Emulsion containing clevidipine and preparation process and application thereof
A technology of clevidipine and preparation process, applied in the field of medicine, can solve problems such as poor water solubility, and achieve the effects of stable quality and uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
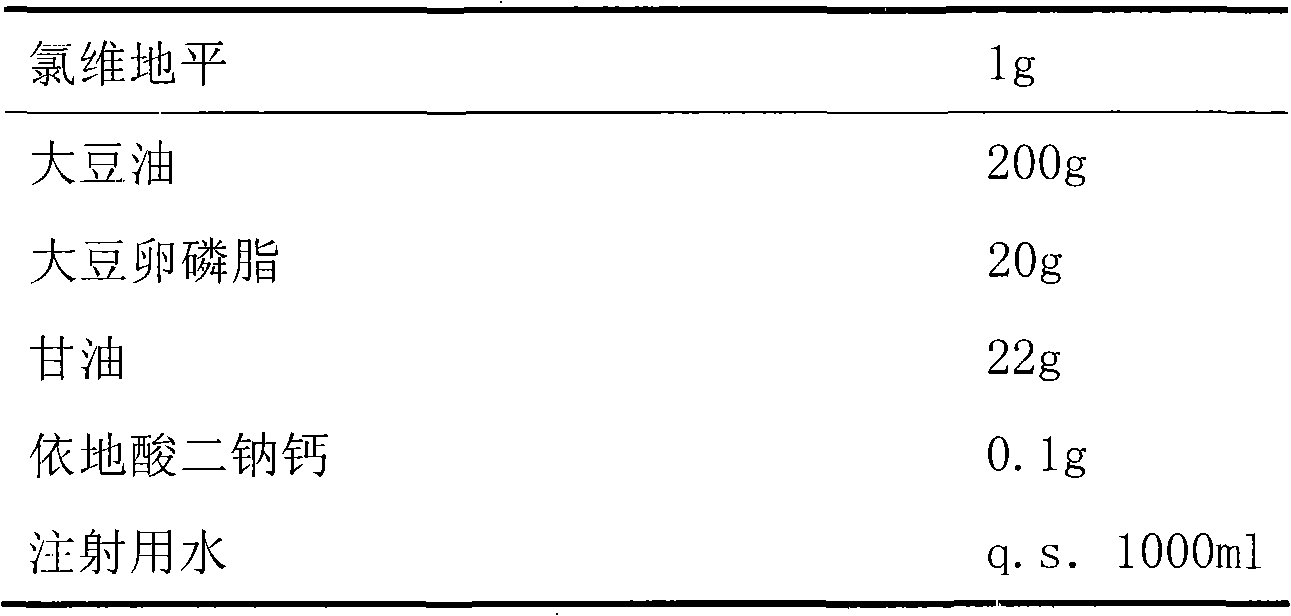
[0017]
[0018] Preparation:
[0019] First, measure 500ml of water for injection, dissolve the prescribed amount of glycerin and edetate disodium calcium in it, and dissolve clevidipine and soybean phosphatidylcholine in soybean oil, and heat appropriately to promote dissolution. Then add the water phase and the oil phase to the high-pressure homogenizer in turn for circulation and homogenization until the particle size distribution is 50-250nm, take it out, adjust the pH value to 6-8 with 0.1mol / L NaOH, and then add the remaining A certain amount of water for injection was filtered 5 times with a 200nm microporous membrane to further homogenize the particle size and remove larger particles.
Embodiment 2
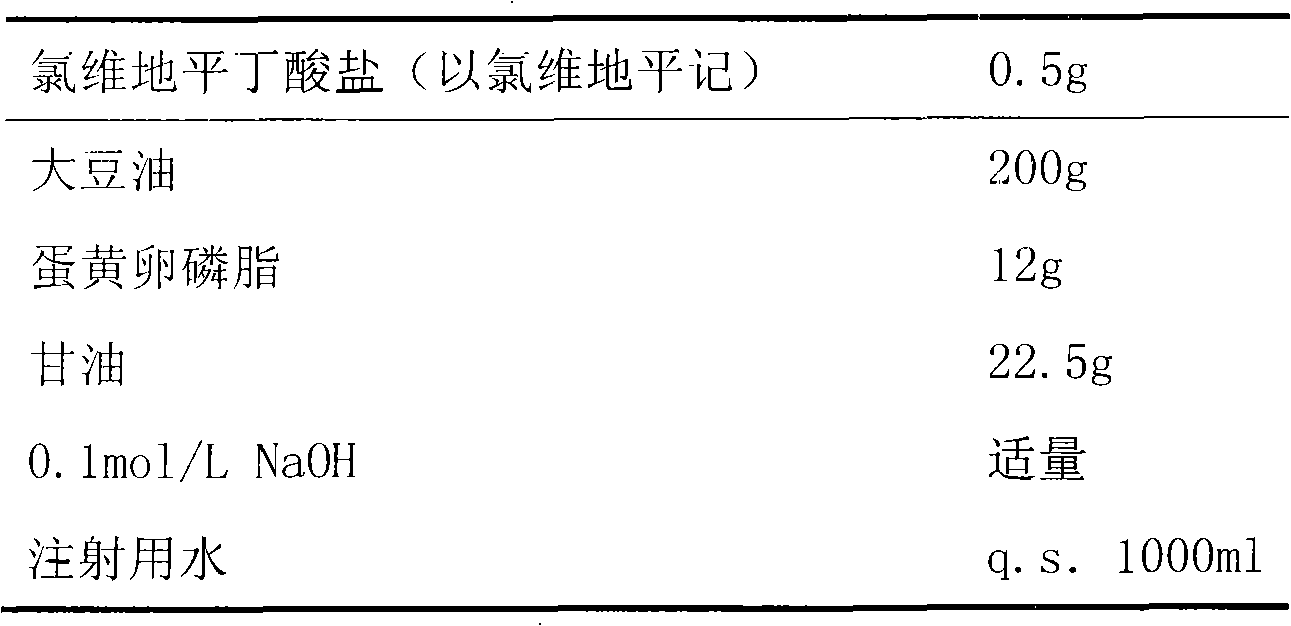
[0021]
[0022] Preparation:
[0023] First, measure 500ml of water for injection, dissolve the prescribed amount of glycerin in it, dissolve clevidipine butyrate and egg yolk lecithin in soybean oil, and heat appropriately to promote dissolution. Then add the water phase and the oil phase to the high-pressure homogenizer in turn for circulation and homogenization until the particle size distribution is 50-250nm, take it out, adjust the pH value to 6-8 with 0.1mol / L NaOH, and then add the remaining A certain amount of water for injection was filtered 5 times with a 200nm microporous membrane to further homogenize the particle size and remove larger particles.
Embodiment 3
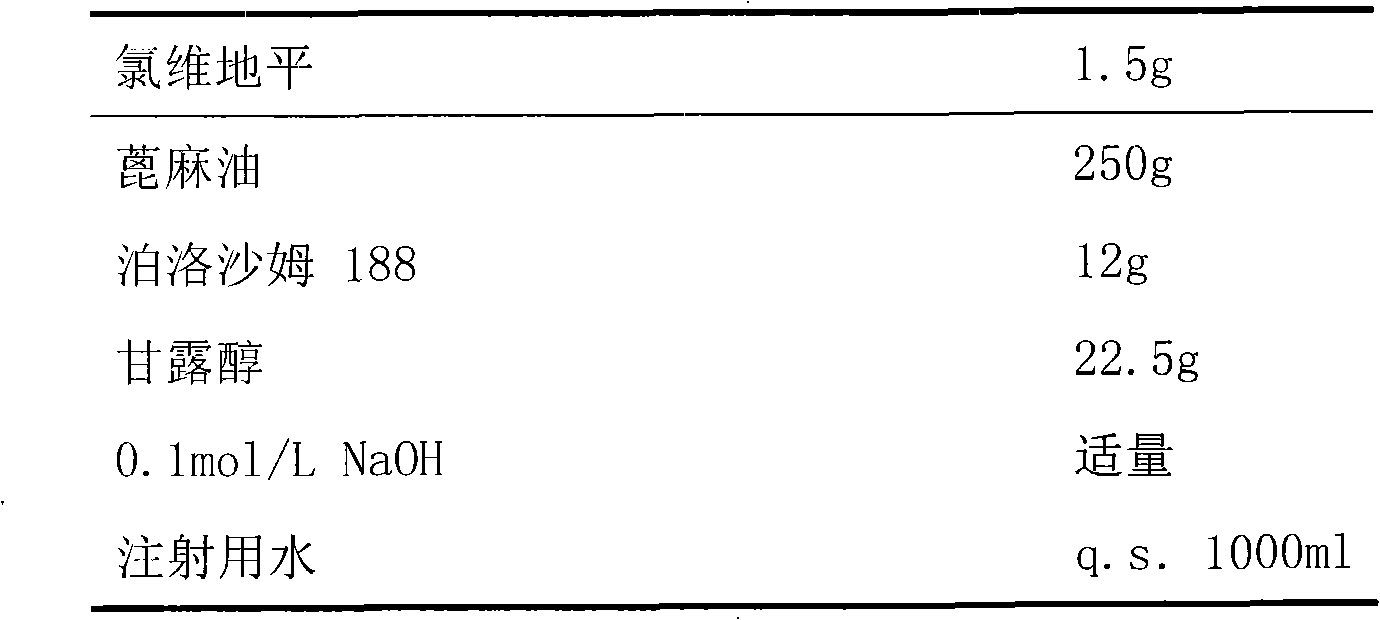
[0025]
[0026] Preparation:
[0027] First, measure 500ml of water for injection, dissolve the prescribed amount of mannitol in it, dissolve clevidipine and soybean lecithin in castor oil, and heat to promote dissolution. Then add the water phase and the oil phase to the high-pressure homogenizer in turn for circulation and homogenization until the particle size distribution is 50-250nm, take it out, adjust the pH value to 6-8 with 0.1mol / L NaOH, and then add the remaining A certain amount of water for injection was filtered 5 times with a 200nm microporous membrane to further homogenize the particle size and remove larger particles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com