Novel compound and pharmaceutically acceptable salt thereof
A compound and pharmaceutical technology, applied in organic chemistry, drug combination, anti-tumor drugs, etc., can solve the problems of brain cancer treatment effect to be improved, limited anti-cancer effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
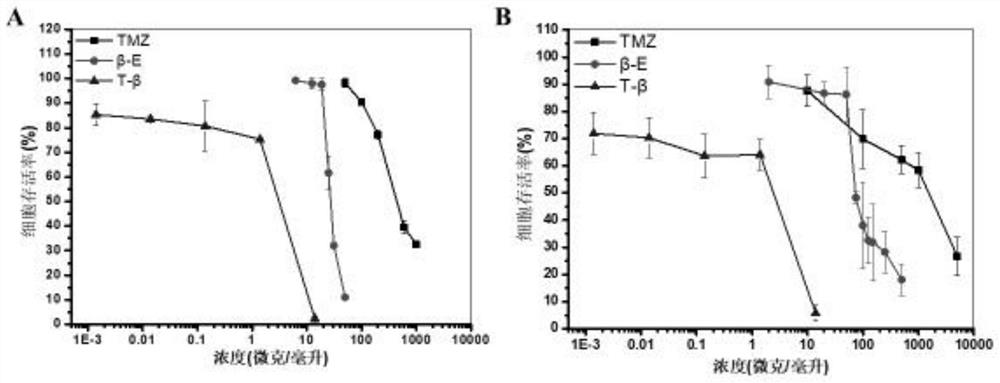
Image
Examples
preparation example Construction
[0088] The preparation of compounds of the present application may involve the protection and deprotection of various chemical groups. Those skilled in the art can readily determine the need for protection and deprotection and select appropriate protecting groups. Protecting group chemistry can be found in, eg, T.W. Greene and P.G.M. Wuts, Protective Groups in Organic Synthesis, 3rd Ed., Wiley & Sons, Inc., New York (1999), the entire contents of which are incorporated herein by reference.
[0089] Reactions may be monitored or purified according to any suitable method known in the art. For example, spectroscopic methods such as nuclear magnetic resonance spectroscopy (e.g. 1 H or 13 C) Infrared spectroscopy, spectrophotometry (e.g. UV-visible), mass spectrometry, or chromatographic methods such as high performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LCMS) or thin layer chromatography (TLC) for the product formation is monitored. Those sk...
Embodiment 1
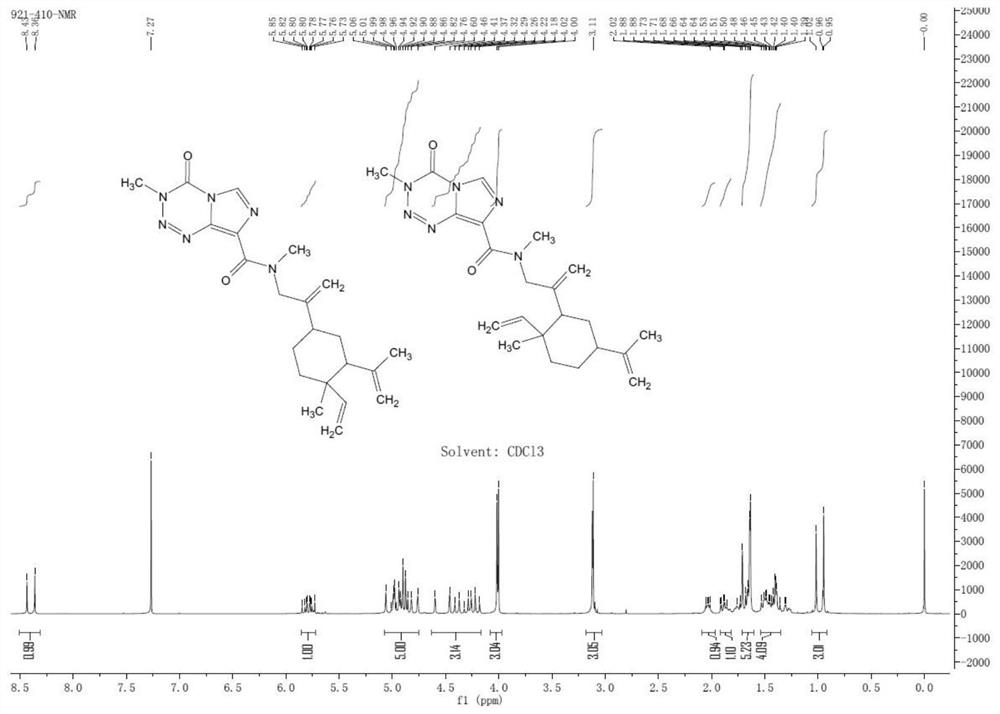
[0114] The synthesis of embodiment 1 compound I
[0115] Dissolve 1-methyl-2,4-bis(prop-1-en-2-yl)-1-vinylcyclohexane (2g) in 10mL of dichloromethane, add 2mL of acetic acid at 0°C, stir for ten minute. Then, slowly add 15 mL of sodium hypochlorite solution to the system at 0°C. The reaction solution was stirred under ice bath for 5 hours. The reaction solution was extracted three times with dichloromethane, the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was removed to obtain 4-(3-chloroprop-1-en-2-yl)-1-methyl-2 -(prop-1-en-2-yl)-1-vinylcyclohexane and 2-(3-chloroprop-1-en-2-yl)-1-methyl-4-(prop-1- En-2-yl)-1-vinylcyclohexane mixture 1g, light yellow liquid, was directly used in the next step without purification, yield 42.1%.
[0116]
[0117] 1 g of the mixture obtained in the previous step was dissolved in 20 mL of acetonitrile, and 570 mg of methylamine hydrochloride and 670 mg of sodium hydroxide were added ...
Embodiment 2
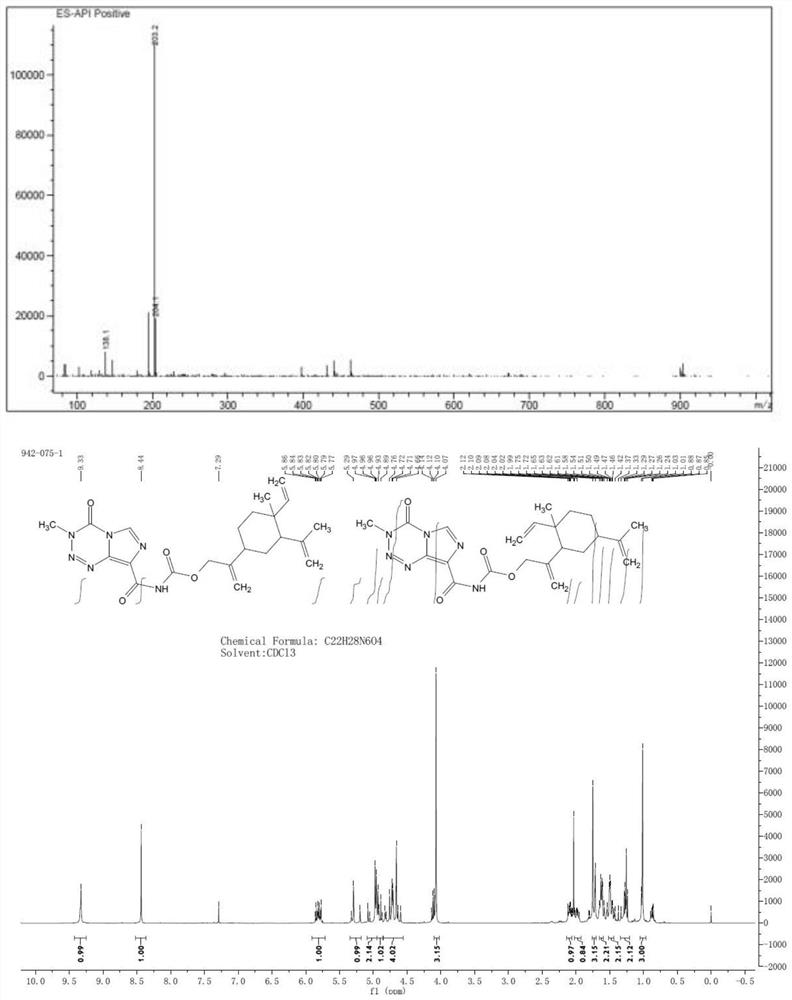
[0122] The preparation of embodiment 2 compound II
[0123] Dissolve 1-methyl-2,4-di(prop-1-en-2-yl)-1-vinylcyclohexane (2 g) in 50 mL of acetonitrile, add 2.76 g of potassium carbonate, and stir for ten minutes. Then, slowly add 2 g of m-chloroperoxybenzoic acid in batches to the system under ice-cooling. The reaction solution was stirred under ice bath for 2 hours, then raised to room temperature and stirred overnight. After filtration, the filter cake was washed with dichloromethane, and the filtrate was concentrated to obtain crude product 1, which was purified by silica gel column (petroleum ether: ethyl acetate = 5:1) to obtain compound 2, 1.1 g of light yellow liquid, with a yield of 50%.
[0124] Dissolve 1.1 g of the mixture obtained in the previous step in 20 mL of tetrahydrofuran, slowly add LDA (10 mmol) at 0°C, stir for 1 hour, quench the reaction with saturated ammonium chloride, extract the organic phase with ethyl acetate, and dry over anhydrous sodium sulfate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com