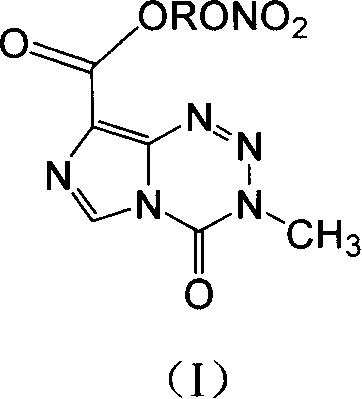
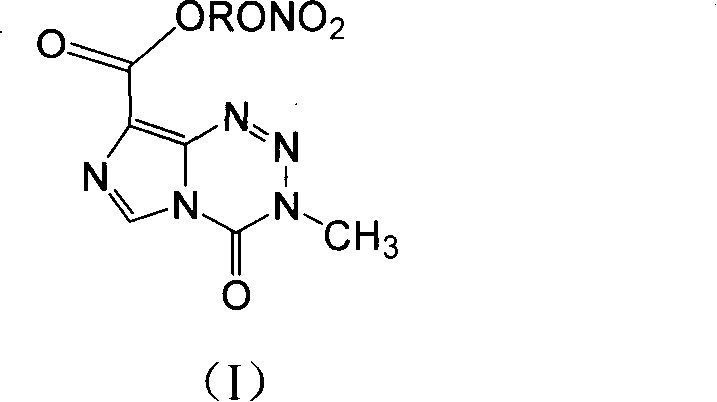
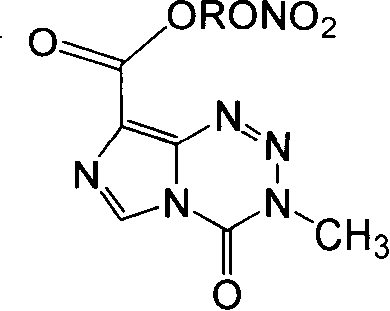
Anticancer compound, preparation method and use thereof, and composition containing the compound
A compound and composition technology, applied in the field of medicine, can solve the problems of large oral dose, inconvenient use, and instability of temozolomide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1I 1 Compound preparation
[0054] 1) 3,4-dihydro-3-methyl-4-oxoimidazol[5,1-d]bromoethyl 1,2,3,5-tetrazine-8-carboxylate (Ia 1 )
[0055] Dissolve 1.95 g (10 mmol) of 3,4-dihydro-3-methyl-4-oxoimidazol[5,1-d]-1,2,3,5-tetrazine-8-carboxylic acid in 15 ml of acetone , add 1,2-dibromoethane and triethylamine (3ml) under stirring, react at room temperature for 3 hours, filter off insoluble matter, concentrate the filtrate, column chromatography [ethyl acetate:petroleum ether=1:6(V: V)], 1.74g of white solid was obtained, the yield was 57.6%, mp: 102.7~104.5°C; the same method could be obtained by 3,4-dihydro-3-methyl-4-oxoimidazole [5,1-d] And 1,2,3,5-tetrazine-8-carboxylic acid and 1,4-dibromobutane, 1,5-dibromopentane can prepare other two intermediate compounds Ia 2 and Ia 3 .
[0056] 2) 3,4-dihydro-3-methyl-4-oxoimidazo[5,1-d]1,2,3,5-tetrazine-8-carboxylate nitroethyl ester (I1)
[0057] Ia 1 0.5g (1.66mmol) was dissolved in tetrahydrofuran solvent, 0....
Embodiment 2
[0063] Example 2I 2 Compound preparation
[0064] 1) 3,4-dihydro-3-methyl-4-oxoimidazol[5,1-d]bromobutyl 1,2,3,5-tetrazine-8-carboxylate (Ia 2 )
[0065] Refer to Ia 1 The preparation method, by 3,4-dihydro-3-methyl-4-oxoimidazol[5,1-d] and 1,2,3,5-tetrazine-8-carboxylic acid and 1,4-two Bromobutane reaction preparation, yield 43.6%,
[0066] mp: 73.5~75.6℃
[0067] 2) 3,4-dihydro-3-methyl-4-oxoimidazo [5,1-d] and 1,2,3,5-tetrazine-8-carboxylate nitrobutyl ester (I 2 ) refer to I 1 The preparation method is prepared by reacting Ia2 with silver nitrate, the yield is 55.8%, mp: 82.8~84.5°C
[0068] ESI-Ms: 334.8[M+Na] +
[0069] IR (KBr, v (cm -1 )): 3100(O-H); 2959(C-H); 1734(C=O); 1606(C=N); 1456(N=O); 1252, 1052(C-O);
[0070] 1 H-NMR (300MHz, CDCl 3 ), δ (ppm): 1.97 (m, 4H, CH 2 ), 4.07 (s, 3H, CH 3 ), 4.52(t, 2H, OCH 2 ), 4.56(t, 2H, CH 2 O), 8.47 (s, H, CH)
[0071] Elemental Analysis C 10 h 12 o 6 N 6 Found: (%)N 26.98 C 38.60 H 3.91
[0072] ...
Embodiment 3
[0073] Example 3I 3 Compound preparation
[0074] 1) 3,4-dihydro-3-methyl-4-oxoimidazol[5,1-d]bromopentyl 1,2,3,5-tetrazine-8-carboxylate (Ia 3 )
[0075] Refer to Ia 1 The preparation method, by 3,4-dihydro-3-methyl-4-oxoimidazol[5,1-d] and 1,2,3,5-tetrazine-8-carboxylic acid and 1,5-bromo Prepared by pentane reaction, yield 56.7%,
[0076] mp: 80.5~82.7℃
[0077] 2) 3,4-dihydro-3-methyl-4-oxoimidazo[5,1-d] and 1,2,3,5-tetrazine-8-carboxylate nitropentyl ester (I 3 )
[0078] Refer to I 1 The preparation method of Ia 3 Prepared by reacting with silver nitrate, the yield is 47.6%, mp: 74.6~76.8℃
[0079] ESI-Ms: 348.8[M+Na] +
[0080] IR (KBr, v (cm -1 )): 3090(O-H); 2953(C-H); 1752(C=O); 1636(C=N); 1459(N=O); 1257, 1054(C-O); .
[0081] 1 H-NMR (300MHz, CDCl 3 ), δ (ppm): 1.67 (m, 2H, CH 2 ), 1.80 (m, 2H, CH 2 ), 1.92 (m, 2H, CH 2 ), 4.06 (s, 3H, CH 3 ), 4.46(t, 2H, OCH 2 ), 4.52(t, 2H, CH 2 O), 8.47 (s, H, CH)
[0082] Elemental Analysis C 11 h 14 o ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com