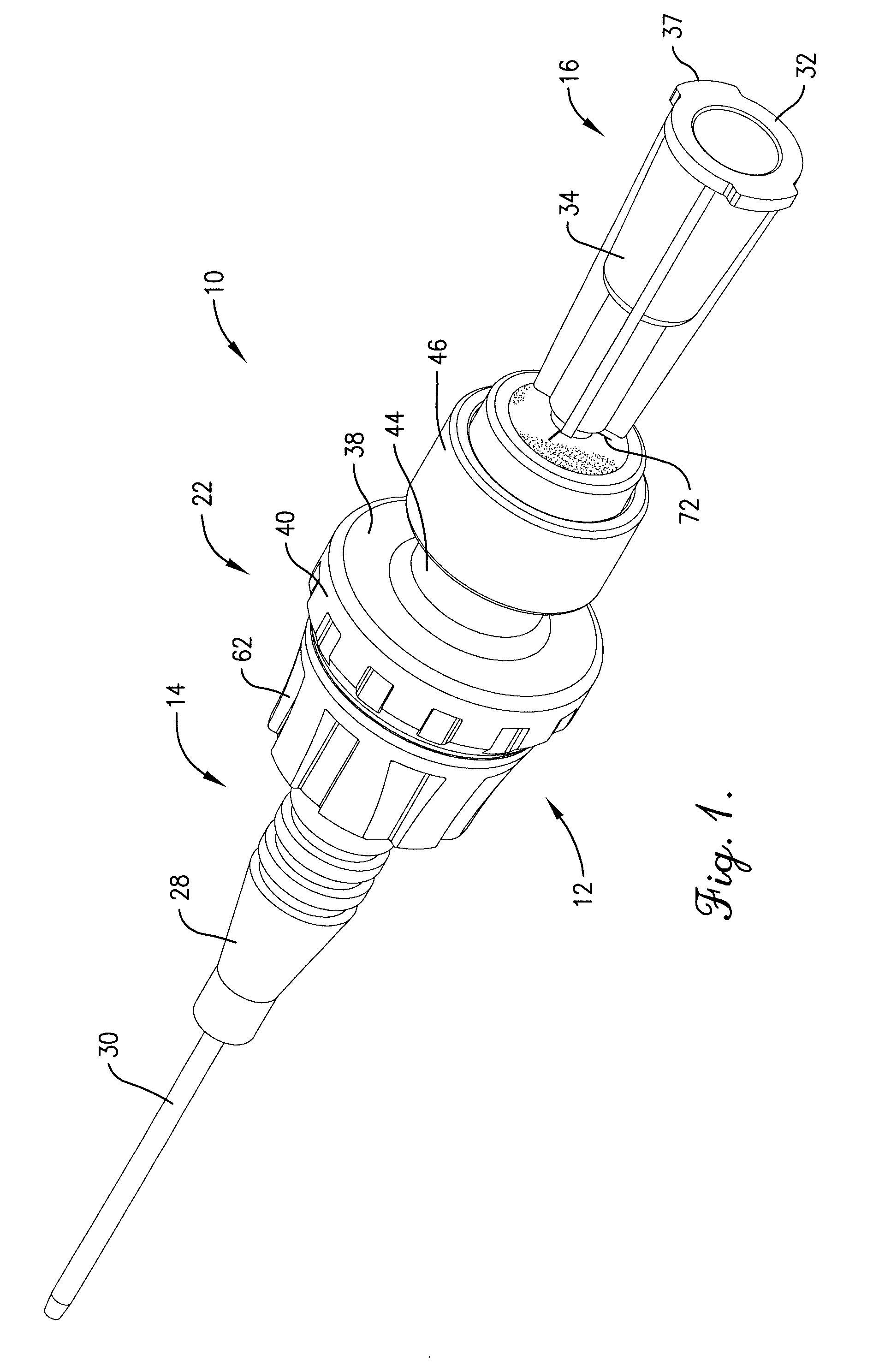
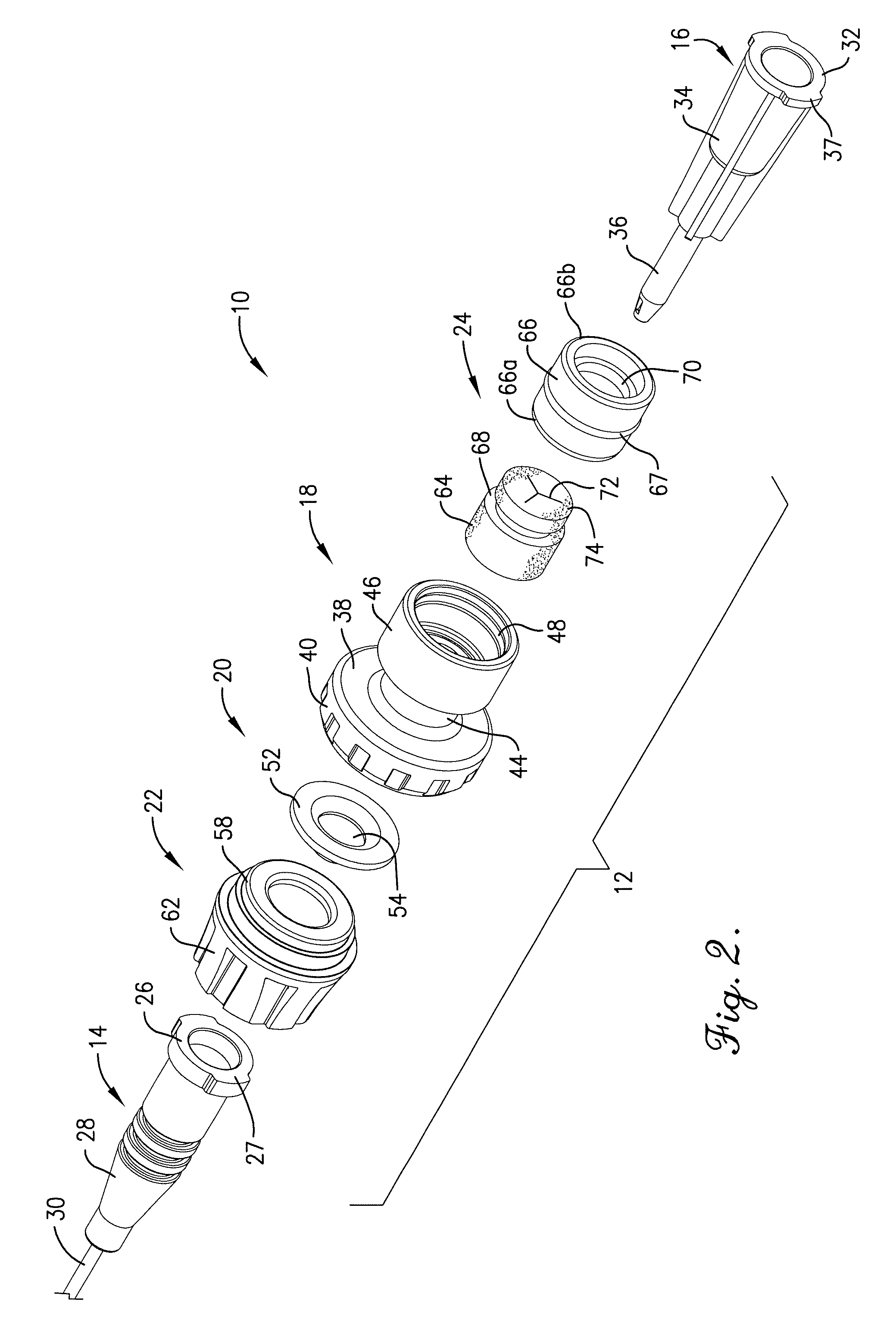
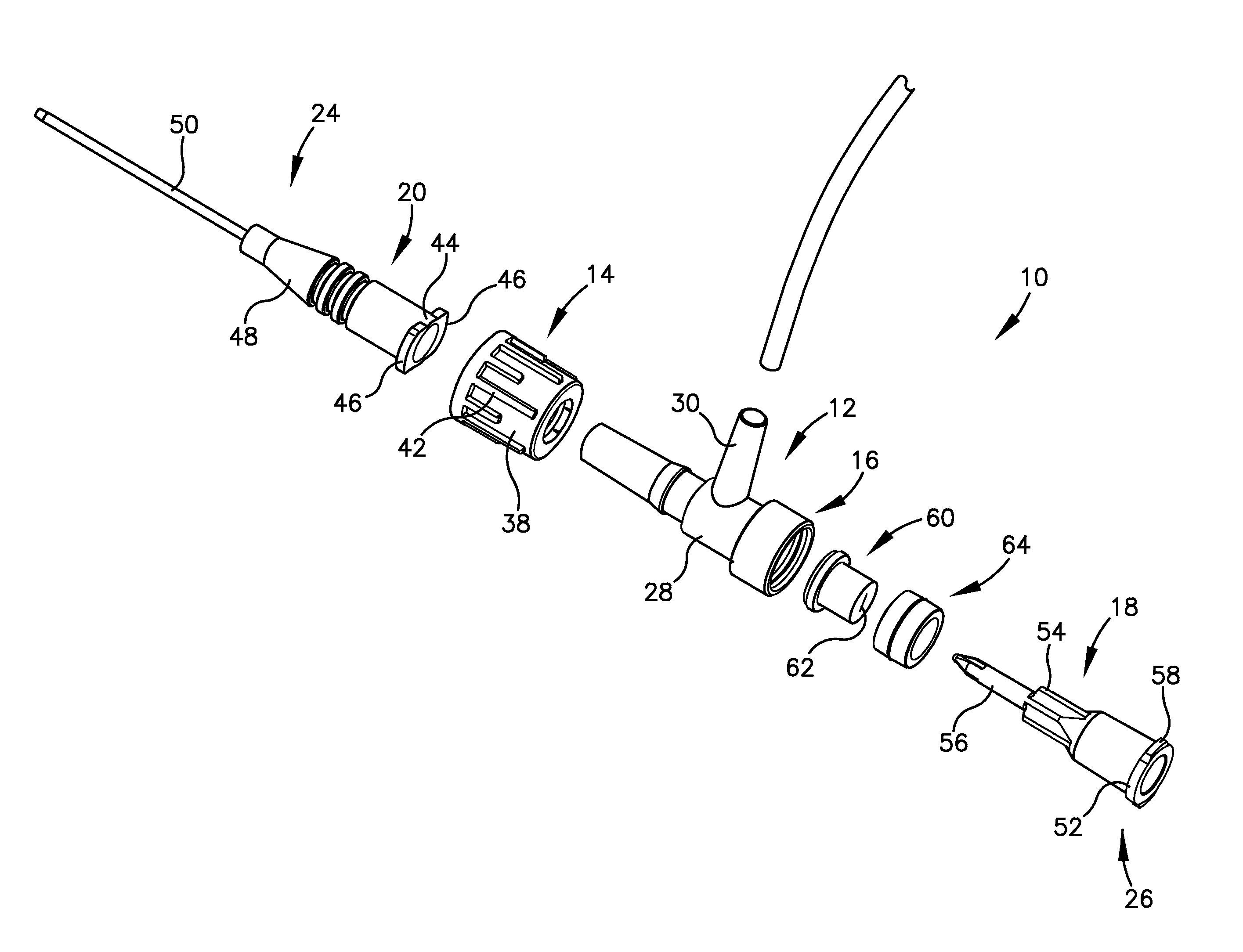
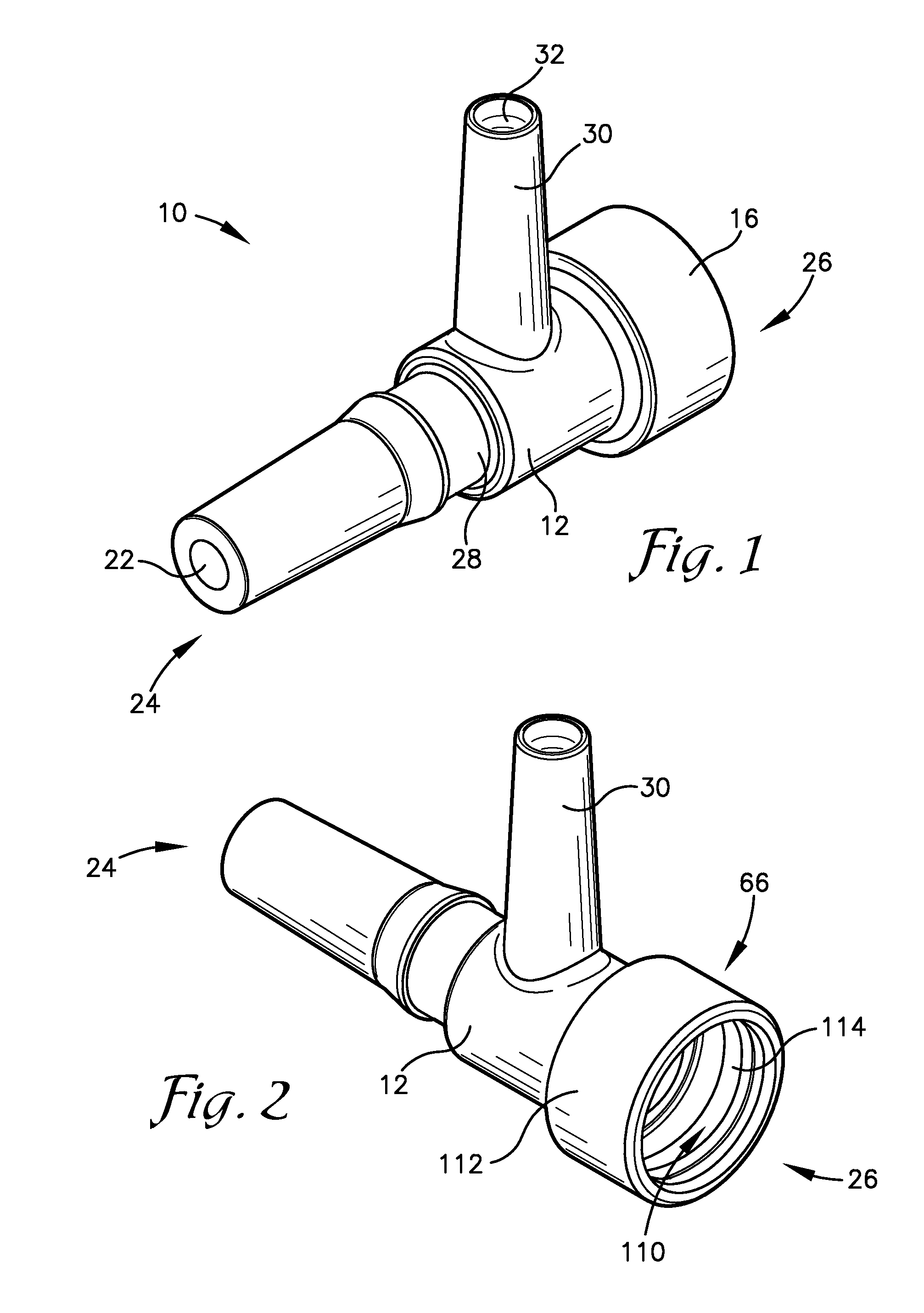
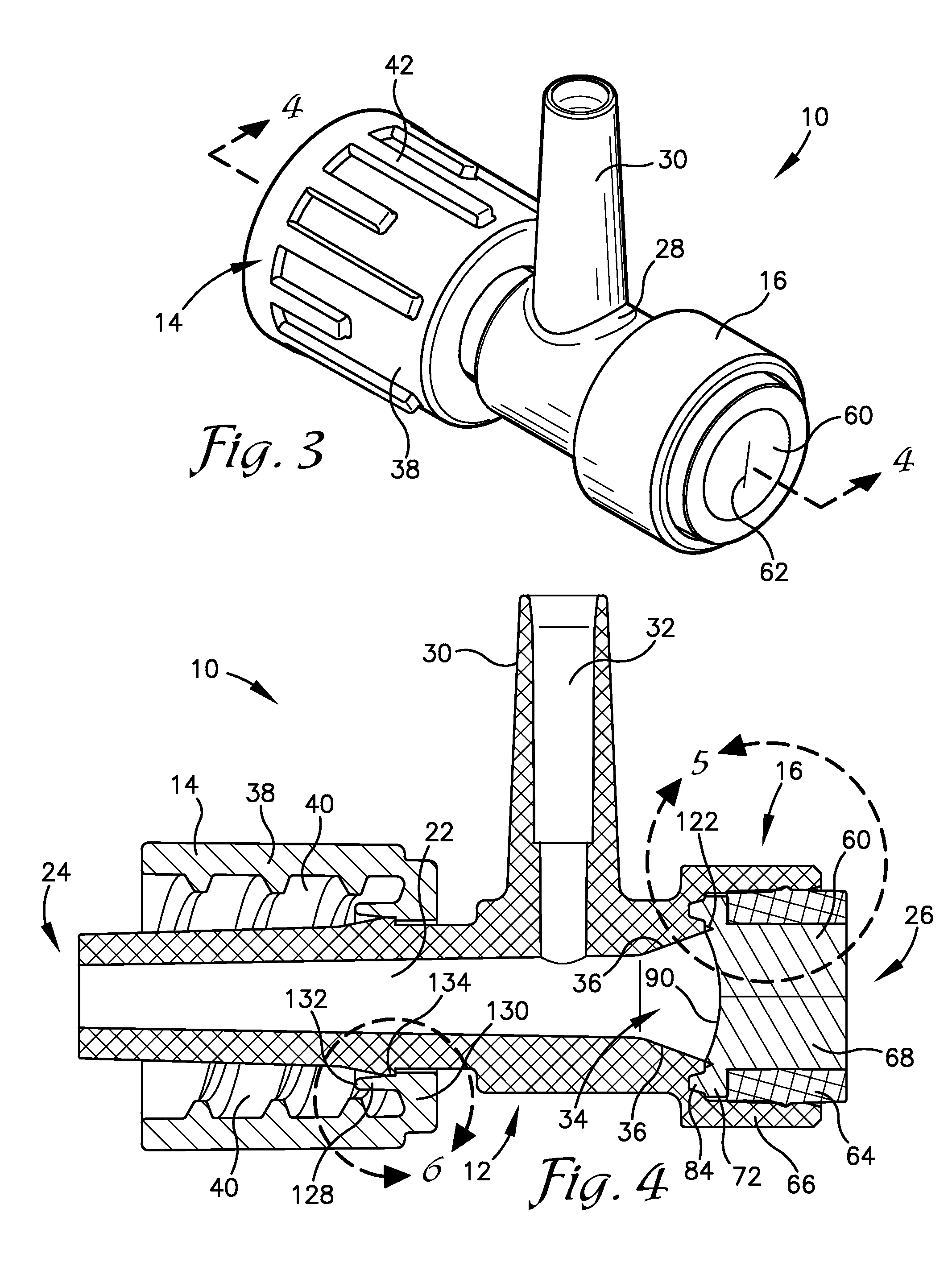
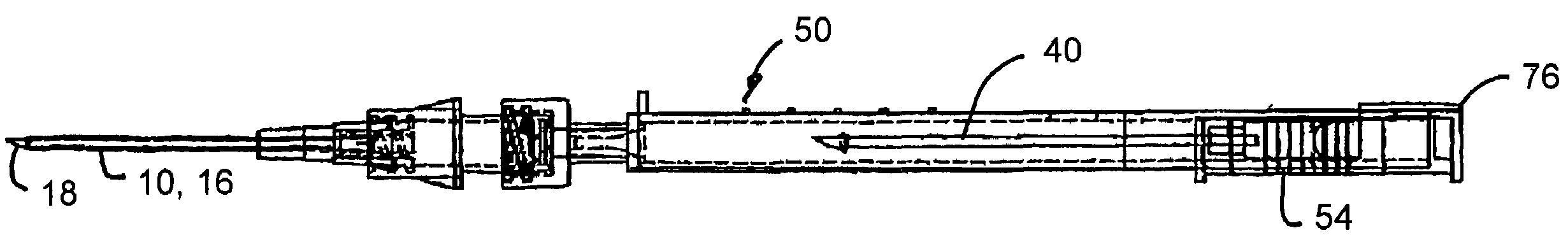
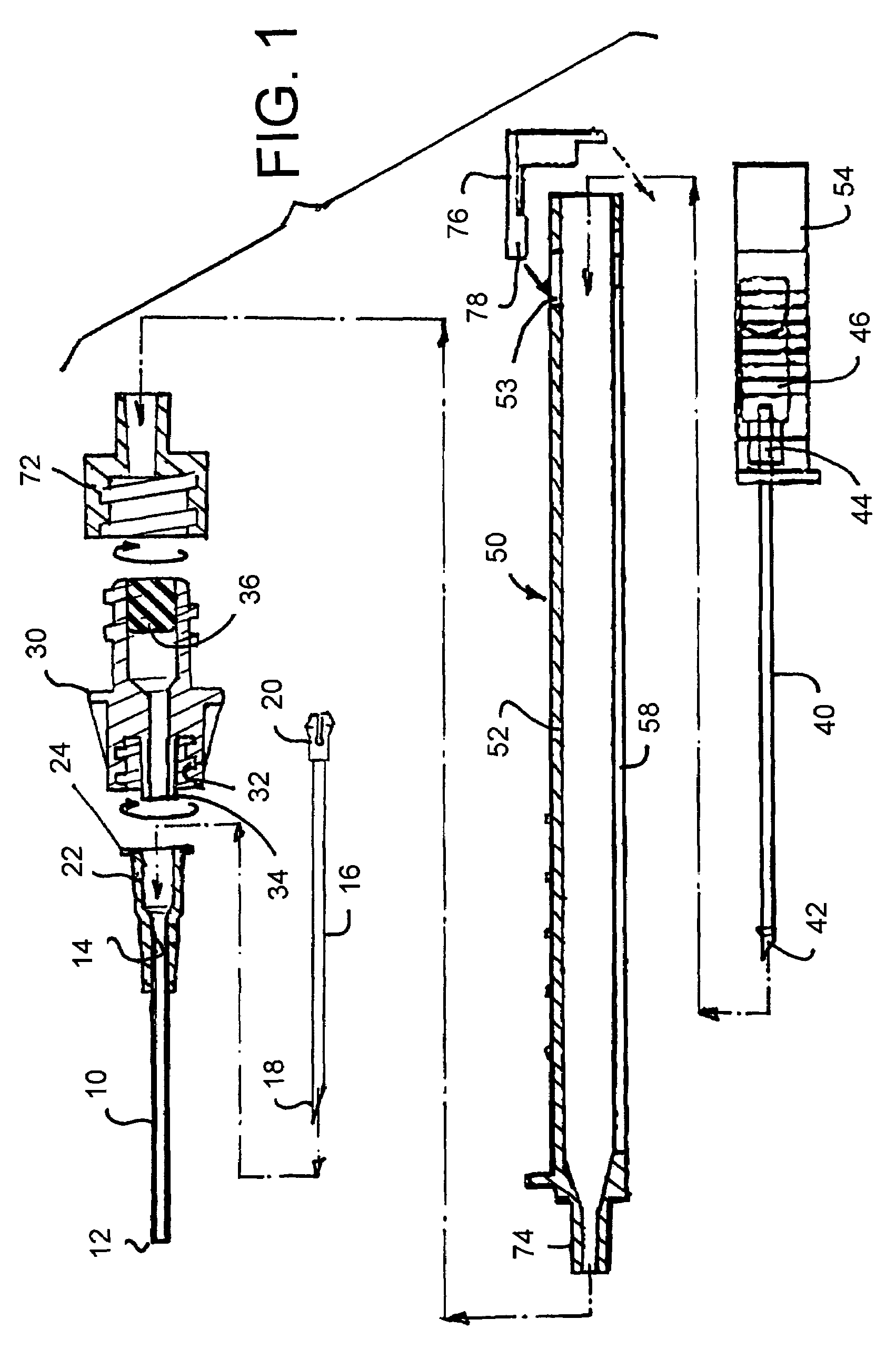
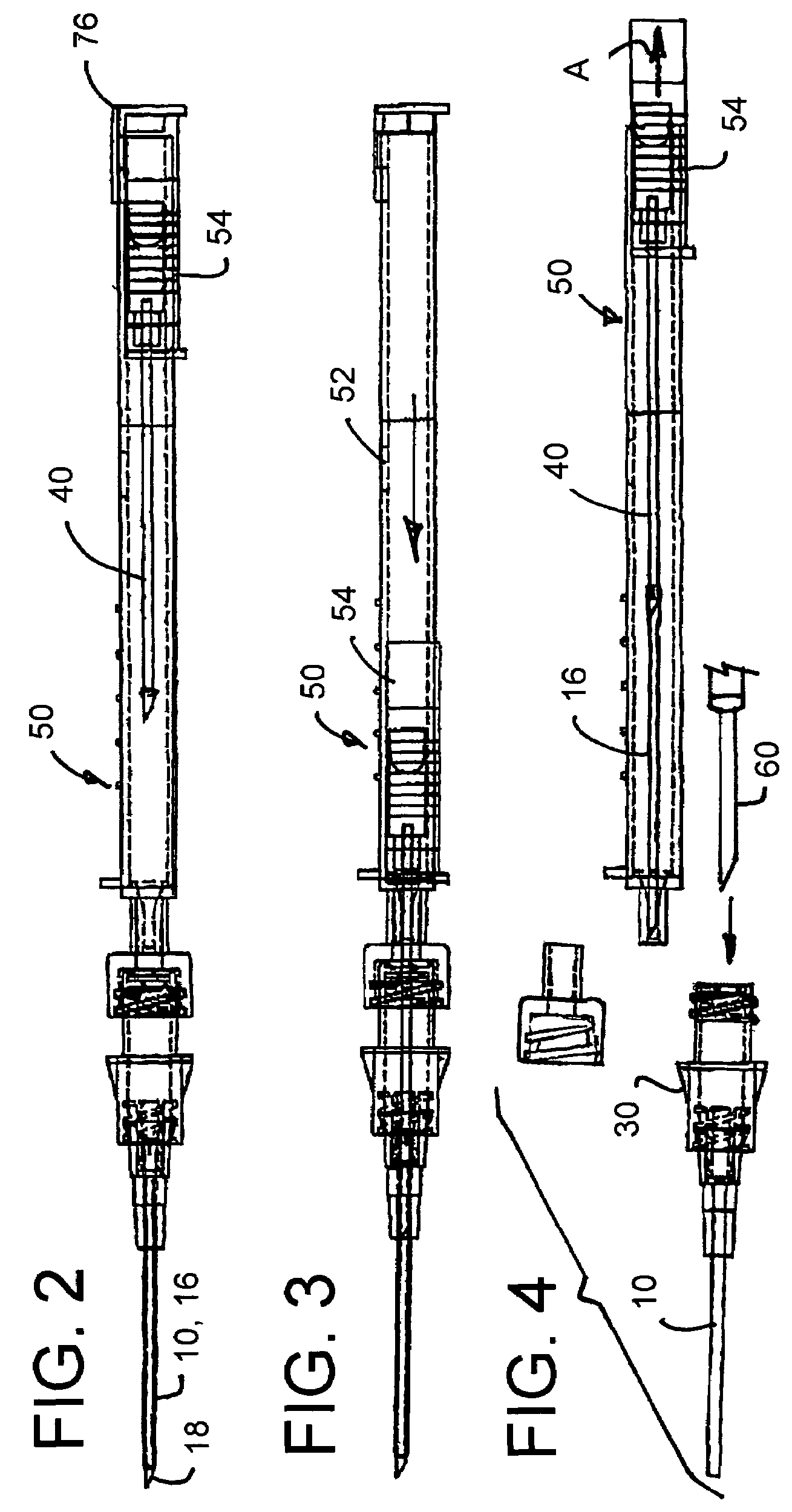
Intravenous injection site with split septum and pressure activated flow control valve
ActiveUS20070225648A1Safety managementRule out the possibilityInfusion syringesCatheterRefluxControl valves
An improved injection site (12) for infusion of parenteral fluids and the like is provided, having a pressure-actuated valve (20) and a novel split septum unit (24), which effectively prevent reflux of blood into the assembly (10). The septum unit (24) includes a resilient split septum body (64) which is precompressed so that the septum body (64) is caused to protrude proximally (78) upon insertion of a cannula (16). Consequently, upon removal of the cannula (16), there is essentially no “drumming” or creation of friction-induced negative pressures sufficient to generate blood reflux. The preloaded septum body (64) also has its proximal surface (74) essentially flush and coplanar with the adjacent proximal end (66b) of the tubular septum holder (66) to enhance the cleanliness of the unit (24). The specialized well (46) and septum unit (24) afford a resilient seal between the periphery of the septum body (64) and the surface (50), and a separate hard-surface seal between the outer margin of the surface (50) and the septum holder (66).
Owner:NEXUS MEDICAL LLC
Apparatus for conveying a light source to an intravenous needle to kill blood pathogens
A tip of a needle is inserted into large vein of a patient. The needle is mounted in a butterfly device axially engaged to a generally cylindrical housing containing a Y-connector. A pharmaceutically acceptable solution is fed to the needle through the Y-connector to the housing. A quartz optic fiber is fed through the housing and needle to the tip of the needle in the patient's venous system. A source of UV and visible light directs both UV and visible light alternatively through the optic fiber to the patient's venous system to kill pathogens in the venous system.
Owner:DISTEFANO JOSEPH
Trapping of intravenous needle associated with a long catheter, and related methods
Apparatus and methodology are disclosed for entrapping a used intravenous needle in a needle trap and removing the needle and the trap from a catheter tube so as to avoid risk of injury to both the patient and the medical attendant.
Owner:BOSTON SCI SCIMED INC
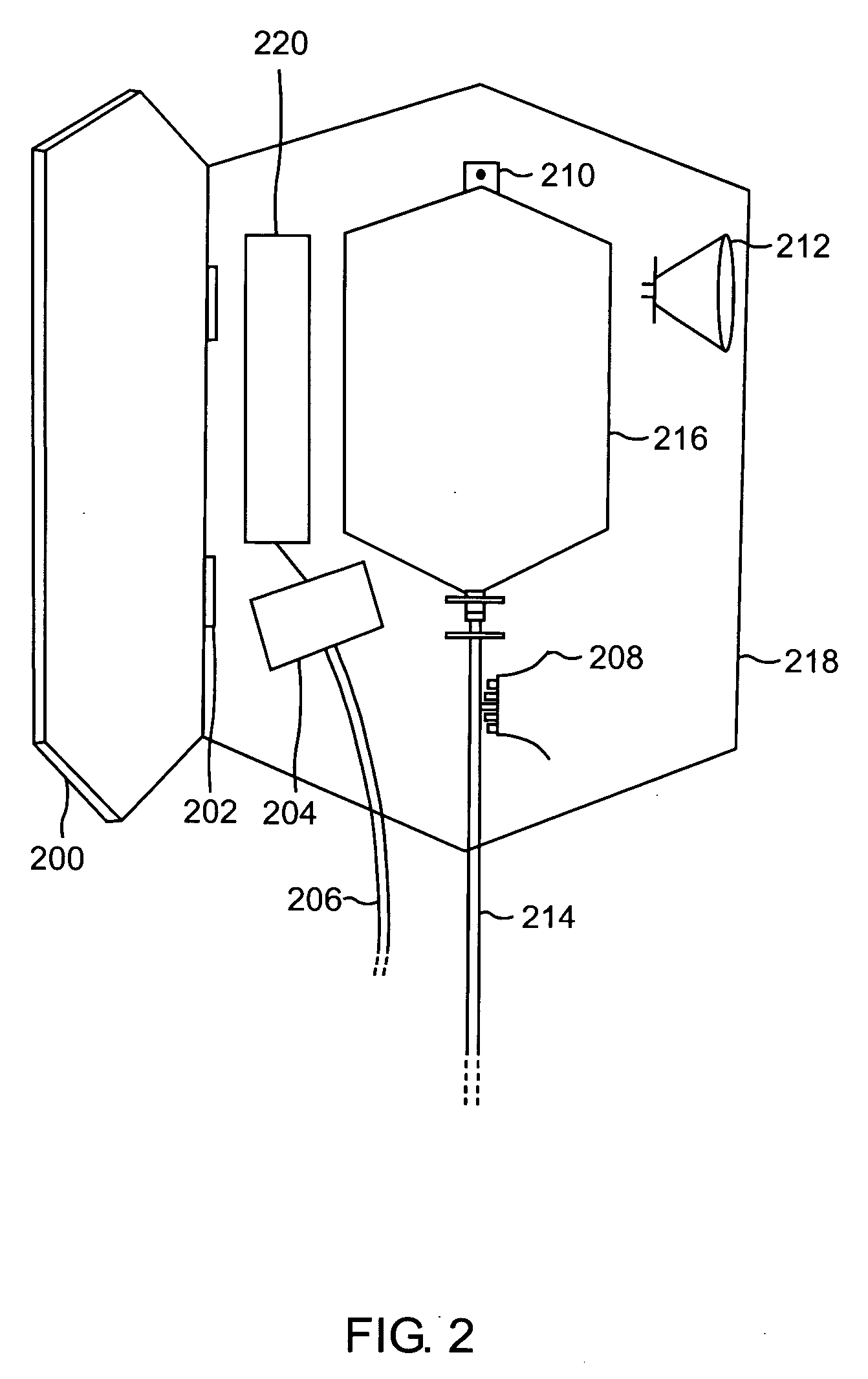
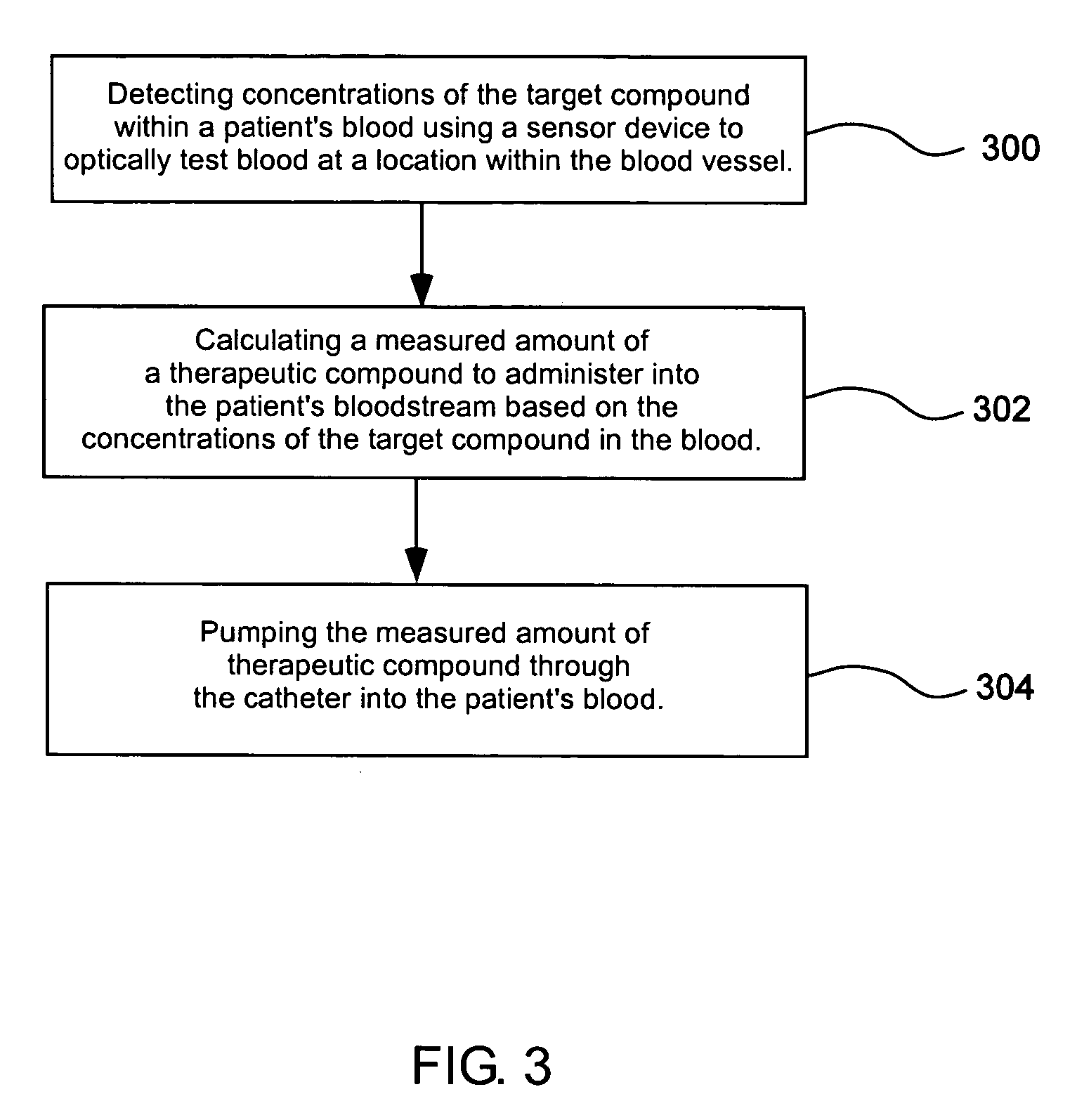
Blood testing and therapeutic compound delivery system
A system and method are provided for determining intravenous blood levels of a target compound contained in a blood vessel of a patient. The method includes the operation of detecting concentrations of the target compound within a patient's blood using a sensor device configured to optically test blood at a location within the blood vessel. Another operation is calculating a measured amount of a therapeutic compound to administer into the patient's bloodstream based on the concentrations of the target compound in the blood. The measured amount of therapeutic compound may then be pumped through the catheter into the patient's blood.
Owner:JONES CHRISTOPHER W
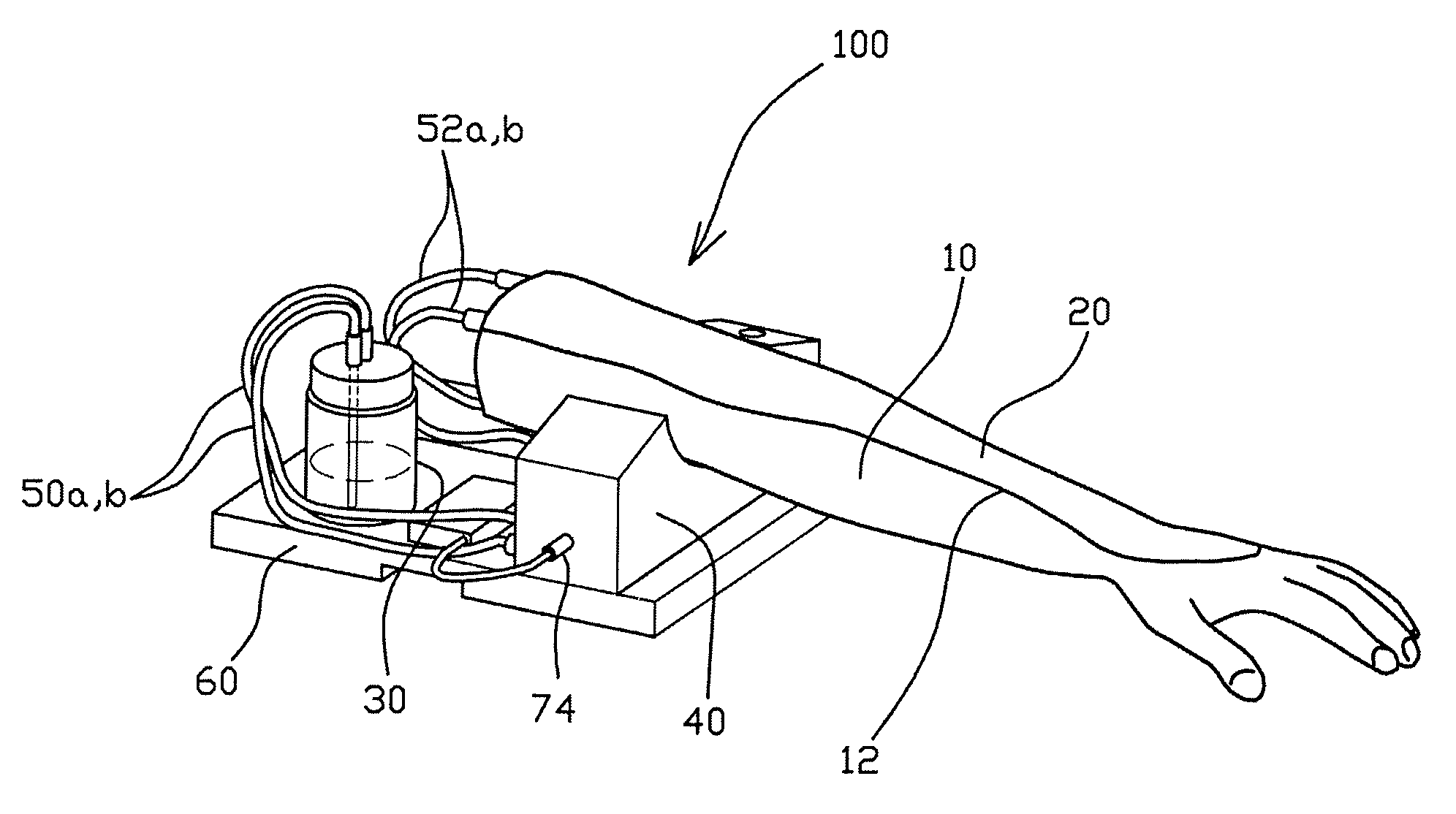
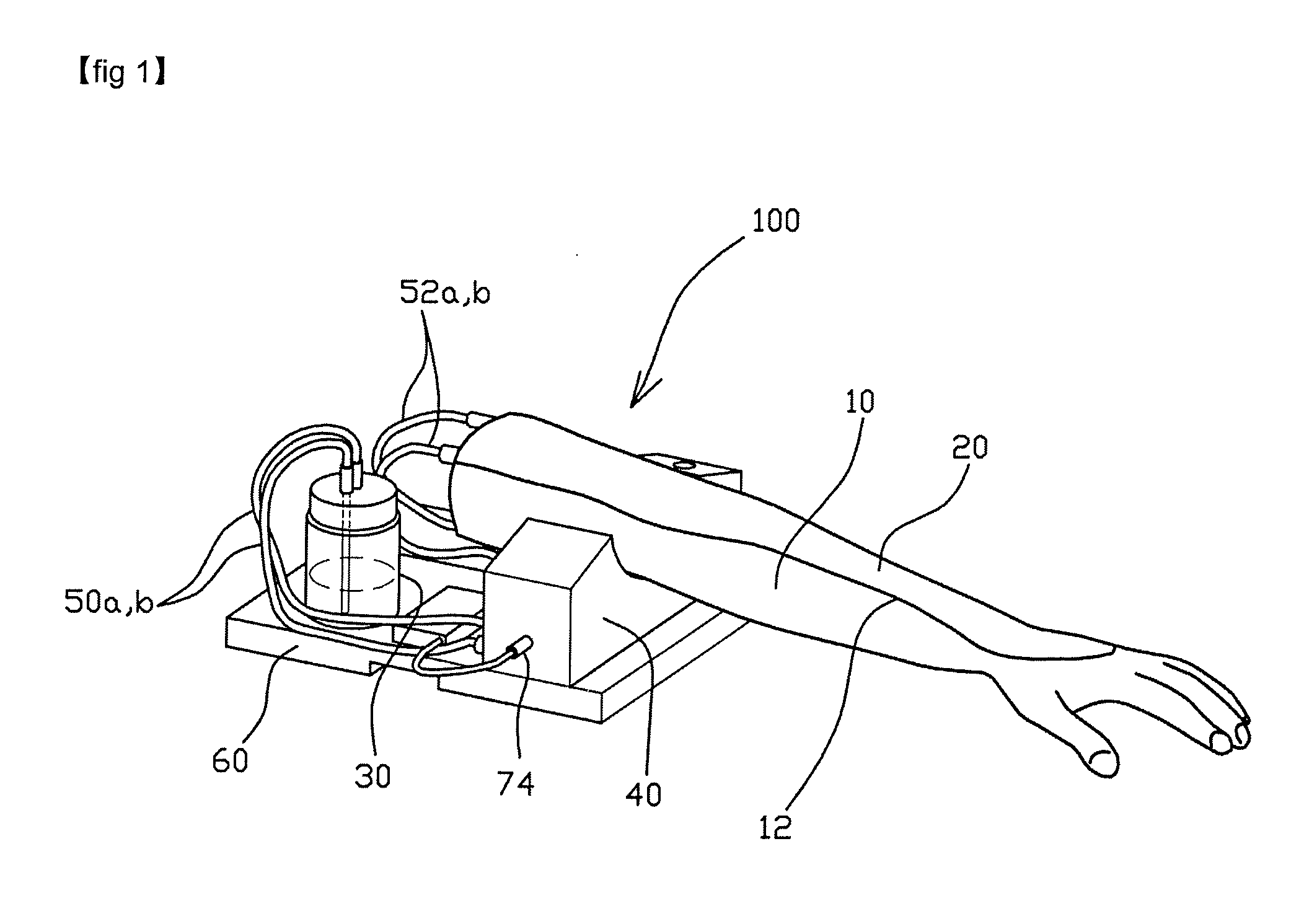
Arm model apparatus for intravenous injection training
The present invention provides an arm model apparatus for intravenous injection training, comprising: an arm model having an insertion grooved recess formed on a top thereof, the arm model being formed such that the arm model are twisted so as to allow the back of a hand part and a cubital fossa to be oriented upwardly; a skin pad detachably mounted into the insertion grooved recess and having a blood vessel-imitating tube formed therein; a pump drive unit connected to the a blood vessel-imitating tube by a pad connecting tube; and a liquid blood supply container connected to the pump drive unit by a container connecting tube and configured to supply blood stored therein to the blood vessel-imitating tube. The inventive arm model apparatus implements a realistic skin sensation as if a syringe needle penetrated through the blood vessel of the human body through the arm model.
Owner:BT INC
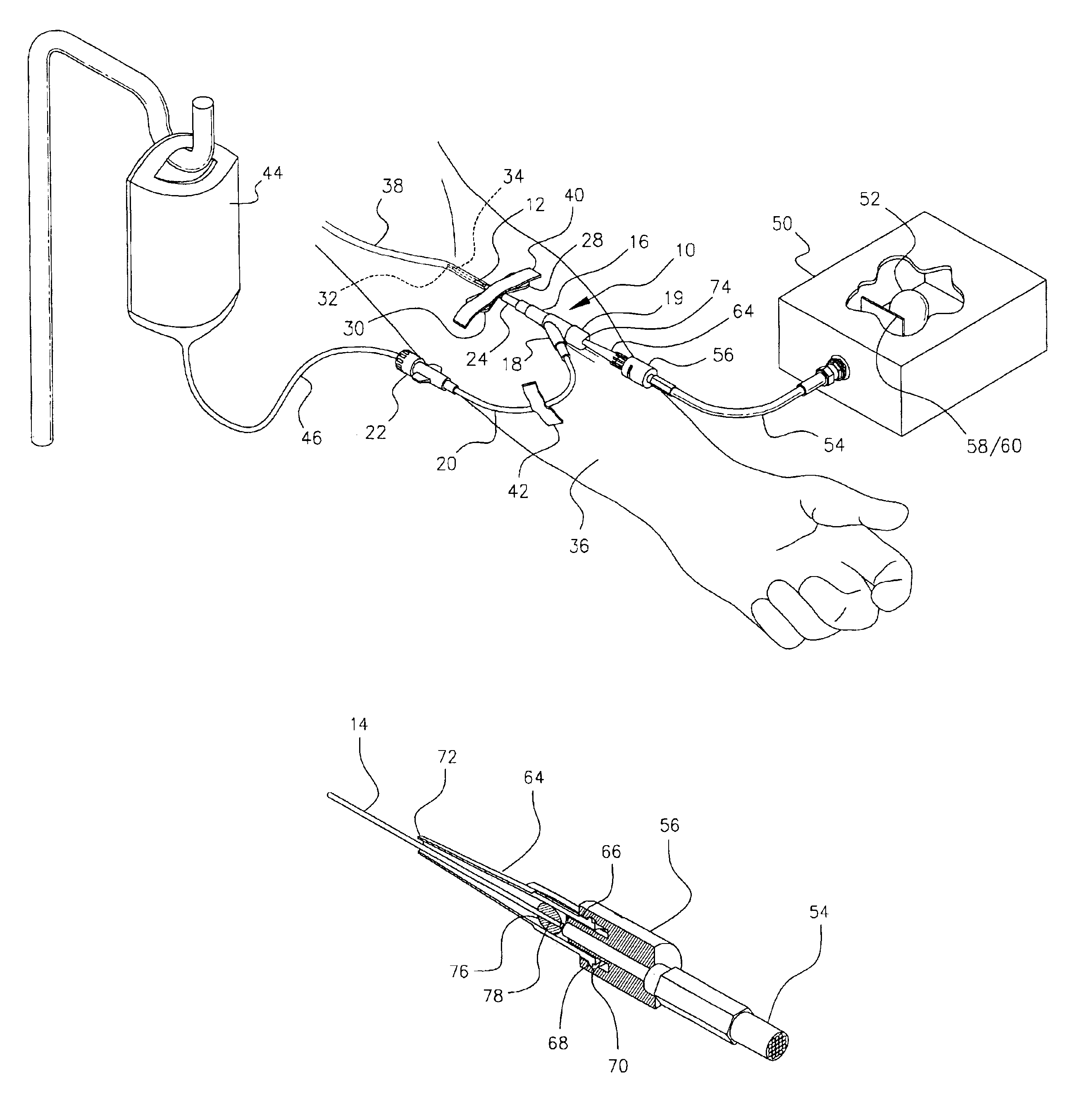
Intravenous injection site with split septum and pressure activated flow control valve
ActiveUS8211089B2Safety managementRule out the possibilityMedical devicesCatheterRefluxVein injection
An improved injection site (12) for infusion of parenteral fluids and the like is provided, having a pressure-actuated valve (20) and a novel split septum unit (24), which effectively prevent reflux of blood into the assembly (10). The septum unit (24) includes a resilient split septum body (64) which is precompressed so that the septum body (64) is caused to protrude proximally (78) upon insertion of a cannula (16). Consequently, upon removal of the cannula (16), there is essentially no “drumming” or creation of friction-induced negative pressures sufficient to generate blood reflux. The preloaded septum body (64) also has its proximal surface (74) essentially flush and coplanar with the adjacent proximal end (66b) of the tubular septum holder (66) to enhance the cleanliness of the unit (24). The specialized well (46) and septum unit (24) afford a resilient seal between the periphery of the septum body (64) and the surface (50), and a separate hard-surface seal between the outer margin of the surface (50) and the septum holder (66).
Owner:NEXUS MEDICAL LLC
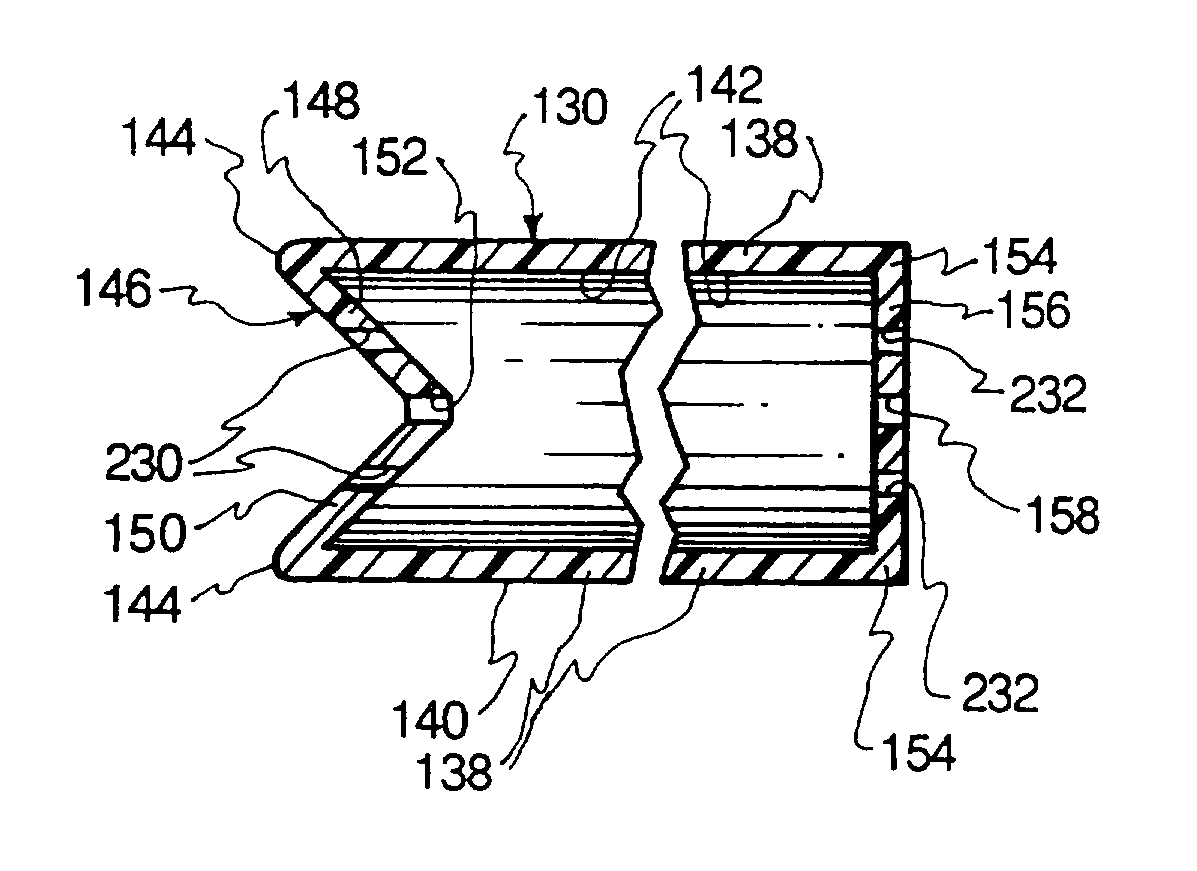
Split septum assembly for an intravenous injection site
ActiveUS20150224296A1Prevent proximal leakage of fluidMedical devicesCatheterAxial compressionEngineering
An intravenous injection site having a split septum assembly interfit with the site for the intravenous administration of fluids to a patient. The split septum assembly has a resilient and compressible split septum having an axially-formed slit for receipt of a blunt cannula, needle, or other medical device through said slit, a septum holder for receipt of the split septum, and a septum housing for receipt of the combined septum holder and split septum. The septum includes a body and a flange extending radially from the body. A projection extends from the flange. The septum is mounted within the septum holder, and the septum holder is mounted within the septum housing to provide axial compression of the flange. This axial compression presents a double hermetic seal that significantly minimizes or prevents proximal leakage of fluid through the slit or around the septum holder when the cannula is removed.
Owner:NEXUS MEDICAL LLC
Uses of icaritin in preparing medicament for preventing and treating endotoxemia
InactiveCN101428015ALower levelReduce mortalityOrganic active ingredientsAntinoxious agentsInflammatory factorsAbnormal macrophage
The invention belongs to the field of traditional Chinese medicine pharmacy, and relates to the novel medical usage of icaritin, in particular to the usage of icaritin in preparation of medicine for preventing and treating endotoxemia. Lipopolysaccharide (LPS) is used for stimulating the macrophage system RAW264.7 of a mouse and establishing an extraneous endotoxic inflammation model, LPS is used for stimulating the C57BL / 6J mouse and establishing the endotoxemia animal model, icaritin is adopted for intervention, and dexamethasone is used as reference. The experiment result shows that the icaritin can reduce the death rate, the level of the inflammatory factor, the inflammatory mediator and the adhesion molecules and infiltration of inflammatory cells of the tissue after the mouse is attacked by endotoxin, and proves that the icaritin can effectively prevent and treat endotoxemia and can be used for further preparing effective drug for preventing and treating endotoxemia, including dosage forms such as oral and enteric capsules or intravenous injections.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
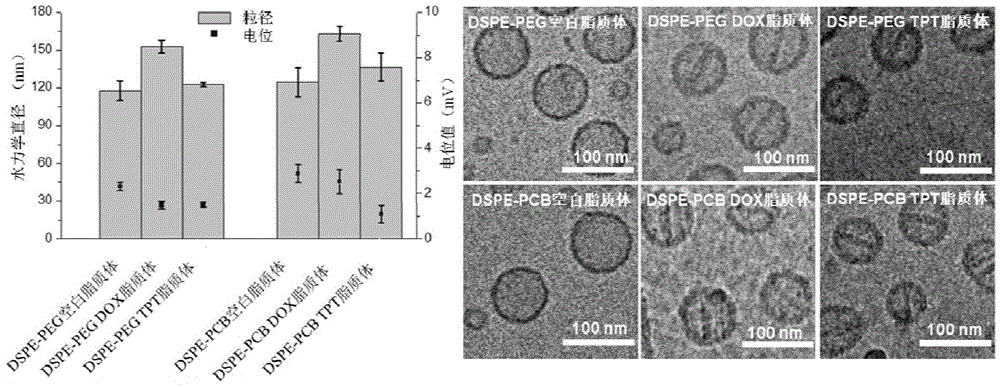
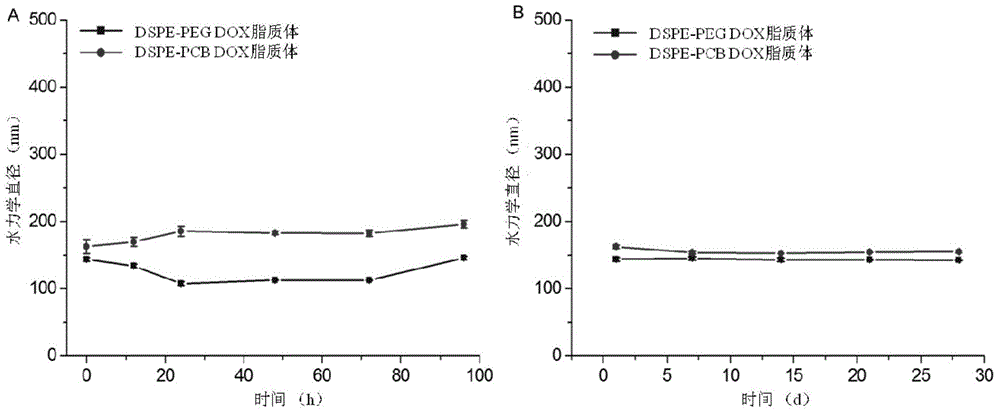
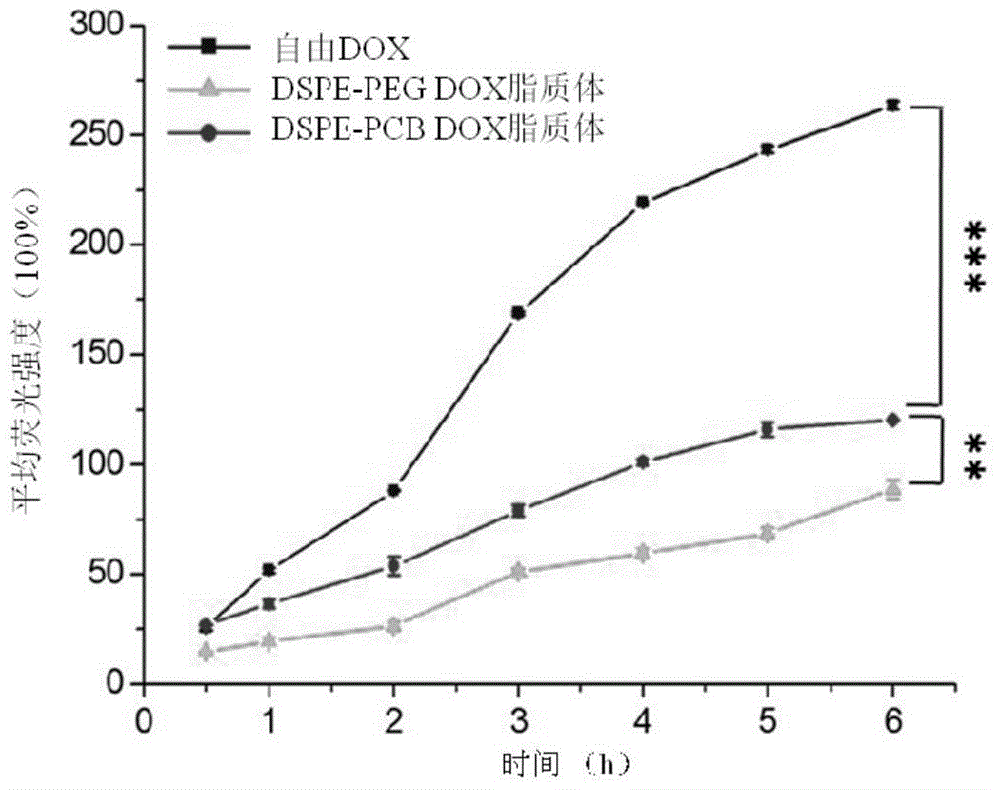
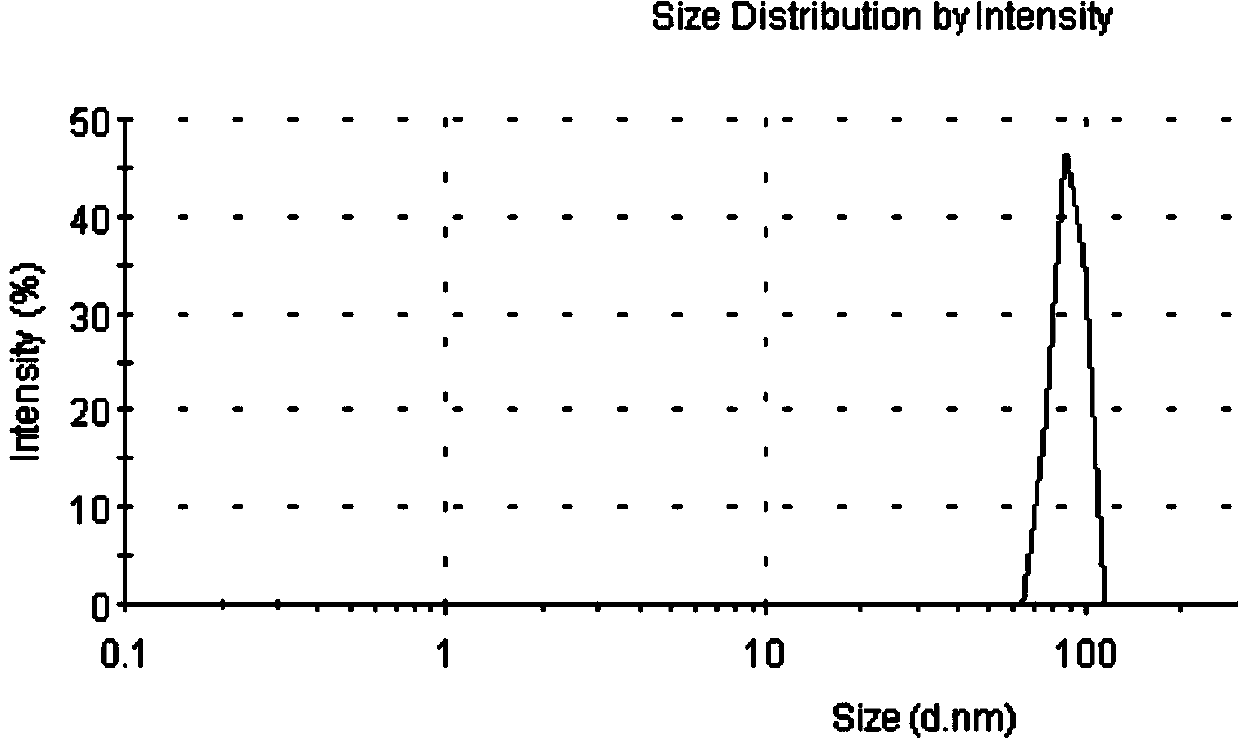
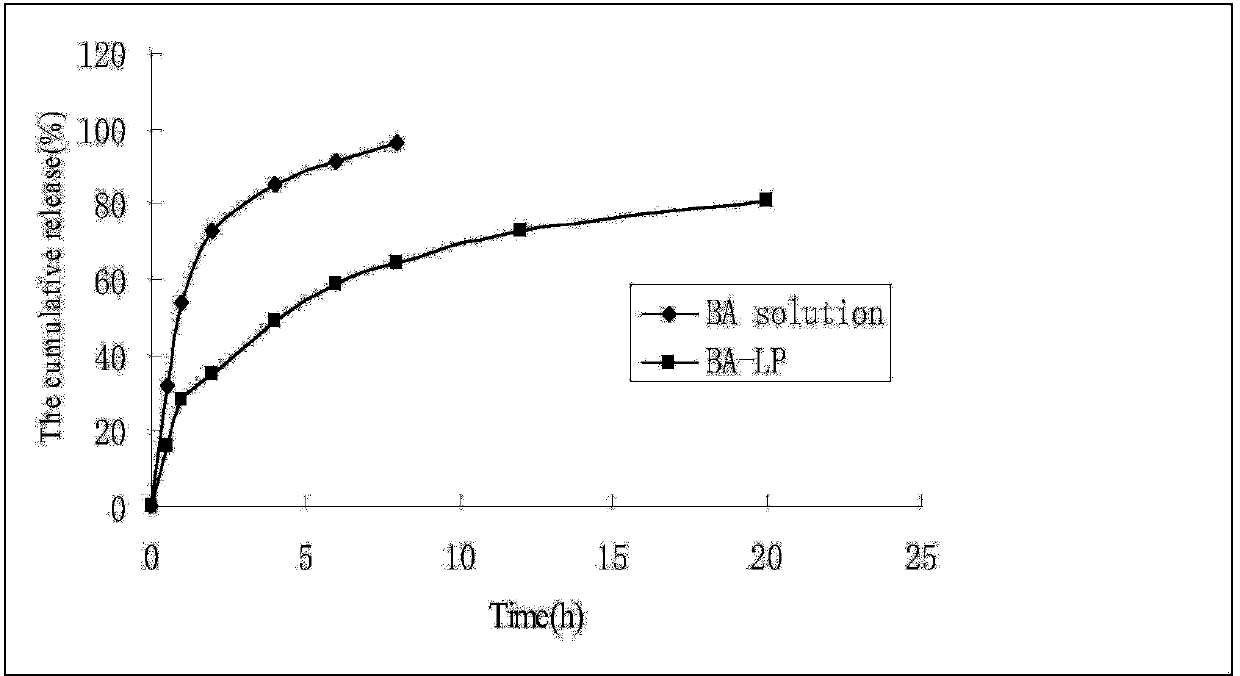
Long-circulating liposome capable of avoiding accelerated blood clearance (ABC) phenomenon, and preparation method and application thereof
InactiveCN104644555APromote escapeIncrease serum stabilityOrganic active ingredientsAntipyreticLipid formationDisease
The invention relates to a long-circulating liposome capable of avoiding an accelerated blood clearance (ABC) phenomenon, a medicine preparation containing the long-circulating liposome, and a preparation method and an application of the long-circulating liposome. The long-circulating liposome provided by the invention comprises phospholipid, auxiliary lipid and poly carboxyl betaine lipid, wherein the long-circulating liposome and the preparation thereof can be applied to medicines for treating related diseases. According to the long-circulating liposome for avoiding the ABC phenomenon provided by the invention, the defect that a common liposome is easily recognized by a reticular endothelium system is overcome; a long-circulating effect of blood is reached; meanwhile, the ABC phenomenon caused by multiple intravenous injections of the PEG-modified long-circulating liposome is avoided; the medicine effect of a therapeutic medicine is effectively improved; and the long-circulating liposome has a high application prospect in the field of medicines.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
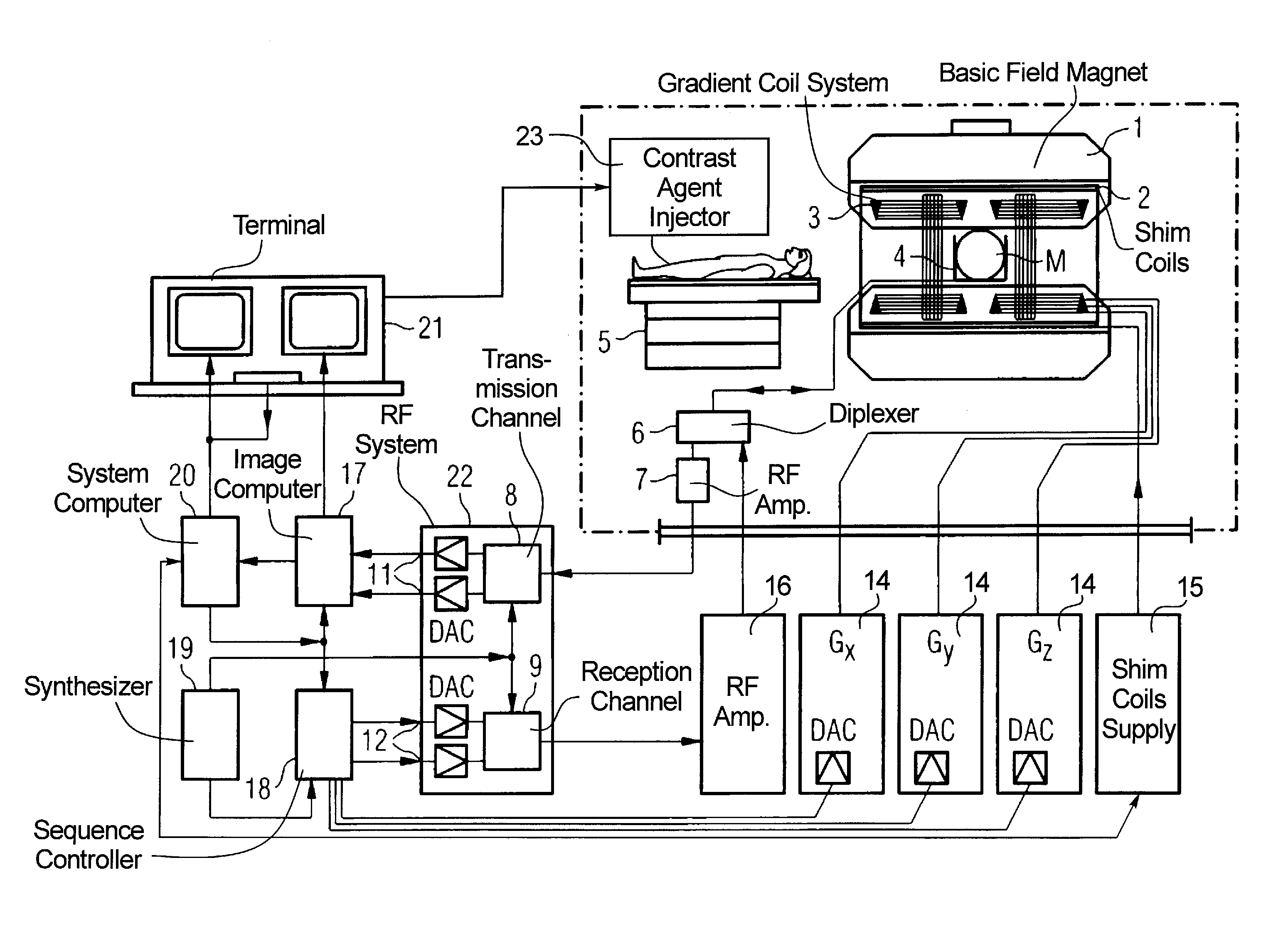
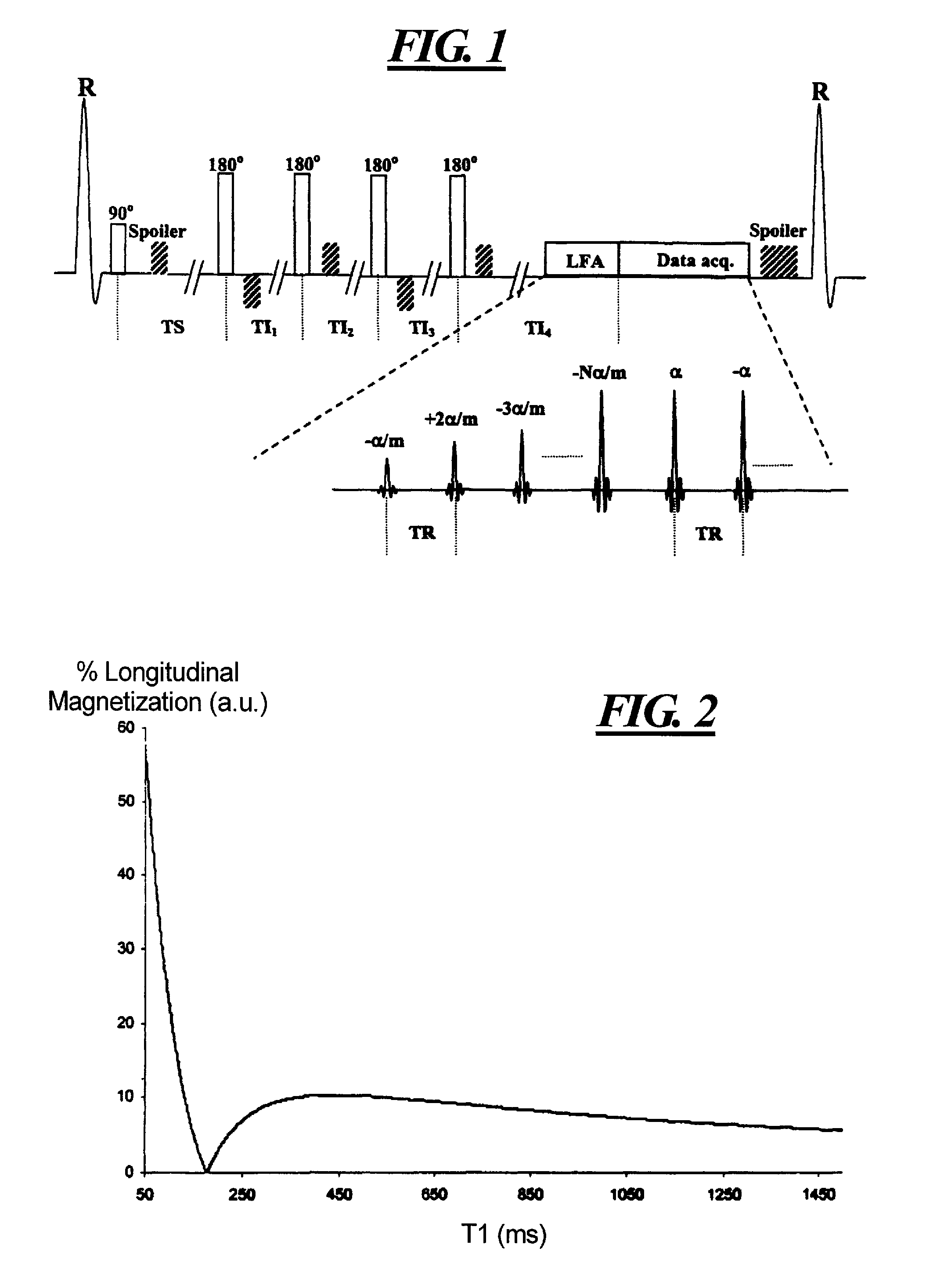
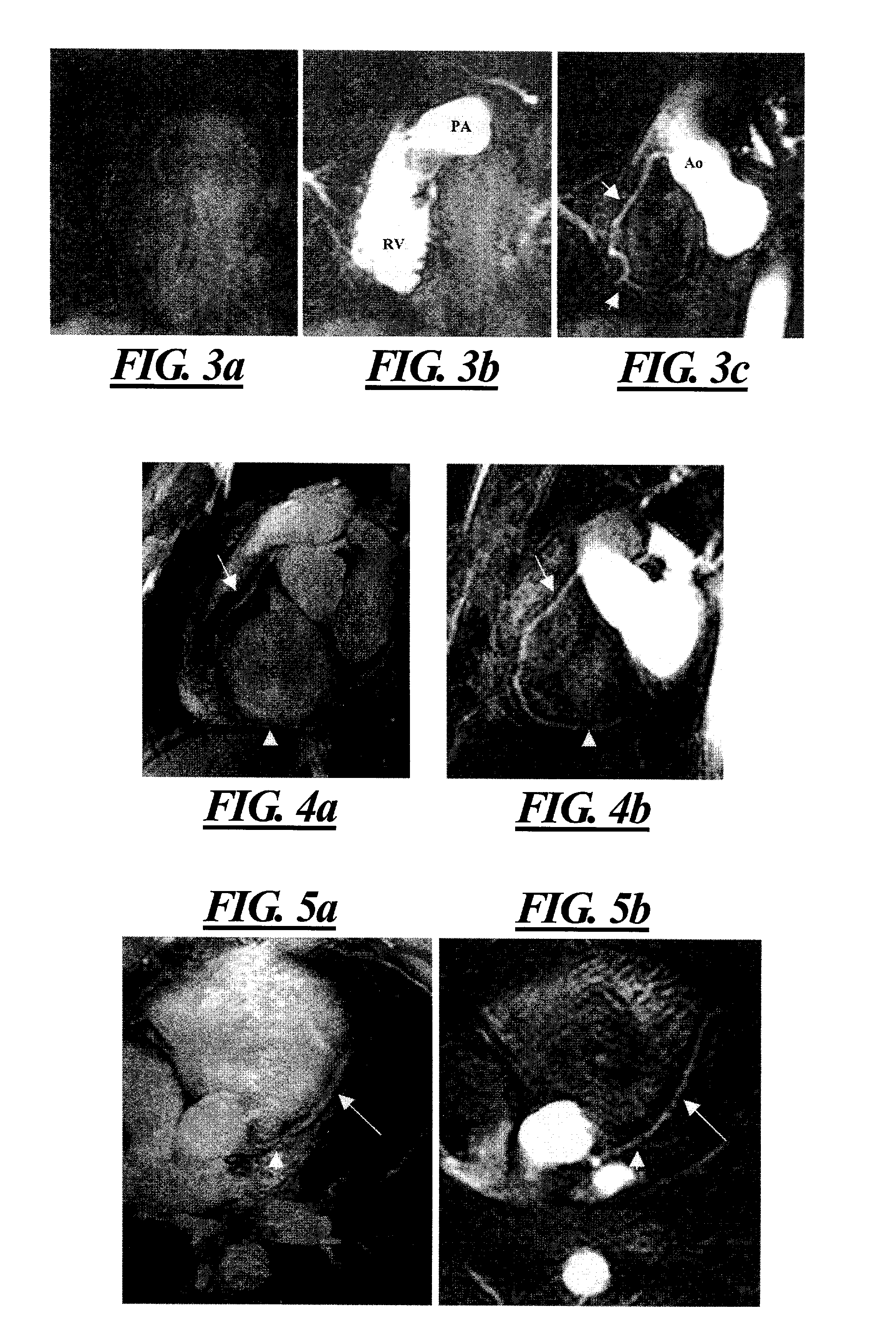
Time resolved contrast-enhanced MR projection imaging of the coronary arteries with intravenous contrast injection
Owner:NORTHWESTERN UNIV
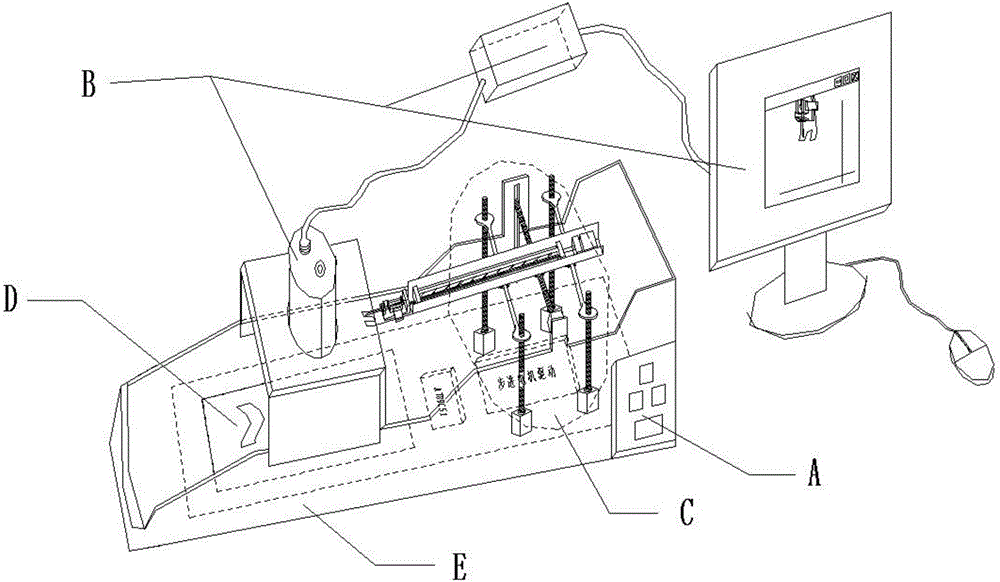
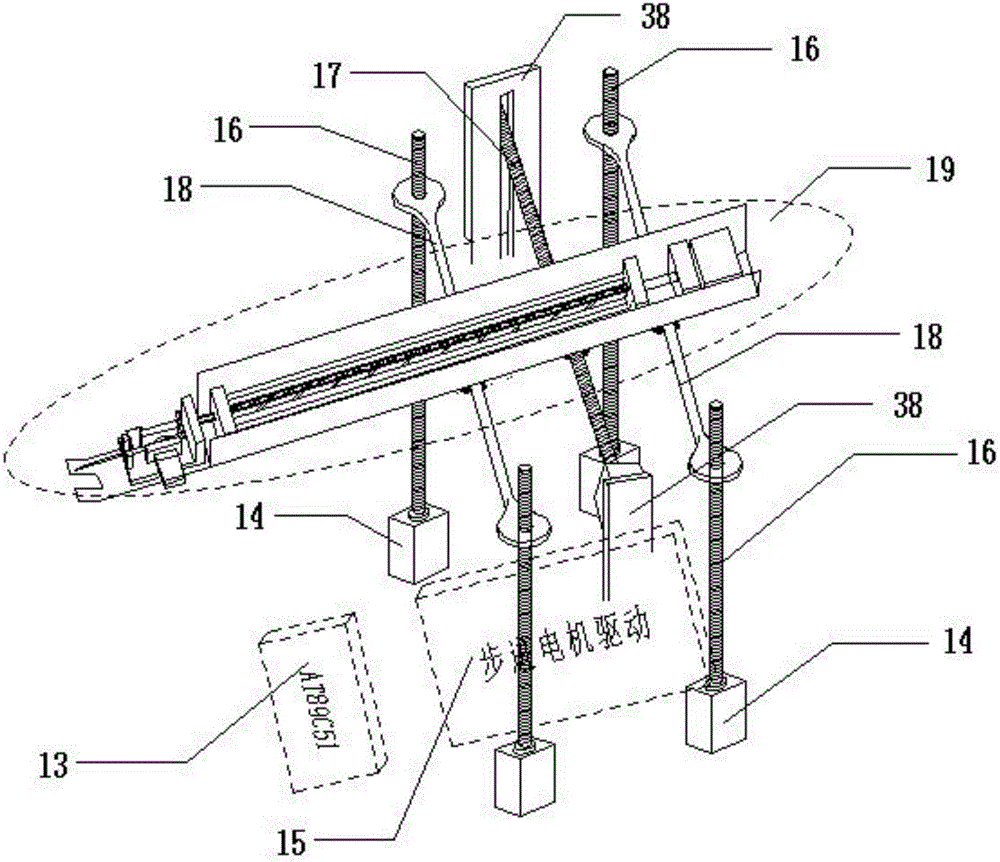
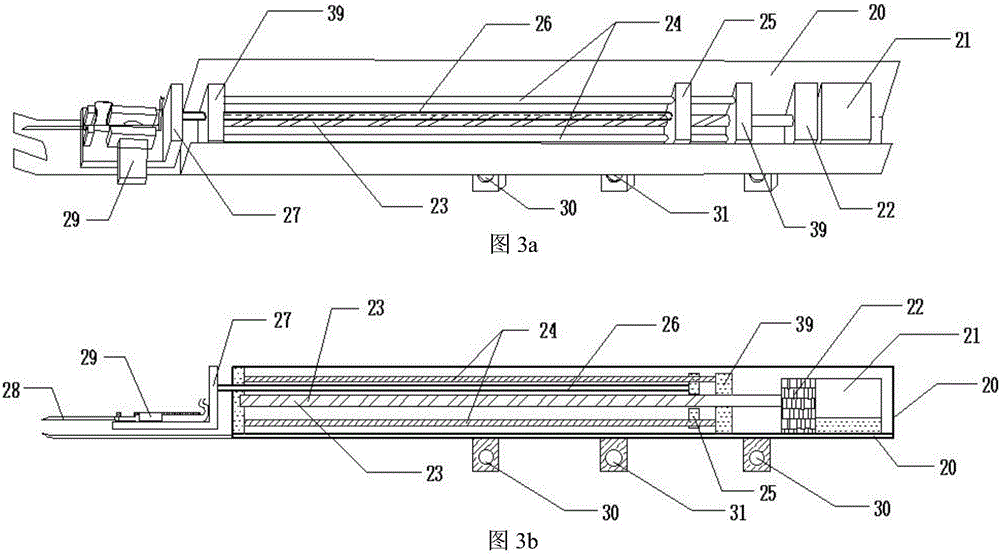
Hand back vein injection instrument based on infrared imaging technology
InactiveCN106267473AEnsure stabilityGuaranteed accuracyDiagnostics using lightAutomatic syringesMicrocontrollerVein puncture
The invention discloses a hand back vein injection instrument based on an infrared imaging technology. The injection instrument comprises a control panel, an infrared imaging part, a mechanical injection part, a fixed part and an instrument framework, wherein a horizontal adjusting rod in the mechanical injection part is parallel with two horizontal sliding rods, and is positioned above a center line position of a rectangle formed by four stepping motors; an injection rod is respectively connected with the horizontal adjusting rod and the horizontal sliding rods; 5 stepping motors are respectively connected with a stepping motor driving module; the stepping motor driving module is connected with a single chip microcomputer; the single chip microcomputer is connected with a computer through a digital serial port. The injection instrument is convenient for adjusting of the injection angle, greatly improves the injection stability of the injection rod, can help patients and medical care personnel to remove the interference of human factors, so as to rapidly and accurately accomplish vein injection, and improves the success rate of hand back vein puncture.
Owner:HEBEI UNIV OF TECH
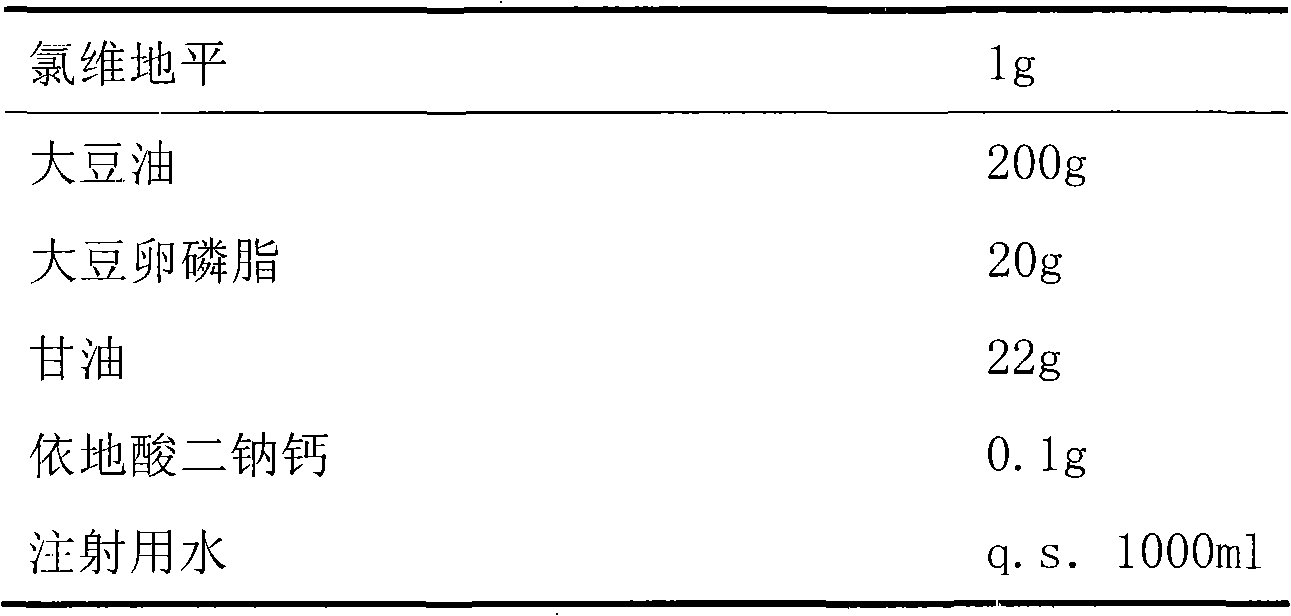
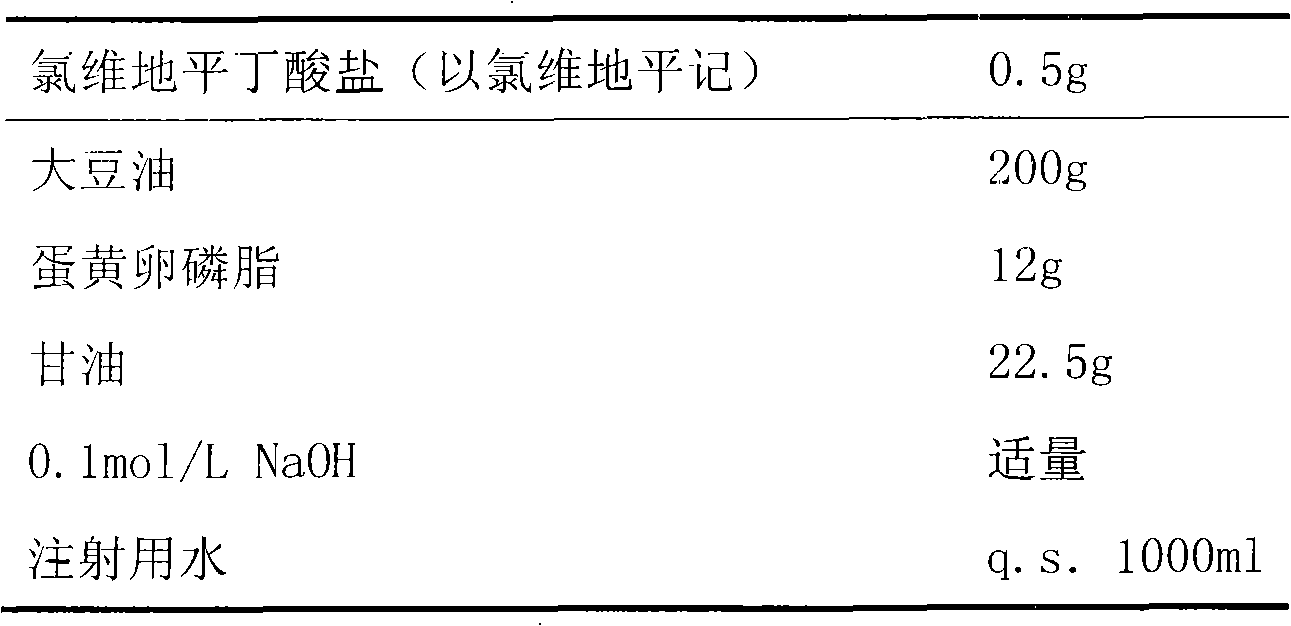
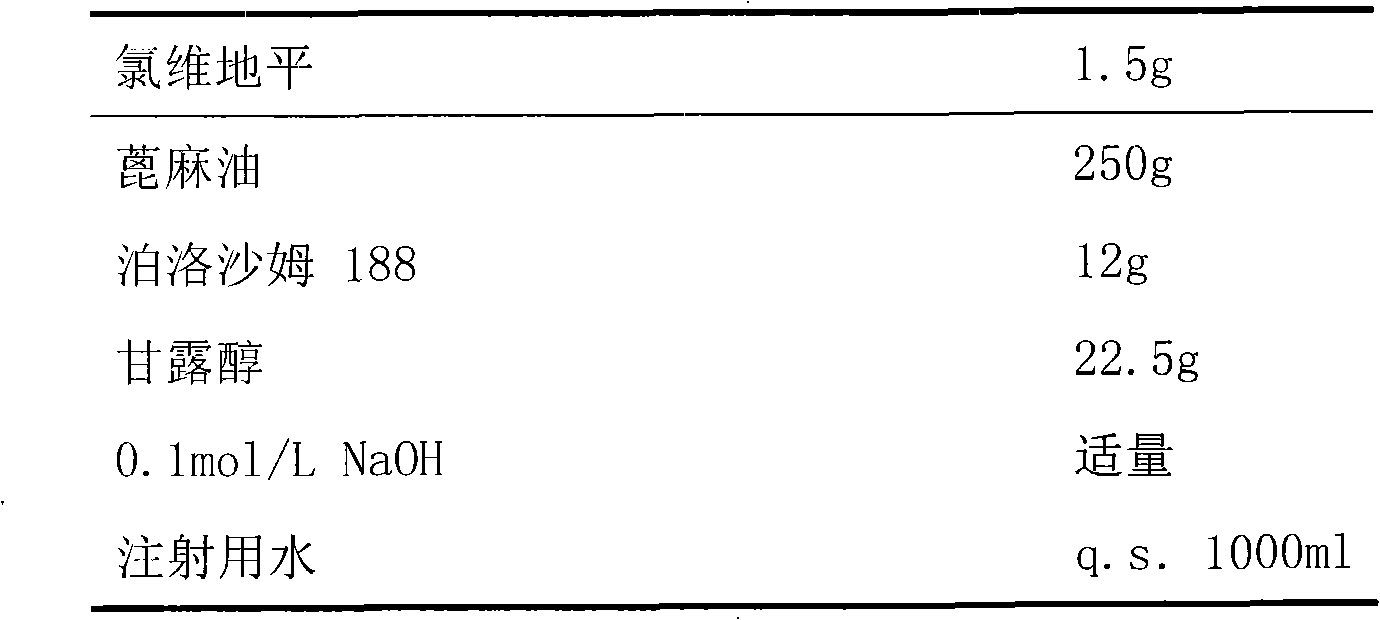
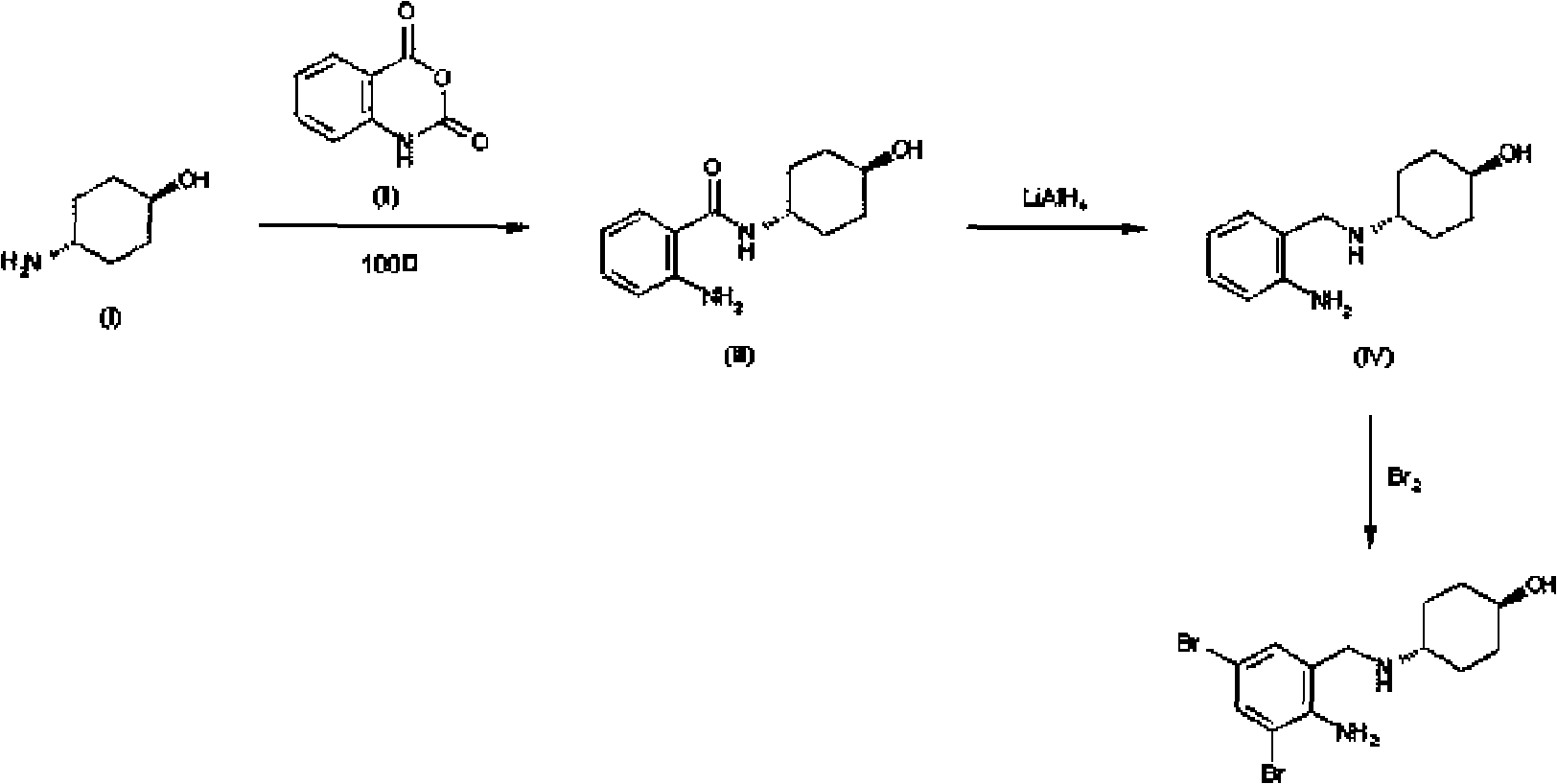
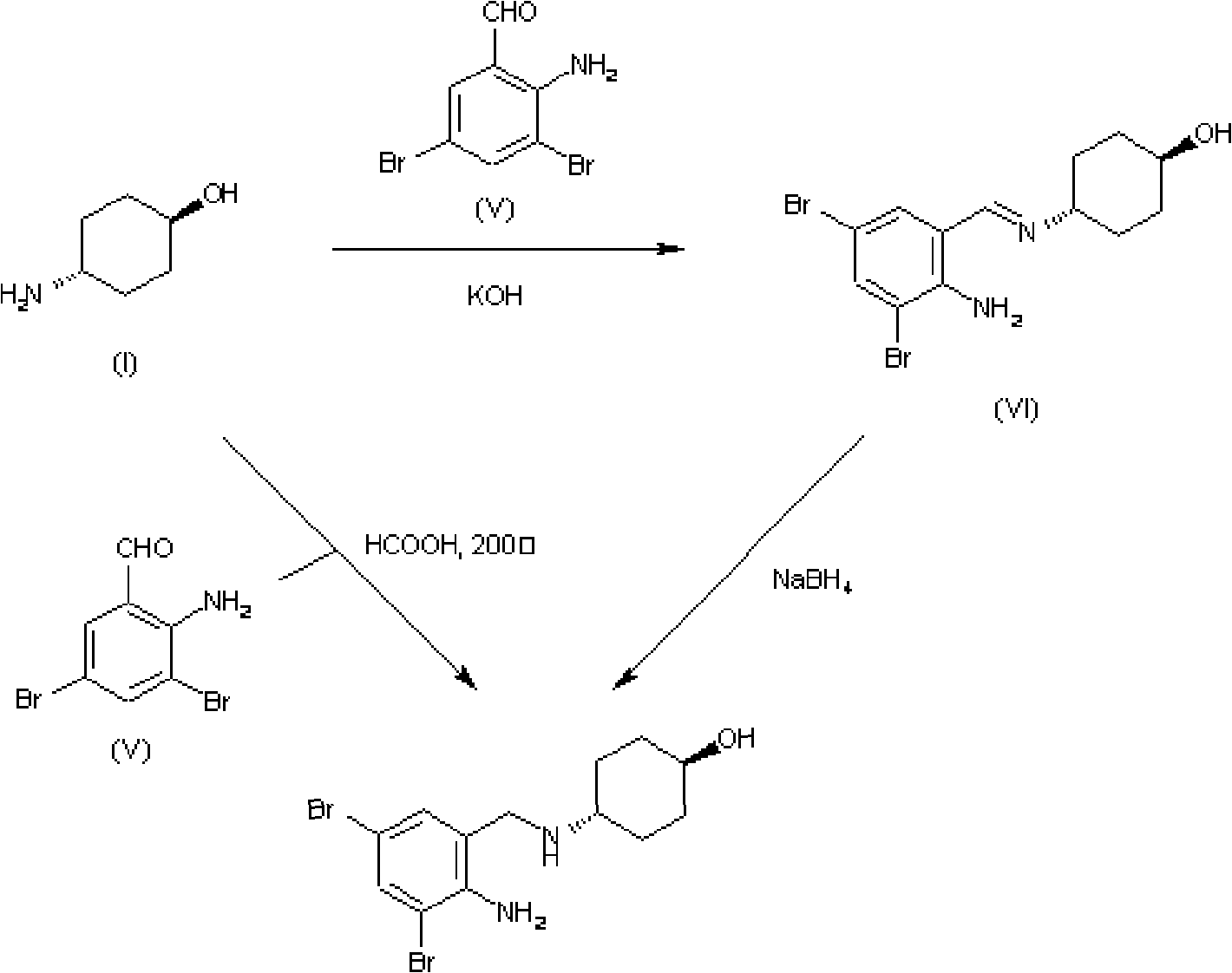
Emulsion containing clevidipine and preparation process and application thereof
InactiveCN101766568AUniform particle size distributionQuality improvementOrganic active ingredientsEmulsion deliveryEmulsionAdditive ingredient
The invention discloses to an emulsion containing clevidipine and a preparation process and application thereof, relating to the emulsion utilizing the clevidipine and pharmaceutically acceptable salt or hydrate as active ingredients and the preparation process and the application thereof. The emulsion used for vein injection, which utilizes the clevidipine and the pharmaceutically acceptable salt or hydrate as the active ingredients, is formed by carrying out a certain preparation process on the active ingredients and pharmaceutically acceptable auxiliary materials, and can be used for rapid depressurization during and after a surgery. In the invention, the clevidipine and the pharmaceutically acceptable salt are taken as the active ingredients and some auxiliary materials with specific varieties and ratios are added, thus the emulsion for vein injection is developed according to the preparation process explained by the patent.
Owner:北京利乐生制药科技有限公司
Culture method, application and combined preparation of hypoxia mesenchymal stem cell
InactiveCN101792737AHigh activityImprove survival rateOrganic active ingredientsMammal material medical ingredientsCell massTherapeutic effect
The invention discloses a culture method, application and a combined preparation of a hypoxia mesenchymal stem cell. By adopting an umbilical or placenta mesenchymal stem cell and combining the means of transfusing compound amino acid injection and the like, the vitality, the implantation survival rate and the treating effect of the mesenchymal stem cell in vivo can be improved; and in order to avoid the possibility of conglutination, conglomerating and cell mass embolism of the mesenchymal stem cell in blood vessel, the matching of saline containing heparin during treatment can be carried out by a mode of intravenous injection or intravenous drip. With more than three times of treatment, physical conditions of curee can be remarkably improved and objective indicators such as immunity, blood fat, blood sugar and the like can be remarkably improved.
Owner:晏泽
Ambroxol hydrochloride injection with small volume and preparation method thereof
InactiveCN101647777AStrong buffer capacityImprove stability and securityOrganic active ingredientsPharmaceutical delivery mechanismChemistryCarbon dioxide
The invention discloses an ambroxol hydrochloride injection with small volume and high stability and a preparation method thereof. In the ambroxol hydrochloride injection with small volume, the weightpercentage concentration of ambroxol hydrochloride is 0.3-4 percent, the ambroxol hydrochloride solution comprises a component capable of forming acid-base buffer pairs and further comprises carbon dioxide, and a part of space in an ampoule is filled with nitrogen gas. By utilizing carbon dioxide to remove oxygen from the solution, the invention provides the ambroxol hydrochloride injection whichhas small volume and longer stable phase and can be prepared by ambroxol hydrochloride material in different syntheticroutes. The ambroxol hydrochloride injection with small volume can be directly injected into a vein and has good buffering capacity for pH value of blood plasma.
Owner:HEILONGJIANG FUHE HUAXING PHARMA GROUP
Device for intravenous injection of mouse tail and superficial intravenous injection of other parts
InactiveCN104799968AEasy to fixEnsure safetyDiagnostic recording/measuringSensorsVein injectionClosed cavity
The invention relates to a device for intravenous injection of a mouse tail and superficial intravenous injection of other parts. The device comprises a base, a transparent tube, a front fixing part and a rear fixing part, wherein a notch is formed in the rear fixing part, and ventilation holes are formed in two side edges of the transparent tube; the base is a closed cavity with a light source inside, the base comprises a top plate, a soleplate and four side plates, an adjustable light-transmitting operation panel which is hinged to the top plate is arranged between the side plate facing one end of the tail part of a mouse and the top plate, the side edge of the top plate is provided with a fixing plate facing the interior of the base, the adjustable light-transmitting operation panel is provided with an angle adjusting screw for adjusting an angle between the adjustable light-transmitting operation panel and the fixing plate, and other three side edges of the adjustable light-transmitting operation panel are respectively provided with an elastic light screening plate which is connected to the side edge of the adjacent base. By adopting the device, the safety of the operator is adequately guaranteed, and the operator can be prevented from being bitten by the mouse when in injection; moreover, the mouse is firmly fixed, the stress response of the mouse can be prevented from affecting the experiment result, and the experiment result is more reliable.
Owner:THE FIRST AFFILIATED HOSPITAL OF HENAN UNIV OF SCI & TECH
I.V. catheter assembly with blood exposure prevention
I.V. catheter assembly was a catheter with a distal needle for insertion into a blood vessel a portion of the catheter. An adapter with a self-sealing or self-closing plug is connected to the catheter, proximally of the distal needle. A proximal needle that can couple to the distal needle is in a guard tube on the other side of the plug. In use the proximal needle is pushed through the plug and into coupling engagement with the distal needle so the distal needle can be inserted into a blood vessel. The coupled needles are then withdrawn and shielded in the guard tube. The guard tube is then disconnected from the adapter and the adapter can be used to discharge blood from, or supply fluid to the blood vessel.
Owner:GUTIERREZ RAYMOND
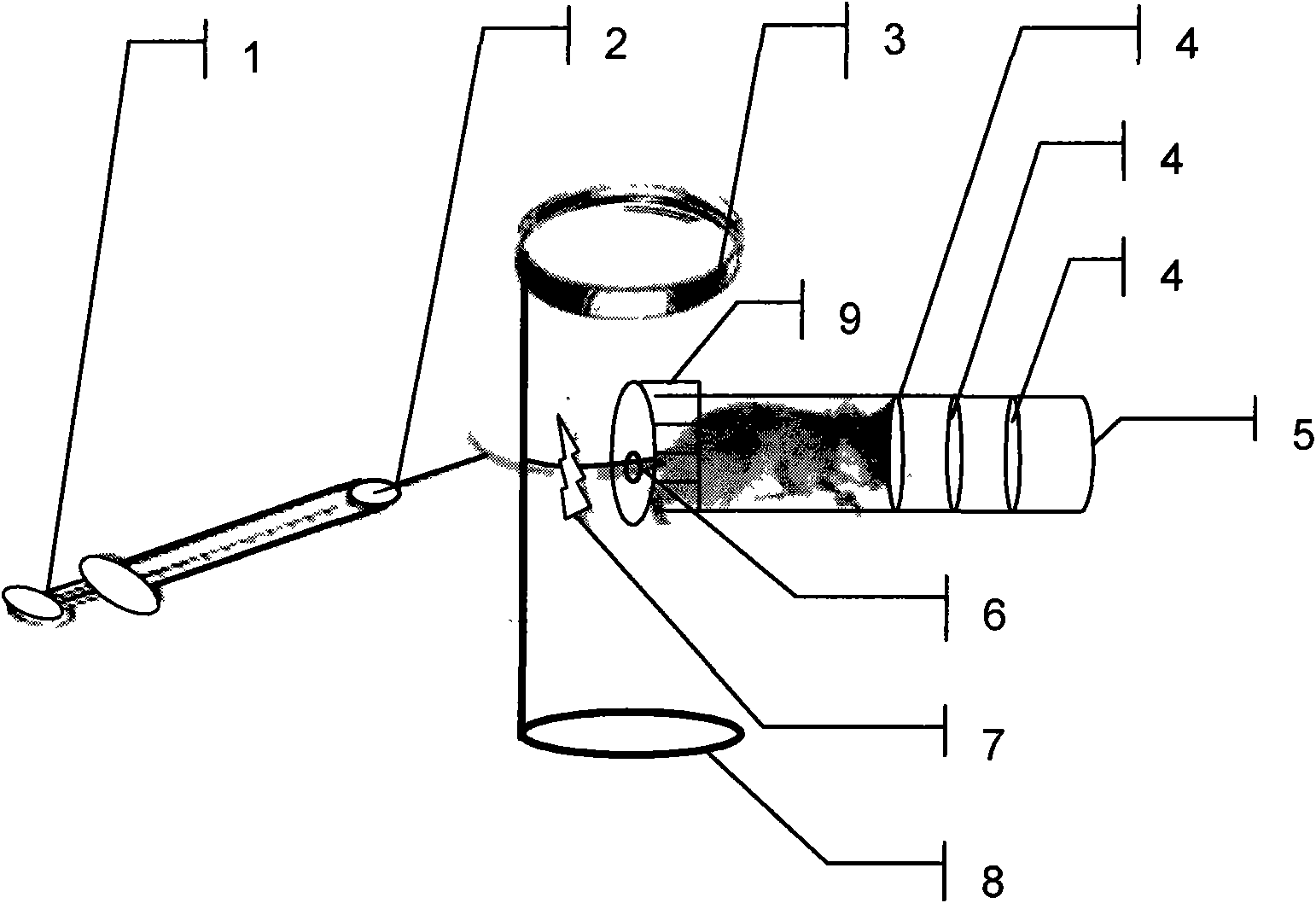
Tiny vein injection drug delivery system on body surface of experimental animal
InactiveCN101637411AEasy to control the lengthAccurate micro-venous injection processVeterinary instrumentsMagnifying glassBlood vessel
The invention relates to a drug delivery system for experimental animals, in particular to a tiny vein injection drug delivery system on body surfaces of experimental animals. The system comprises a fixed chamber 5 for experimental animals, a simple desk type magnifying glass, a vascular clamp 7 and an injector 1 with a superfine injection needle 2, wherein the fixed chamber 5 for experimental animals fixes experimental animals; the vascular clamp 7 is clamped above injection blood vessel; the injector 1 is connected with the superfine injection needle 2; the simple desk type magnifying glassis arranged above vein injection operation, and the injection can be carried out; and component parts of the device can be used solely or be in arbitrarily combined use according to actual operation requirement. The invention has the beneficial effect that the tiny vein injection process of experimental animals is more accurate, efficient, convenient and fast.
Owner:DALIAN UNIV
Doxofylline venous injection with small volume as well as preparation method and quality control method thereof
ActiveCN101647776AReduce health impactReduce the impactInorganic non-active ingredientsSurface/boundary effectDoxofyllineVein injection
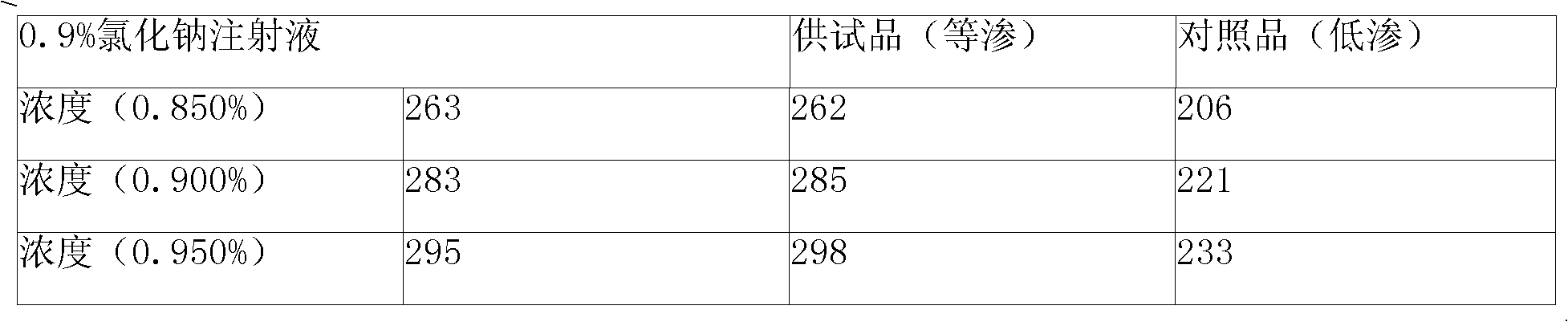
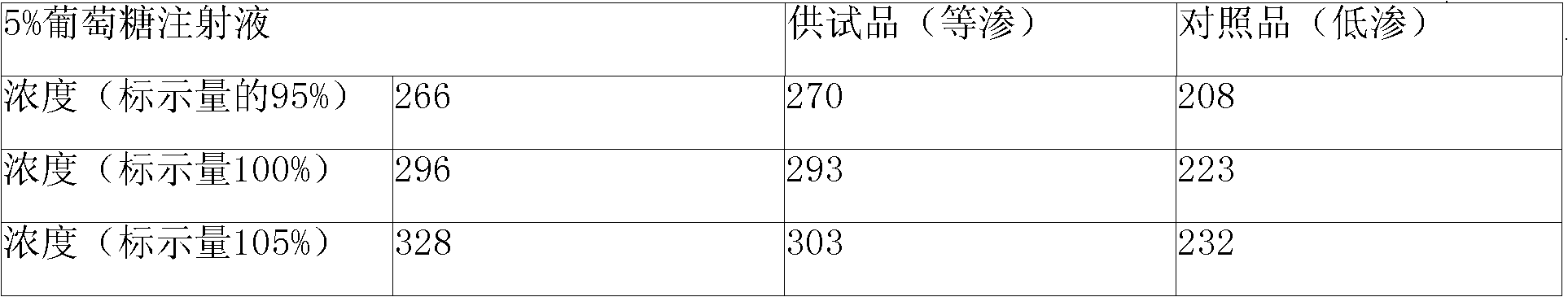
The invention discloses Doxofylline venous injection with a small volume and a preparation method thereof. The preparation unit of the isotonic small volume of doxofylline venous injection is 1-20ml,the weight percentage concentration of doxofylline is 1-4 percent, and the isotonic value of the venous injection is 257-340mosmol / kg. The invention enlarges the optional range of clinical applicationof doxofylline and provides the isotonic doxofylline venous injection with favorable security.
Owner:HEILONGJIANG FUHE HUAXING PHARMA GROUP
Lyophilized injection powder using Lanluodier and its salt as active ingredients and preparing technique therefor
This invention is about a freeze-dried preparation using Llandeilo and its salt as active components and its making method and usages. It is a medicine composition that takes Llandeilo and its salt as active components, and is formed by mixing acceptable finding. It can be used to emergency cure tachycardia arrhythmia (containing atria fibrillation, atria flutter and sinus tachycardia) during operation. It uses Llandeilo and its salt as raw materials, adds some findings of specify kinds and proportions, and develop freeze-dried preparation according to this invention for intravenous injection.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Lipid microvesicle ultrasound angiography powder agent internally containing mixture gas of fluorine carbon/nitrogen gas and production of the same
InactiveCN101130095AEnhanced Ultrasound ImagingEnables mass productionEchographic/ultrasound-imaging preparationsLipid formationDispersity
The invention discloses a hollow lipid microfoam composition with fluorocarbon / nitrogen and preparing method, wherein the microfoam filming material is composed of phosphatide, protective and polymer; the phosphatide is selected from phosphatidyl Choline and phosphatidyl ethanolamine; the protective can be middle and macromolecular hydroxyethyl amidon; the polymer is selected from boluoshamu 188 or medically acceptable surface activator for vein injection; the lipid filming material covers the fluorocarbon liquid to form emulsion particle, which forms hollow lipid microball under high temperature instantaneously through gasifying the liquid fluorocarbon; the fluorocarbon / nitrogen is guided to produce the lipid microball for ultrasonic imaging with effectively reinforcing time over 60 min. The invention has high yield rate and fast manufacturing speed, which improves the microball dispersity and microball density effectively.
Owner:CHONGQING RUNQI PHARMA TECH DEV
Power for intravenous injection with liver-protecting action, and its preparation and quality control method
The present invention discloses a powder injection for intravenous injection for curing several diseases of liver damage with obvious therapeutic effect. Said powder injection has the action of protecting liver, its main component is silibinin-N-methylglucamine whose content is 20-500 mg / g.
Owner:LUNAN PHARMA GROUP CORPORATION
Method and apparatus for monitoring temperature of intravenously delivered fluids and other medical items
InactiveUS8821011B2Facilitate indicationEasy temperatureThermometer detailsThermometers using physical/chemical changesMonitoring temperatureIntravenous solutions
An intravenous solution bag includes a temperature sensing device in the form of a temperature sensing strip. The strip includes a temperature scale and corresponding temperature sensitive substances that change color or illuminate the scale indicators to visually indicate solution temperature. The strip may be formed integral with the bag, may be attached to the bag exterior surface, may be laminated to the bag exterior surface or may be encased with the bag within a solution bag liner. Further, the temperature sensing strip may be affixed to bottles containing intravenous or other solutions, where the strip is attached to the bottle exterior surface or to a label affixed to the bottle to measure and indicate temperature of fluid contained therein as described above. Moreover, the temperature sensing strip may be employed by a receptacle or delivery tube of an infusion apparatus to measure and indicate solution temperature prior to or during infusion. In addition, the temperature sensing strip may be disposed within thermal treatment system compartments to measure and provide a visual indication of medical item temperatures. Alternatively, the strip may be employed by a stand, plate, receptacle or other structure receiving a medical item or container to provide a temperature measurement and indication as described above. The structure may be a stand-alone unit or may be attached to a thermal treatment or other system to facilitate temperature measurement.
Owner:MEDICAL SOLUTIONS
Treatment using dantrolene
ActiveUS7758890B2Rapidly administrableRapidly reconstitutableBiocideOrganic active ingredientsDiseaseTime-Consuming
Owner:LYOTROPICS THERAPEUTICS INC
Methodfor solving the problen of writing medical report and prescription in computer by means of 'form method'
InactiveCN1484186AReduce text inputSolve the problem of illegible handwriting that is difficult to decipherSpecial data processing applicationsVeinMedical record
The invention is a method which uses "the template method" to solve that it is difficult to write the medical record and prescription on the computer. each kind of disease name, the host sues, the present medical history, formerly history, the medical examination, and standard treating plan are inputted into the disease template databank in advance, each kind of medicine name, tablet, capsule, intermuscular injection, the vein injection, vein drip and other dosage-forms information are inputted into the data bank in advance. Through the computer procedure, only need several letters, and make a little change according to the reality, a serial of medical file can accomplished.
Owner:练志芳
Brain targeting gene transfer and release system and its prepn process
InactiveCN1931372AHigh transfection efficiencyImprove expression levelNervous disorderGenetic material ingredientsVascular endotheliumPlasmid dna
The present invention belongs to the field of biotechnology, and is one kind of brain targeting gene transfer and release system and its preparation process. Lactoferrin is first used to modify cationic polymer by means of hydrophilic polymer, and the modified cationic polymer is then compounded with plasmid DNA by means of electrostatic effect to form the gene transfer and release system. The present invention can raise the transfection efficiency and expression efficiency of gene on brain capillary endothelial cell effectively, and raise the brain targeting property of gene transfer and release system and the expression of the gene in brain obviously after the non-invasive administration via intravenous injection. Compared with available technology, the present invention has obviously raised brain targeting effect.
Owner:FUDAN UNIV
Injectio of gastrodine, its preparing process and usage
InactiveCN1634091AGive full play to the pharmacological effectImprove bioavailabilityOrganic active ingredientsNervous disorderVeinMedicine
The invention provides a gastrodine injection, which comprises the forms of gastrodine water for injection and metal chelating agent, the invention also discloses its preparation and use. Wherein the preparation comprises depositing gastrodine with water, cold storing, ultrafiltration technology processing, and sterilizing. The injection can be used for treating brain trauma, megrims, nerve pain, cephalodynia and neurasthenia.
Owner:巴里莫尔制药(通化)有限公司
Intravenous site protection device
Owner:PADILLA JR JAMES D
Solid lipid nanoparticle or liposome and preparation method thereof
InactiveCN104173290ASimple preparation processQuality is easy to controlPowder deliveryInorganic non-active ingredientsOral medicationInhalation
The invention provides a solid lipid nanoparticle or a solid liposome which is prepared from a raw material at least containing active medicines, a lipid carrier material, an effervescent acidic material and an effervescent alkaline material, wherein the lipid carrier material is a material in which active medicines are encapsulated. The medical lipid nanoparticle (the solid lipid nanoparticle or the solid liposome) is successfully prepared by virtue of an effervescent dispersion technology. The method is simple and convenient in preparation process, controllable in quality, low in equipment requirement and easy to industrialize. The lipid nanoparticle prepared according to the method can be in a solid state and in a mixed suspension state, is stable in quality and has the particle diameter within a range of 0.050-20 microns, and can be prepared into various solid-type and solution-type preparations so as to meet clinical medication requirements of oral administration, vein injection, inhalation and transdermal delivery.
Owner:赵领 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com