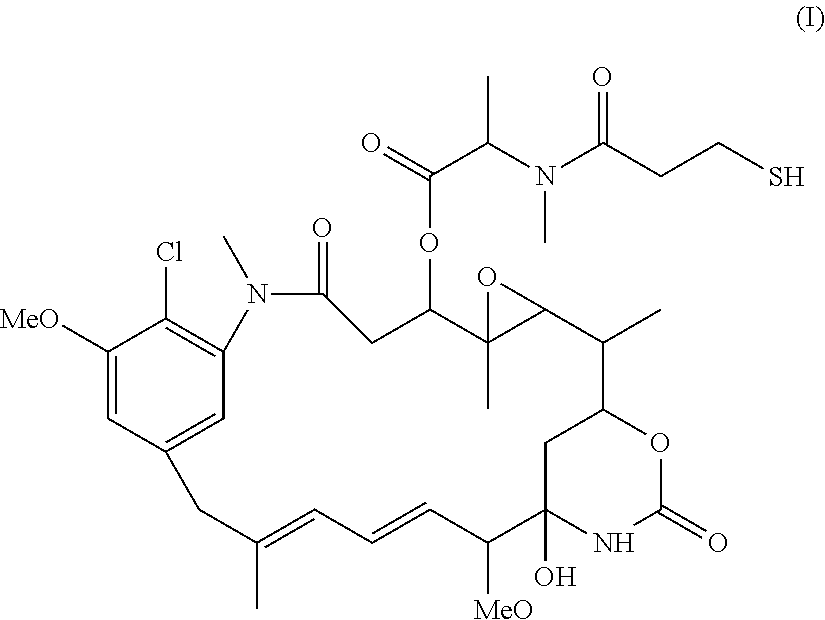
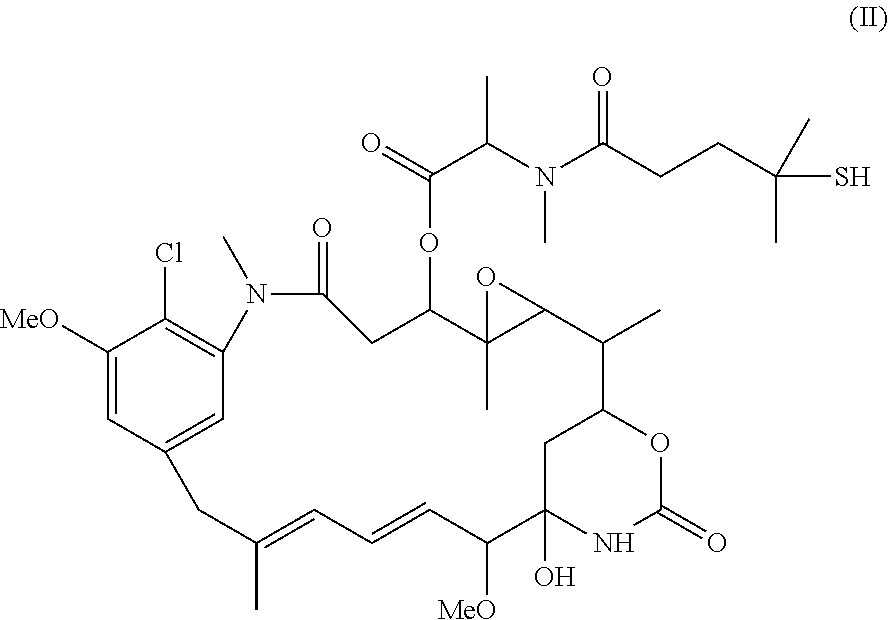
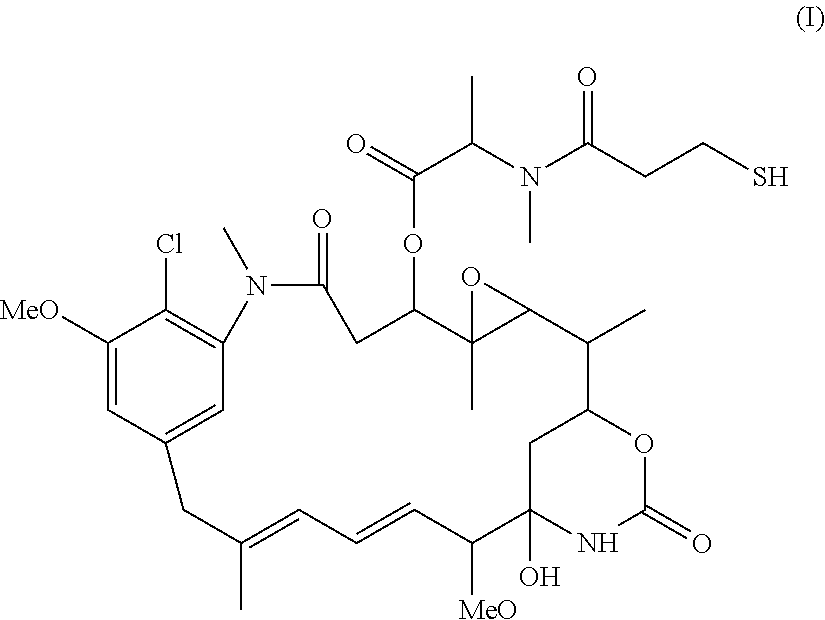
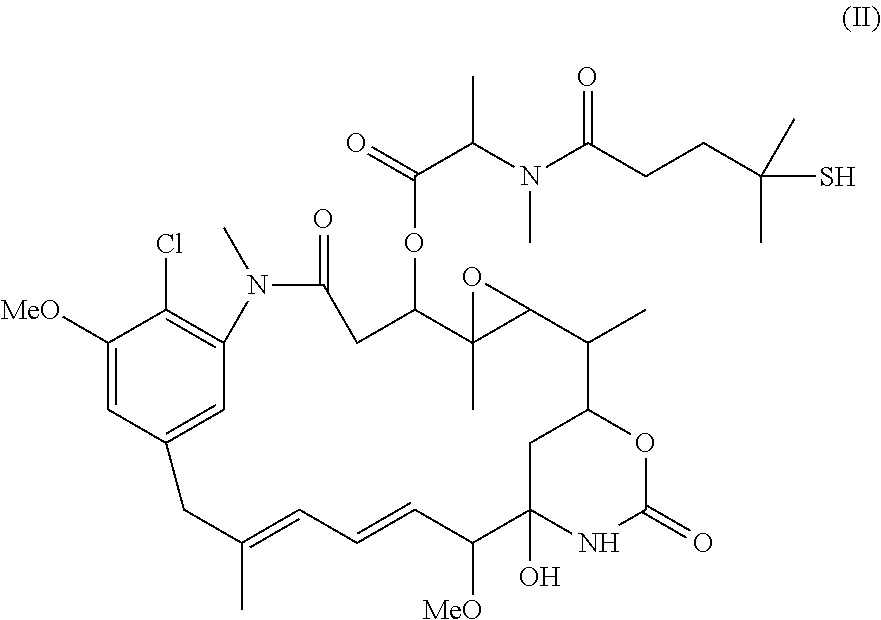
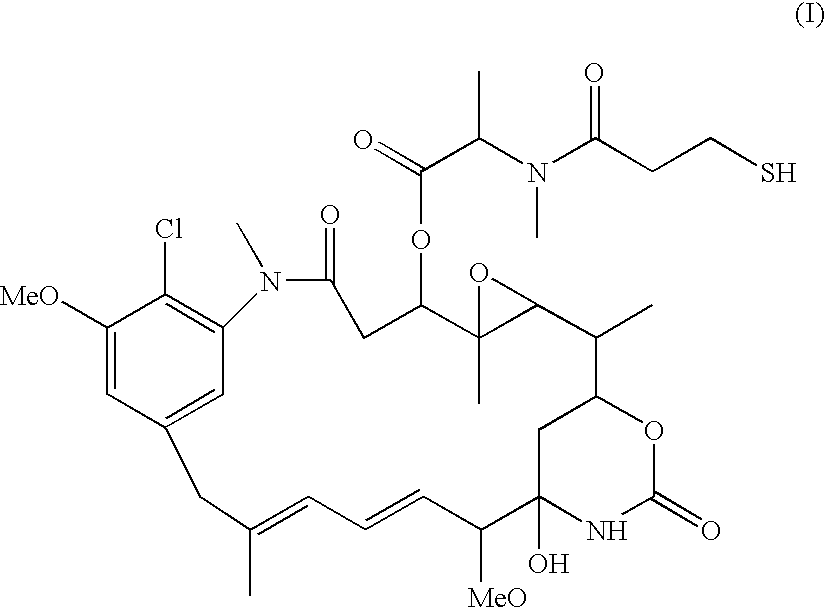
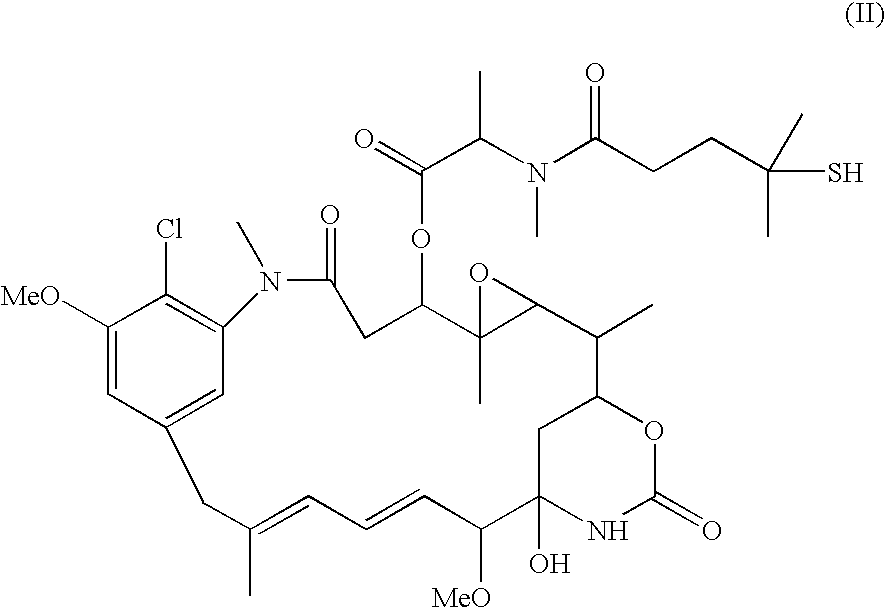
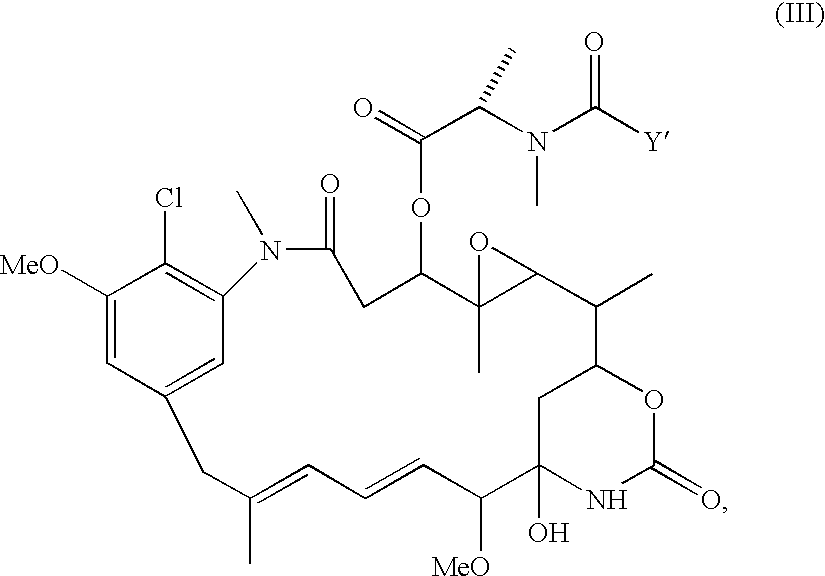
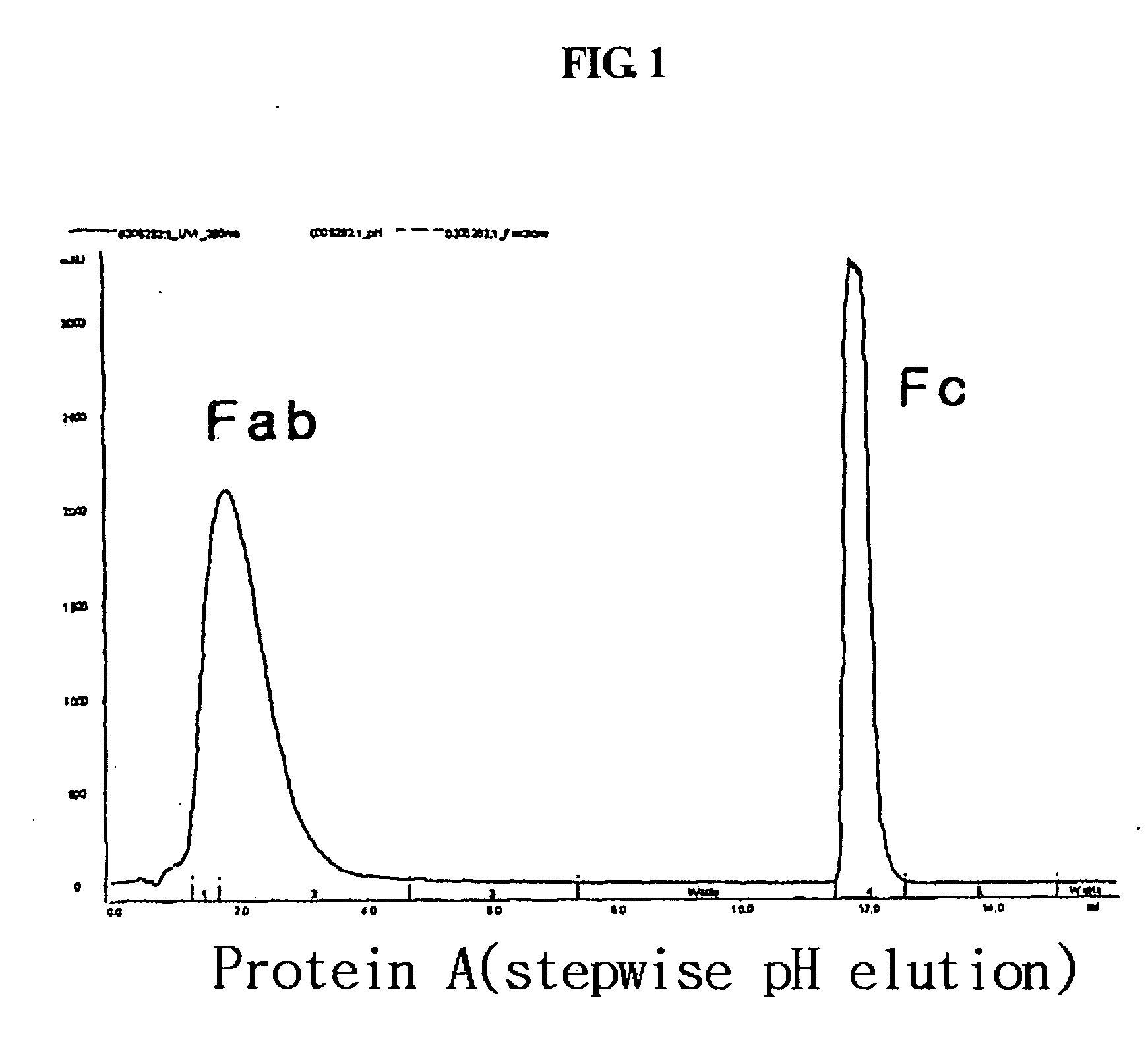
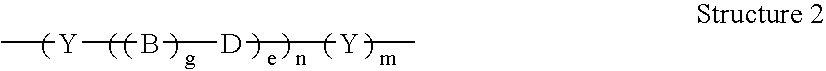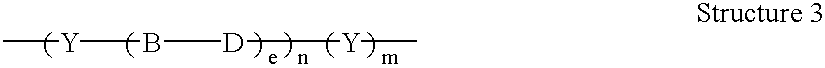Patents
Literature
1943 results about "Covalent modifier" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
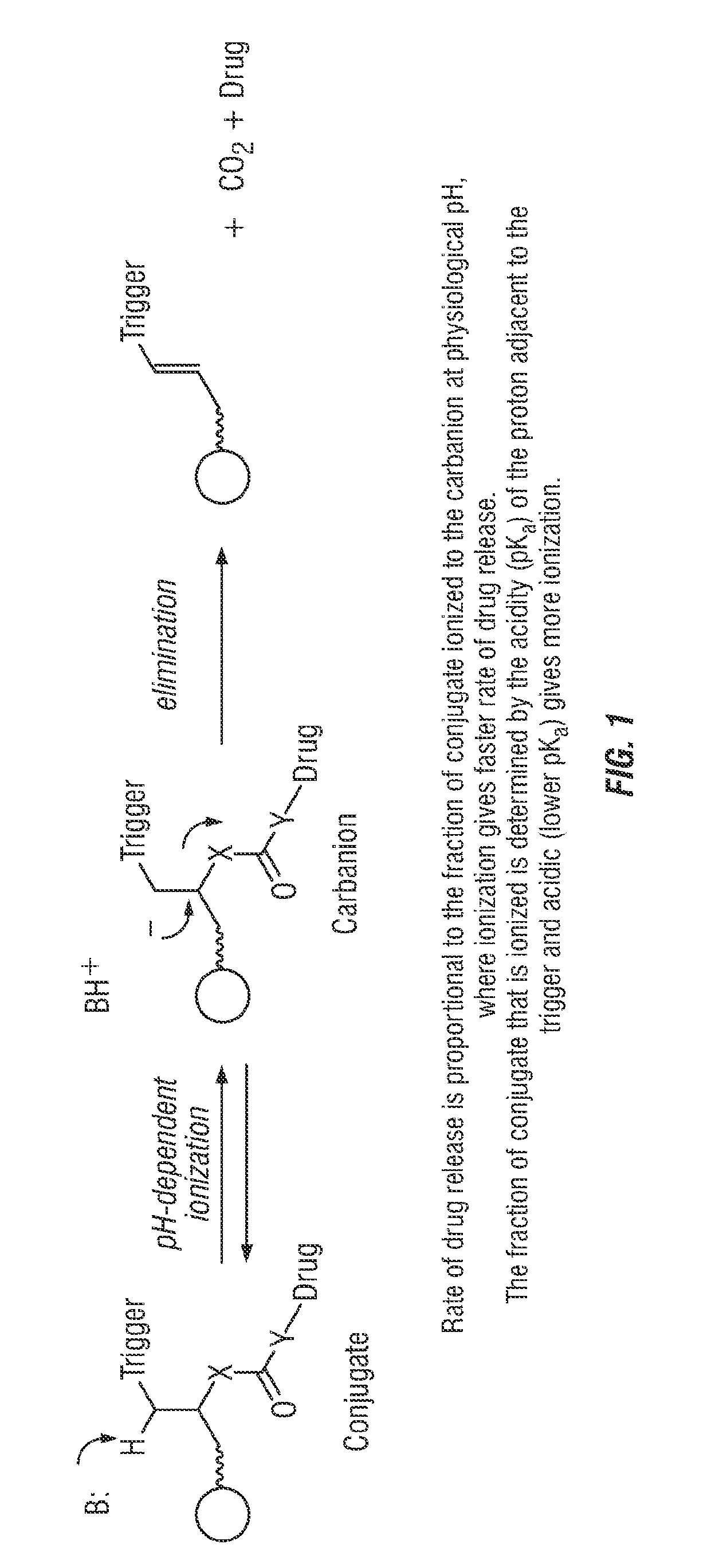
Covalent-modifier drugs bind by forming a chemical bond with their target. This covalent binding can improve the selectivity of the drug for a target with complementary reactivity and result in increased binding affinities due to the strength of the covalent bond formed.
Means to achieve sustained release of synergistic drugs by conjugation
A codrug composition of at least two drug compounds covalently linked to one another via a labile bond to form a single codrug composition, or ionically linked to one another to form a single workings composition, and methods of use of the codrug for the treatment of various medical conditions. The codrug may be administered by itself or in the form of a bioerodible or nonbioerodible substance.
Owner:UNIVERSITY OF KENTUCKY +1
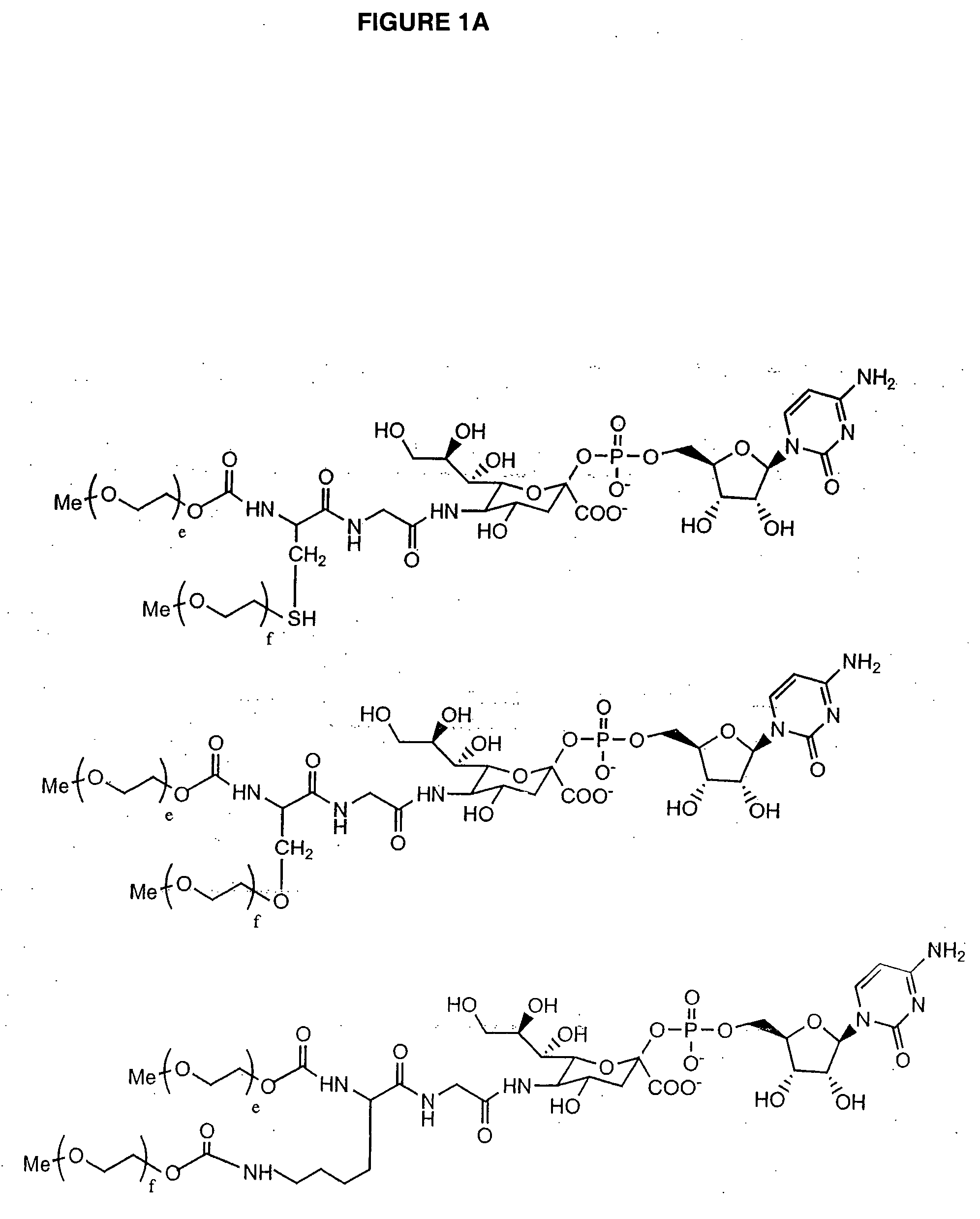
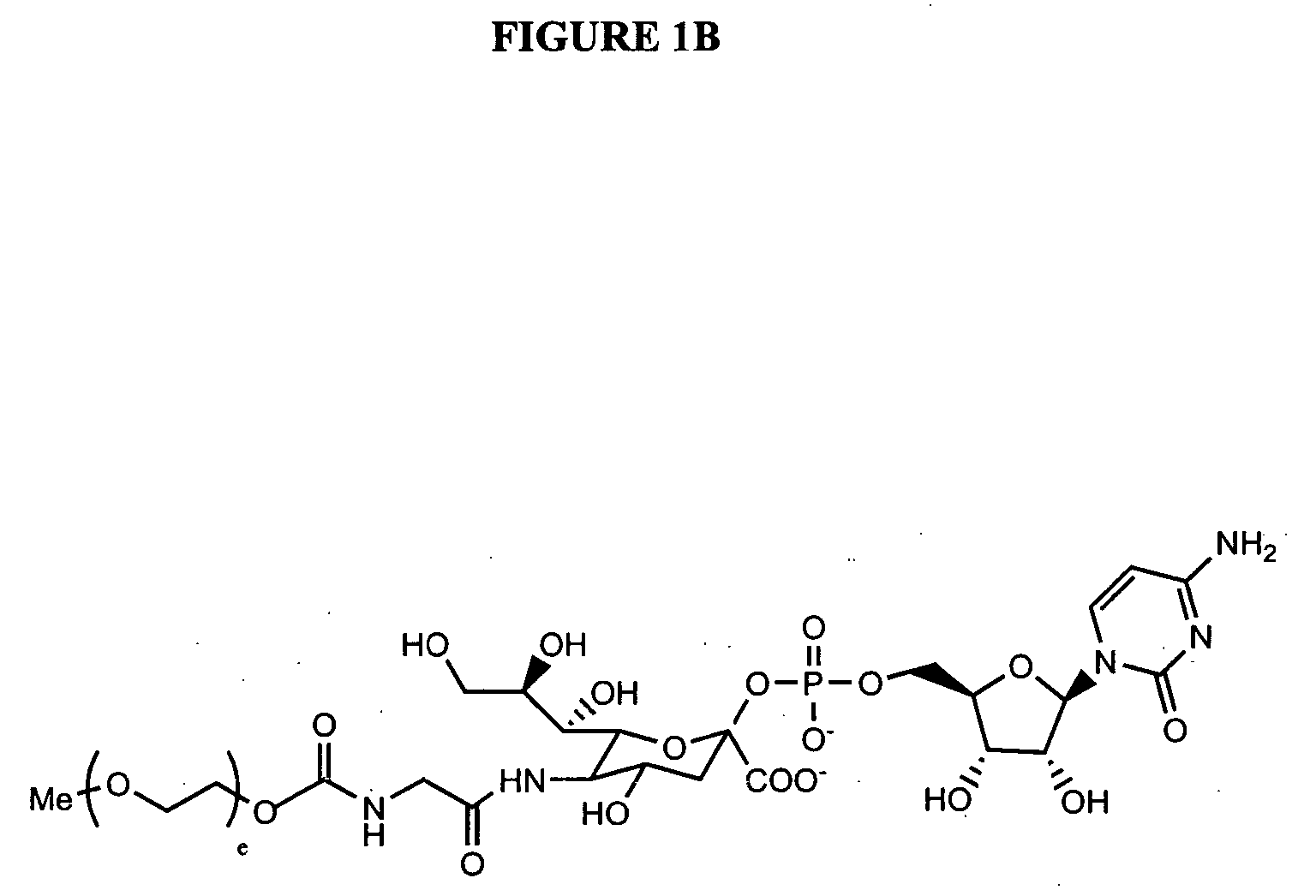
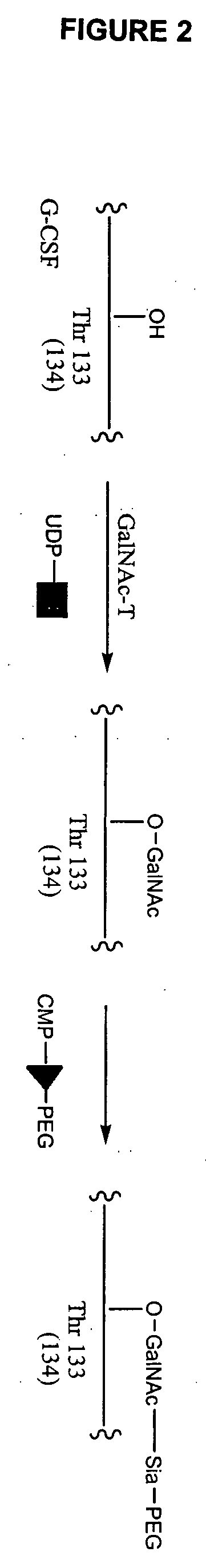
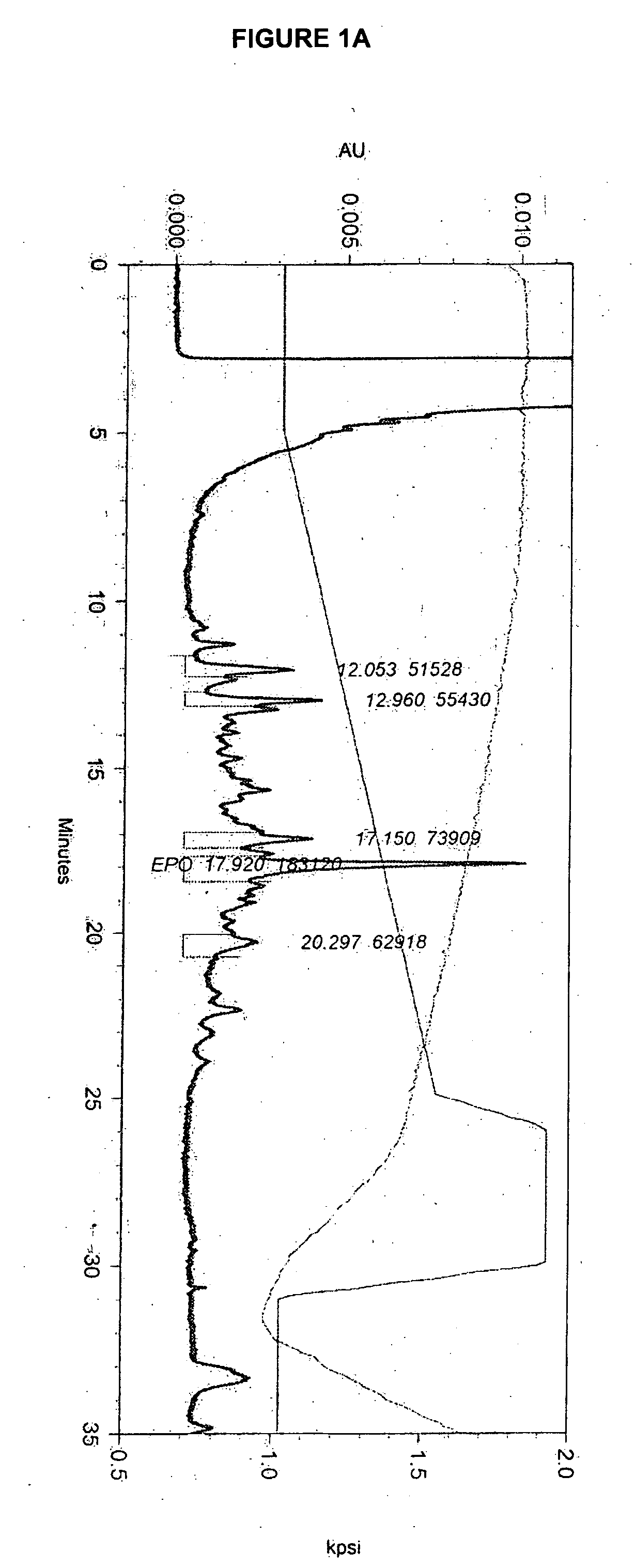
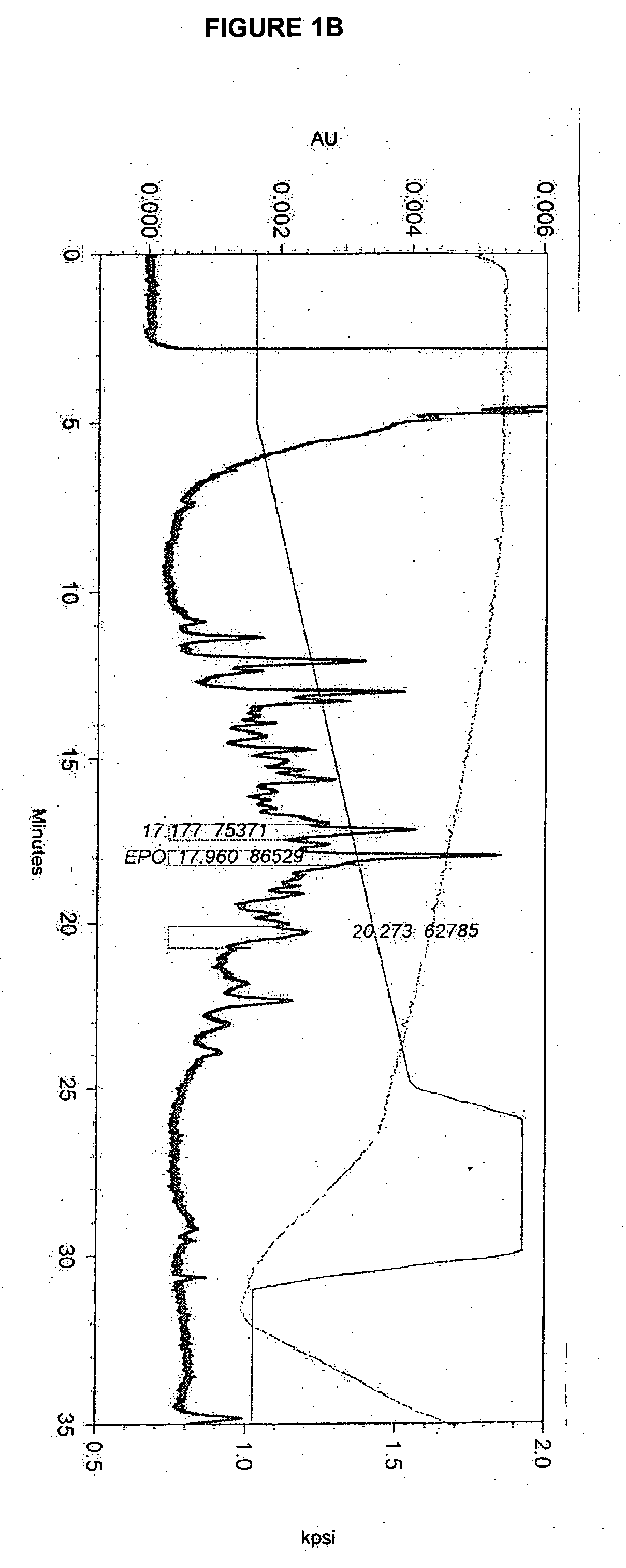
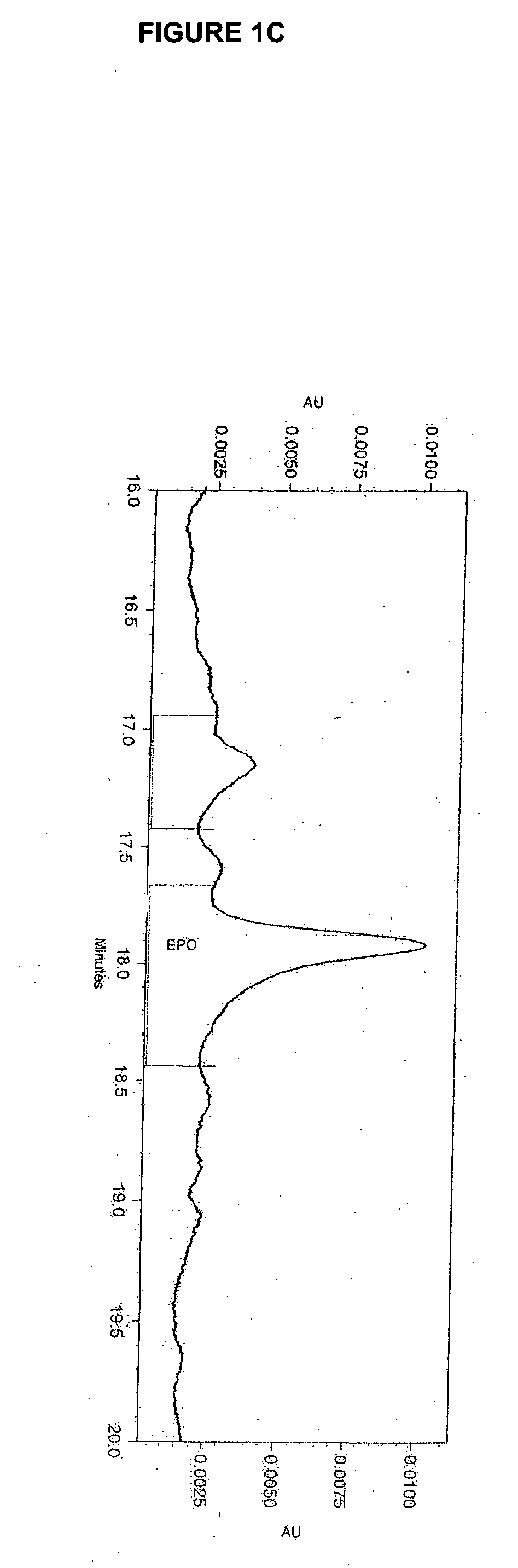
Glycopegylated erythropoietin
InactiveUS20050143292A1Improved pharmacokinetic propertiesCost-effectiveSugar derivativesPeptide/protein ingredientsDiseaseSugar moiety
The present invention provides conjugates between erythropoietin and PEG moieties. The conjugates are linked via an intact glycosyl linking group interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from glycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto a glycosyl residue on the peptide. Also provided are methods for preparing the conjugates, methods for treating various disease conditions with the conjugates, and pharmaceutical formulations including the conjugates.
Owner:NOVO NORDISK AS
Pseudo-antibody constructs
InactiveUS20030211078A1Reduce productionInhibit synthesisOrganic active ingredientsBiocideHalf-lifeIn vivo
This invention relates to novel pharmaceutically useful compositions that bind to a biological molecule, having improved circulatory half-life, increased avidity, increased affinity, or multifunctionality, and methods of use thereof. The present invention provides a pseudo-antibody comprising an organic moiety covalenty coupled to at least two target-binding moieties, wherein the target-binding moieties are selected from the group consisting of a protein, a peptide, a peptidomimetic, and a non-peptide molecule that binds to a specific targeted biological molecule. The pseudo-antibody of the present invention may affect a specific ligand in vitro, in situ and / or in vivo. The pseudo-antibodies of the present invention can be used to measure or effect in an cell, tissue, organ or animal (including humans), to diagnose, monitor, modulate, treat, alleviate, help prevent the incidence of, or reduce the symptoms of, at least one condition.
Owner:CENTOCOR
Biomolecular coupling methods using 1,3-dipolar cycloaddition chemistry
InactiveUS20050032081A1Bioreactor/fermenter combinationsBiological substance pretreatmentsBio moleculesCycloaddition
This invention provides methods for covalently affixing a biomolecule to either a second molecule or a solid surface using 1,3-dipolar cycloaddition chemistry. This invention also provides related methods and compositions.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Covalently Immobilized Enzyme and Method To Make The Same
ActiveUS20100078381A1Avoid disadvantagesRetain activityMembranesOther chemical processesPolymerMacromolecule
A composition of enzyme, polymer, and crosslinker forms a network of covalently bound macromolecules. The covalently immobilized enzyme preparation has enzymatic activity, and retains stable activity when dried and stored at ambient conditions. Methods for preparing an immobilized enzyme and methods for using the enzyme are disclosed.
Owner:FRESENIUS MEDICAL CARE HLDG INC
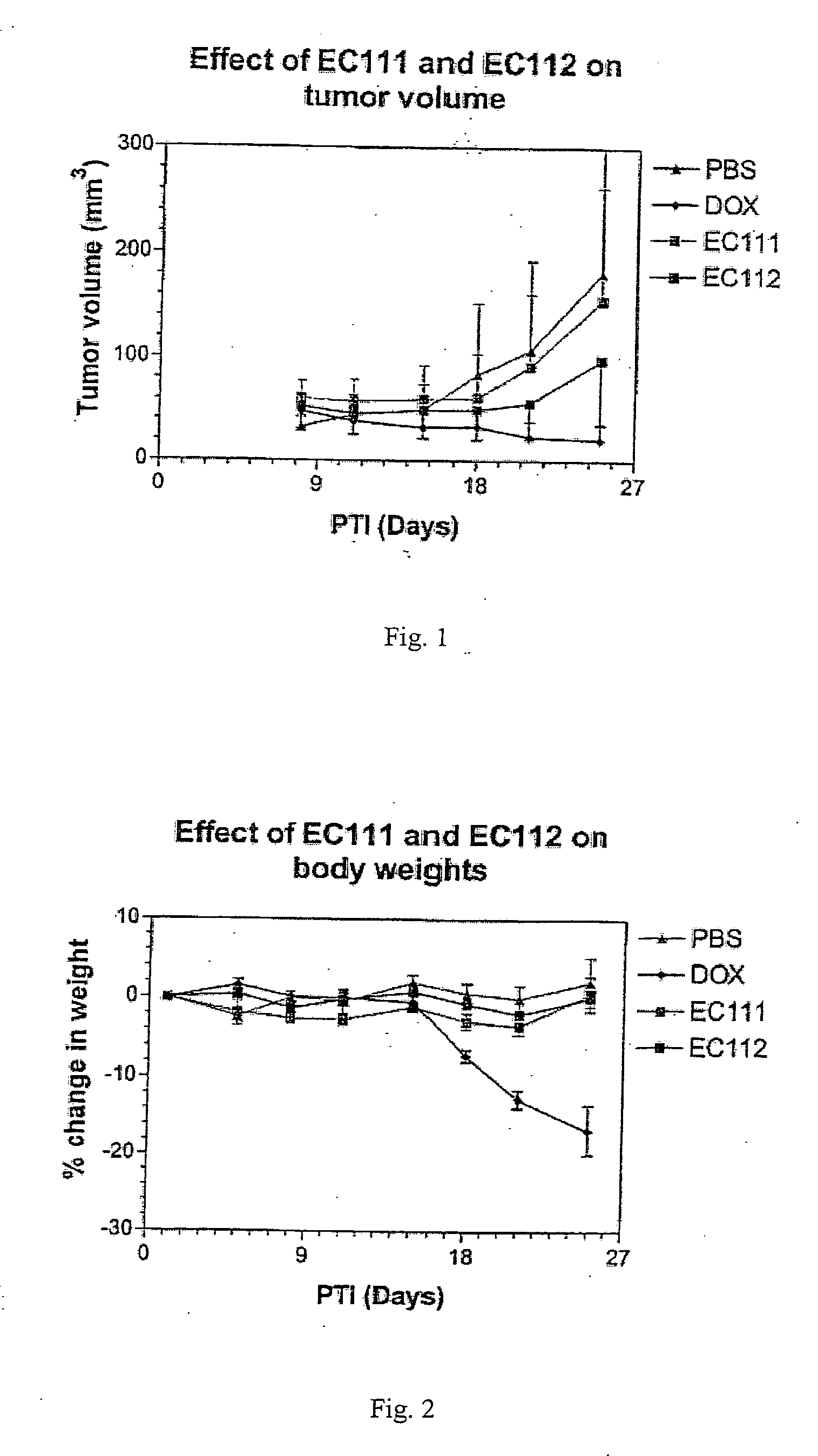
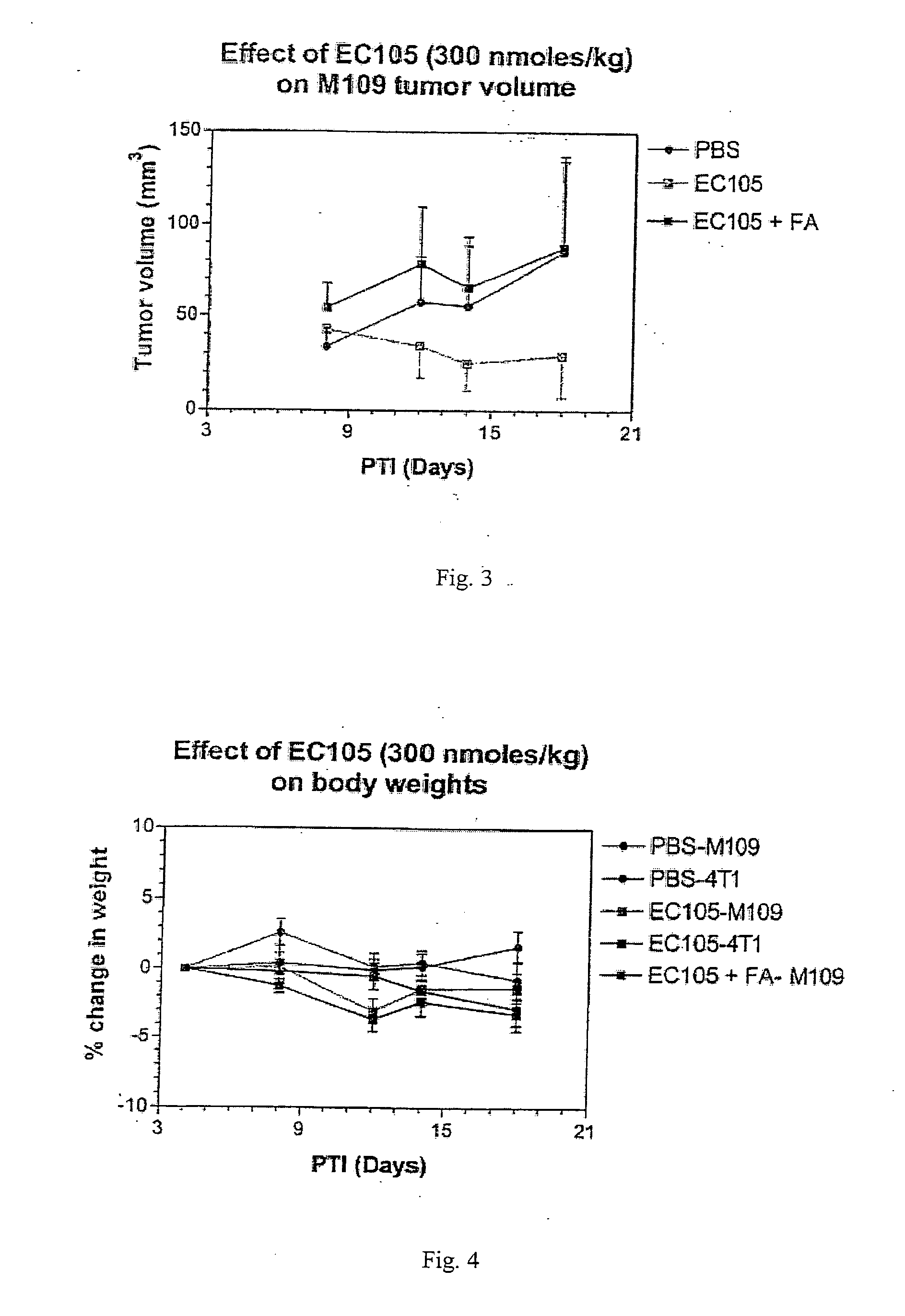
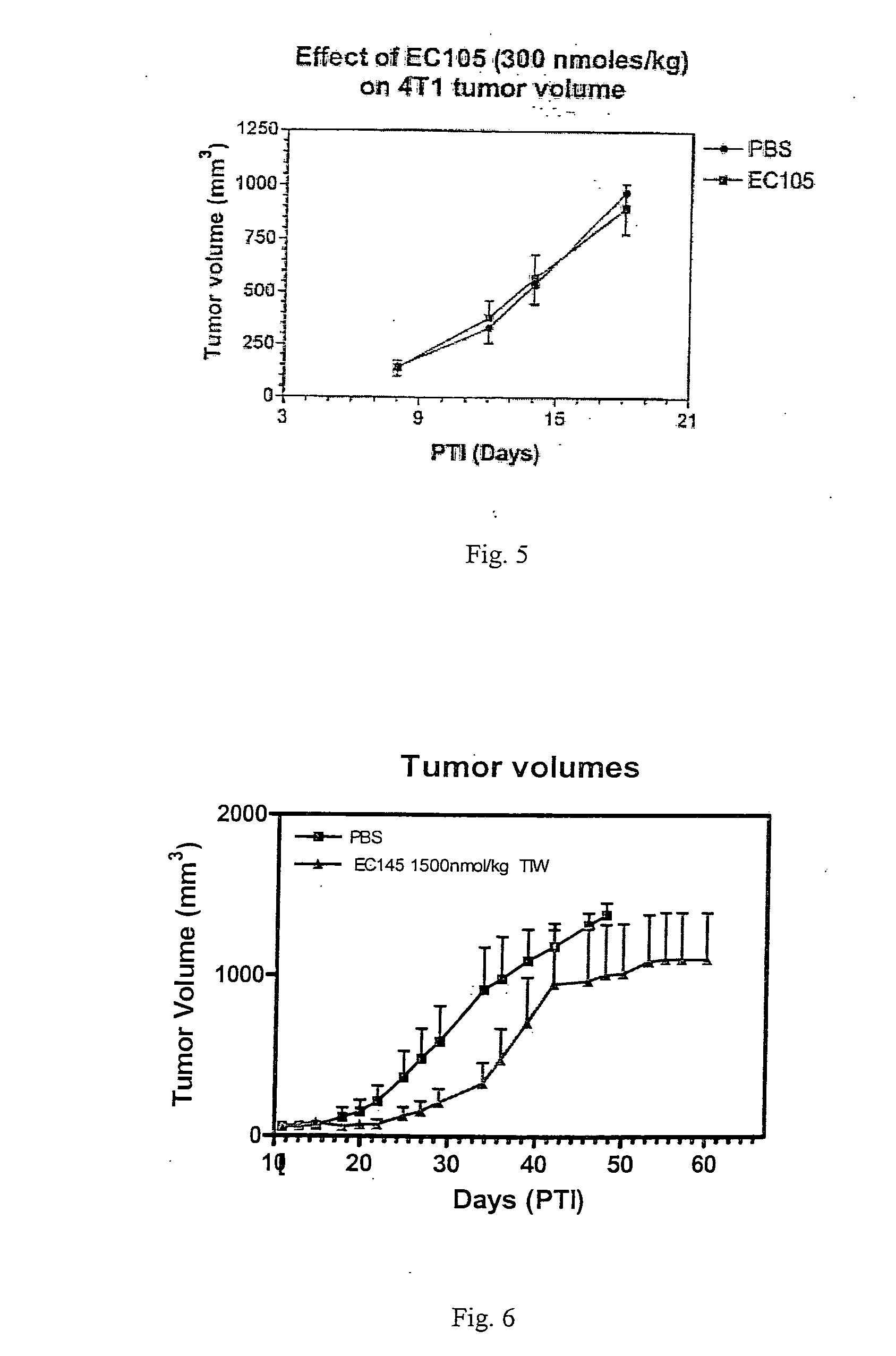
Vitamin receptor binding drug delivery conjugates
The invention describes a vitamin receptor binding drug delivery conjugate, and preparations therefor. The drug delivery conjugate consists of a vitamin receptor binding moiety, a bivalent linker (L), and a drug. The vitamin receptor binding moiety includes vitamins, and vitamin receptor binding analogs and derivatives thereof, and the drug includes analogs and derivatives thereof. The vitamin receptor binding moiety is covalently linked to the bivalent linker, and the drug, or the analog or the derivative thereof, is covalently linked to the bivalent linker, wherein the bivalent linker (L) includes components such as spacer linkers, releasable linkers, and heteroatom linkers, and combinations thereof. Methods and pharmaceutical compositions for eliminating pathogenic cell populations using the drug delivery conjugate are also described.
Owner:ENDOCTYE INC
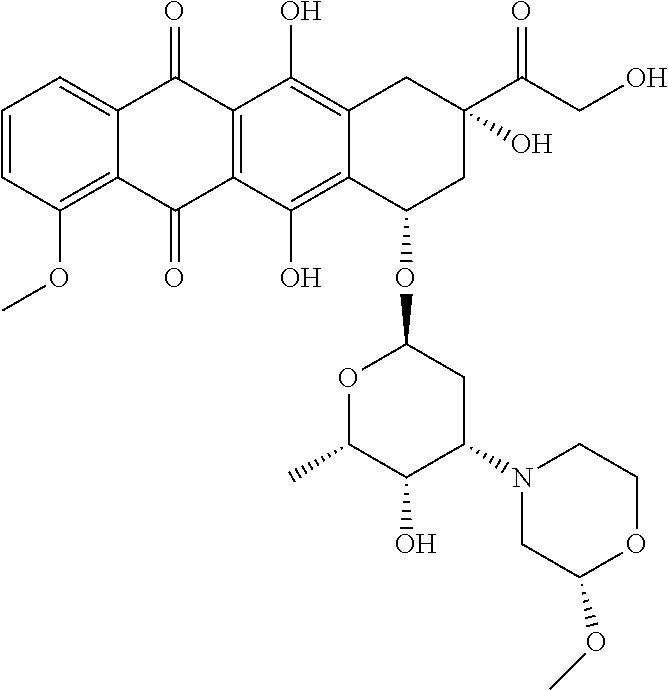
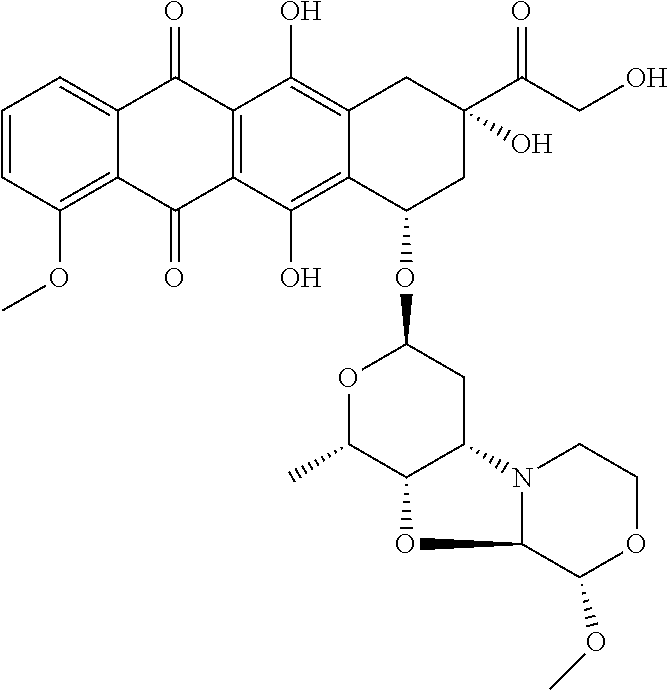
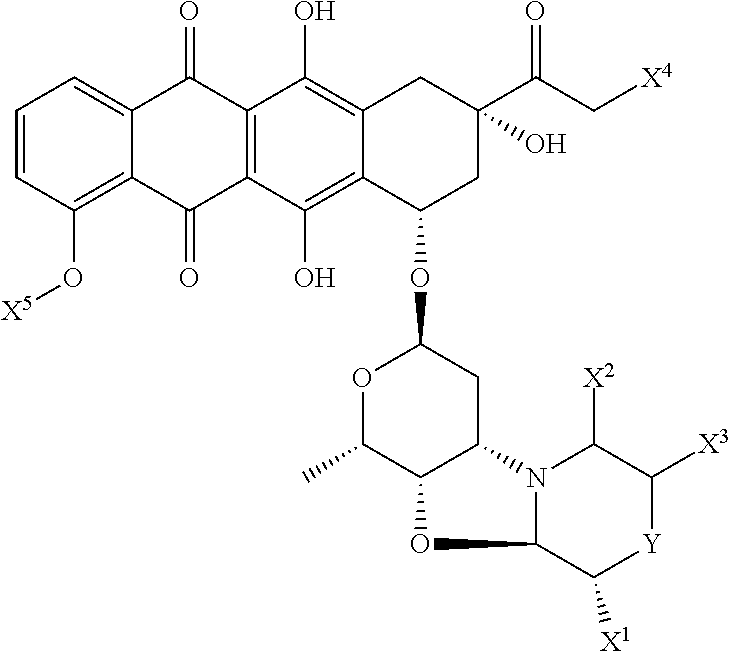
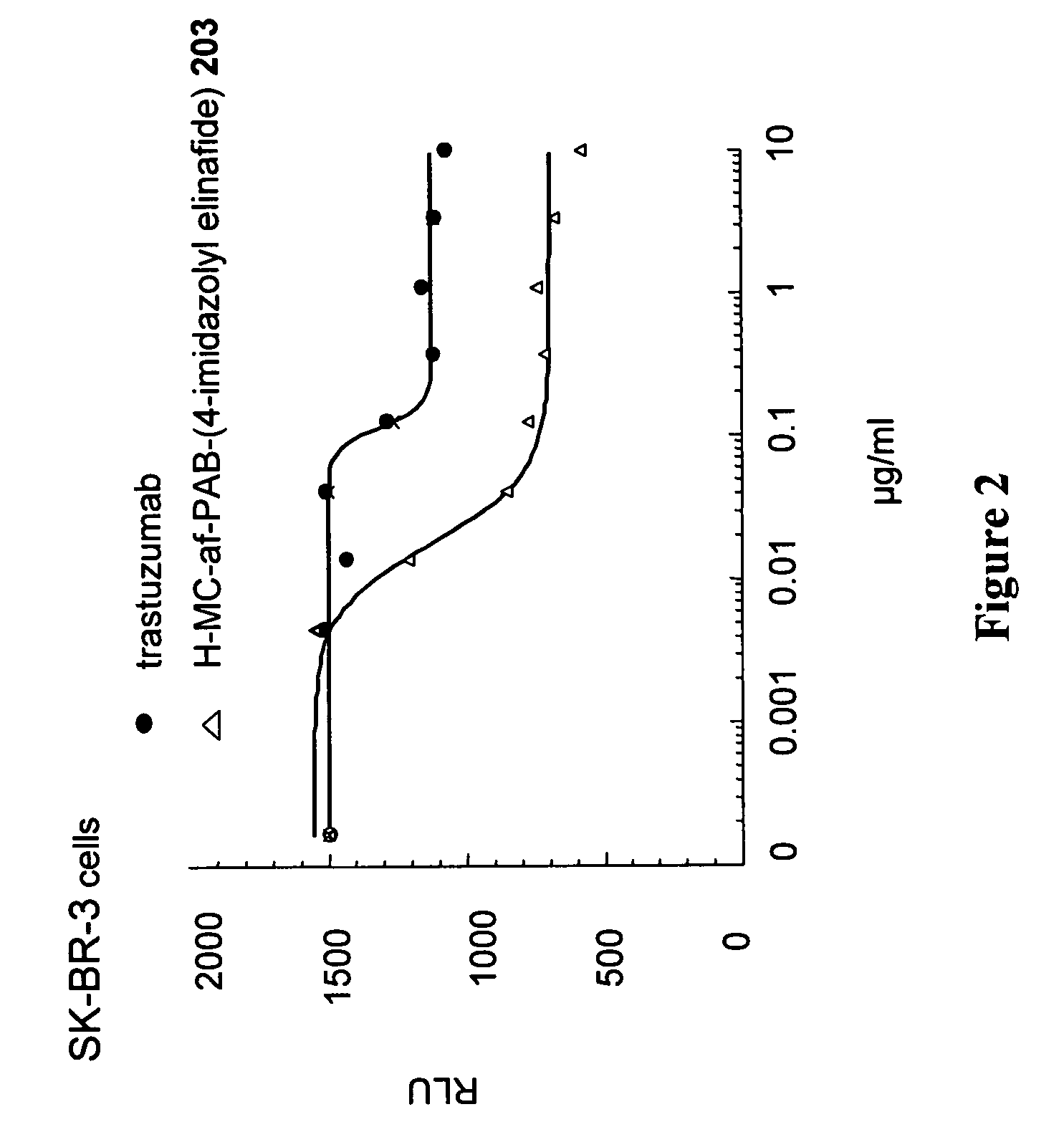
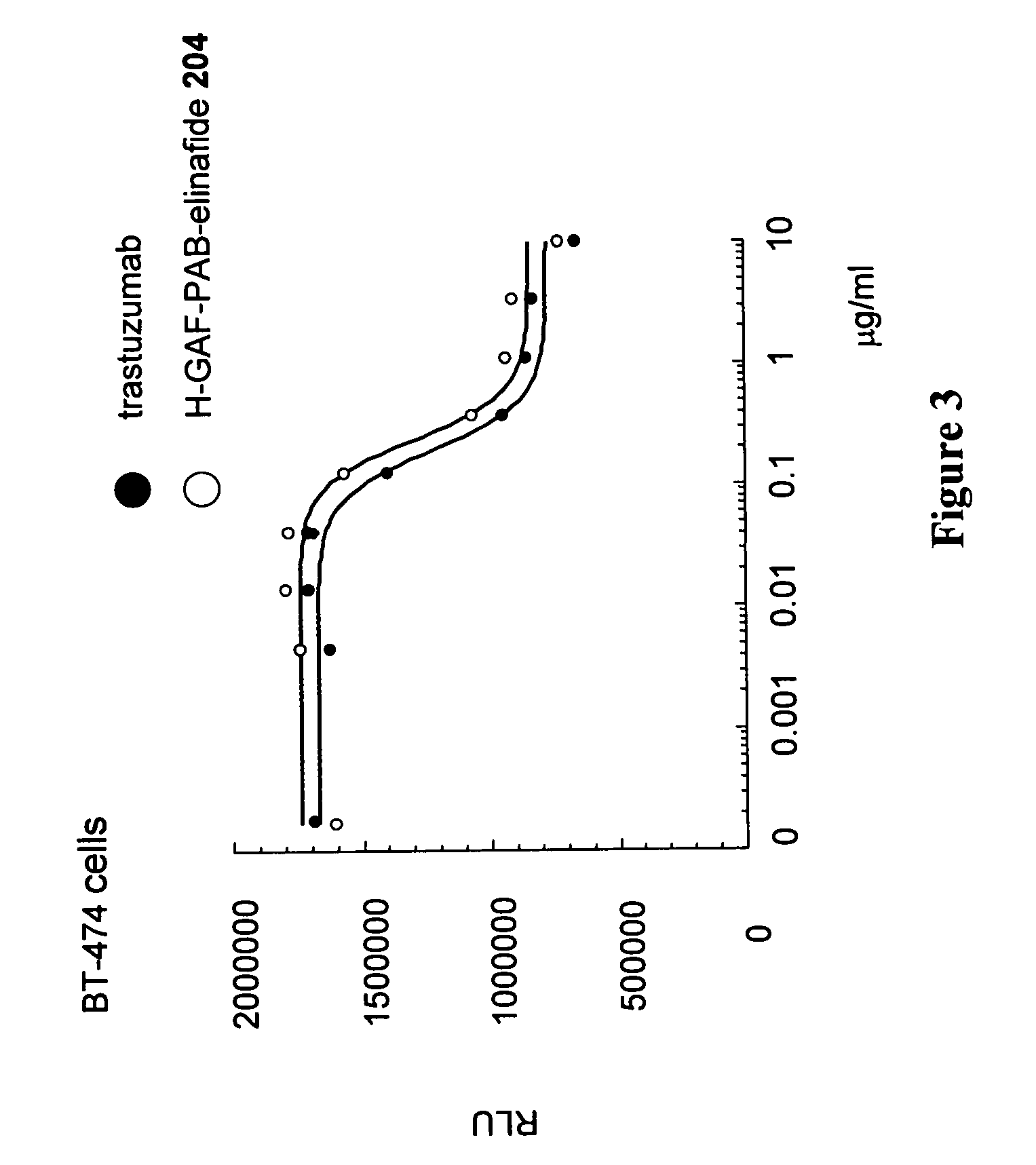
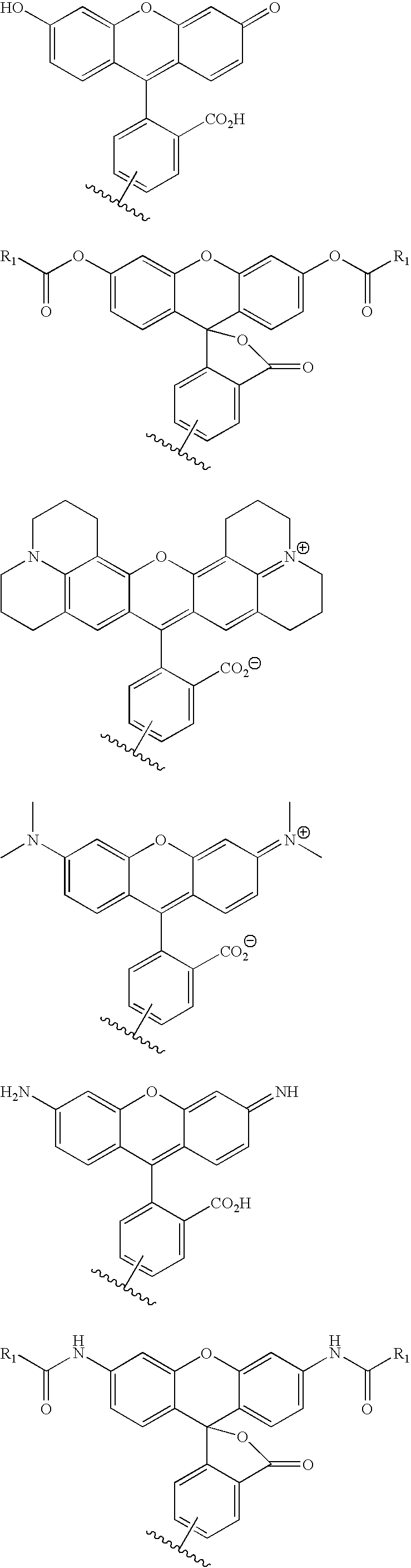
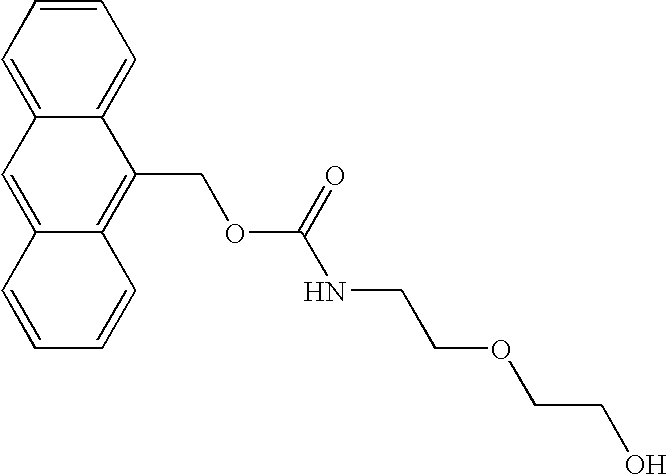
Nemorubicin metabolite and analog reagents, antibody-drug conjugates and methods
ActiveUS20110076287A1Kill and inhibit proliferationOrganic active ingredientsSugar derivativesDiseaseAntiendomysial antibodies
The present invention relates to antibody-drug conjugate compounds of Formula I:Ab-(L-D)p Iwhere one or more nemorubicin metabolite or analog drug moieties (D) are covalently attached by a linker (L) to an antibody (Ab) which binds to one or more tumor-associated antigens or cell-surface receptors. These compounds may be useful in methods of diagnosis or treatment of cancer, and other diseases and disorders.
Owner:GENENTECH INC
Process for preparing stable drug conjugates
InactiveUS20060182750A1Hybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsCell bindingFiltration
The invention provides a process for preparing a conjugate comprising a cell binding agent chemically coupled to a drug. The process comprises covalently attaching a linker to a cell binding agent, which then is conjugated to a drug, as well as purification and holding steps, and optionally a tangential flow filtration (TFF) step.
Owner:IMMUNOGEN INC
Nanoparticles for drug-delivery
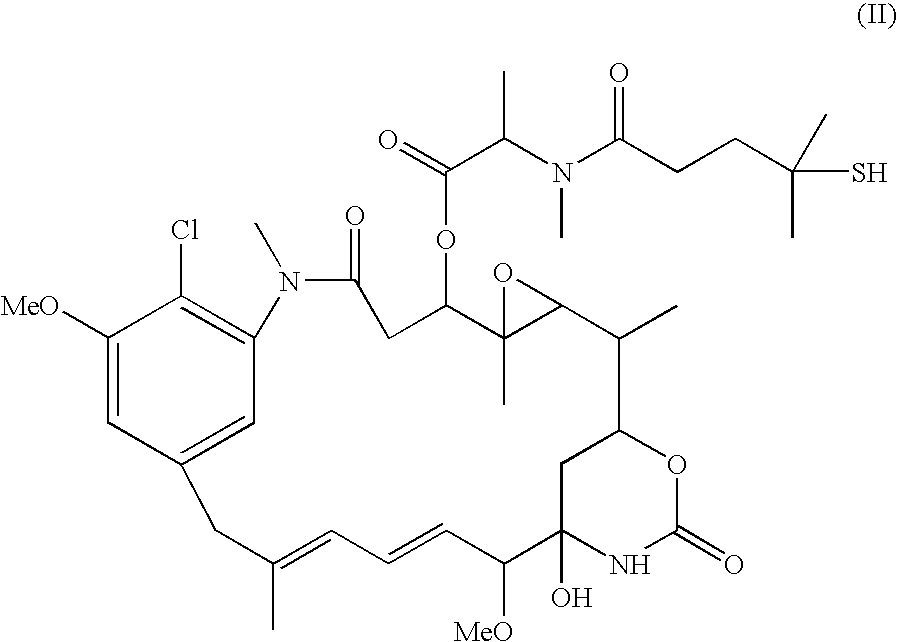
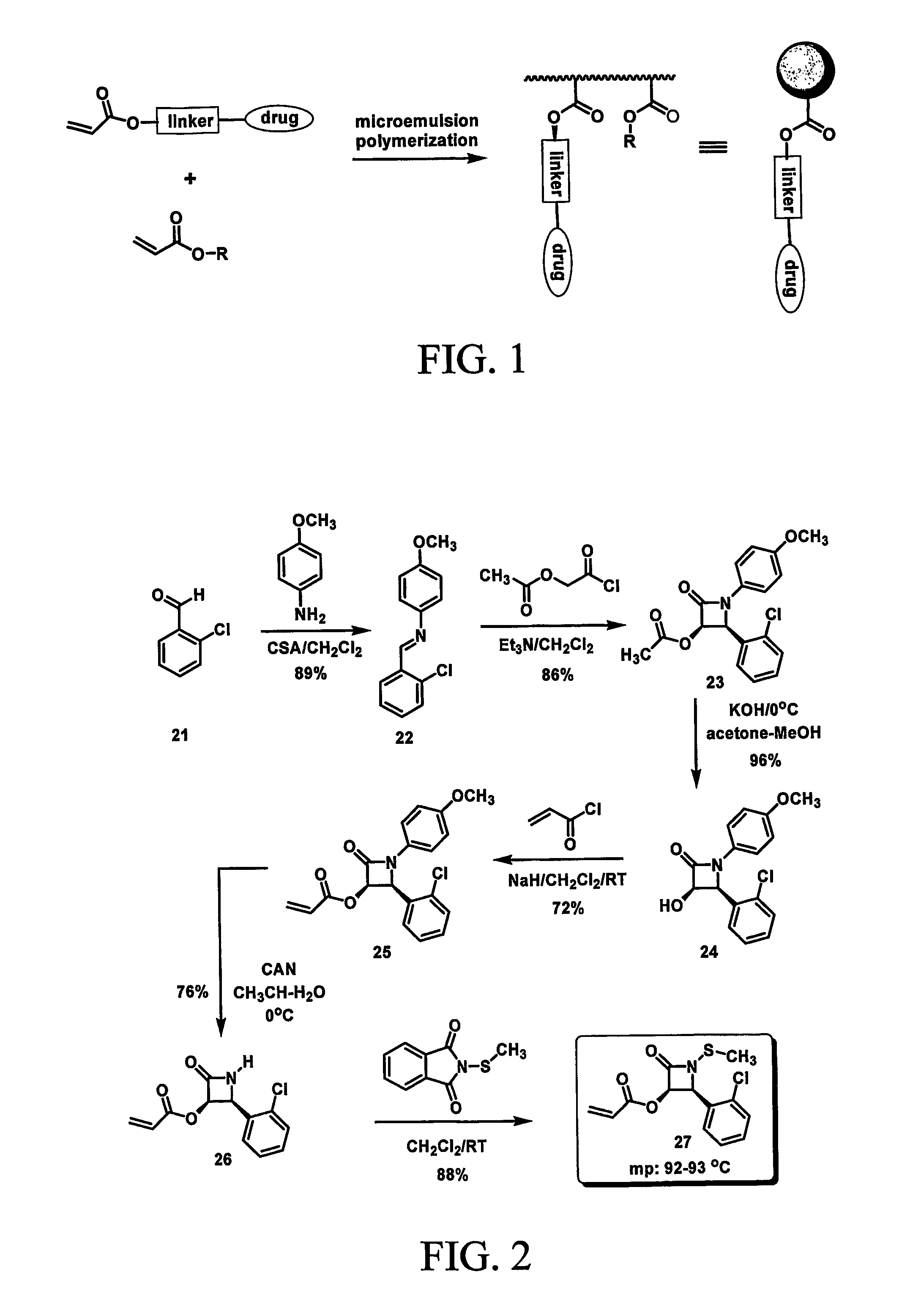
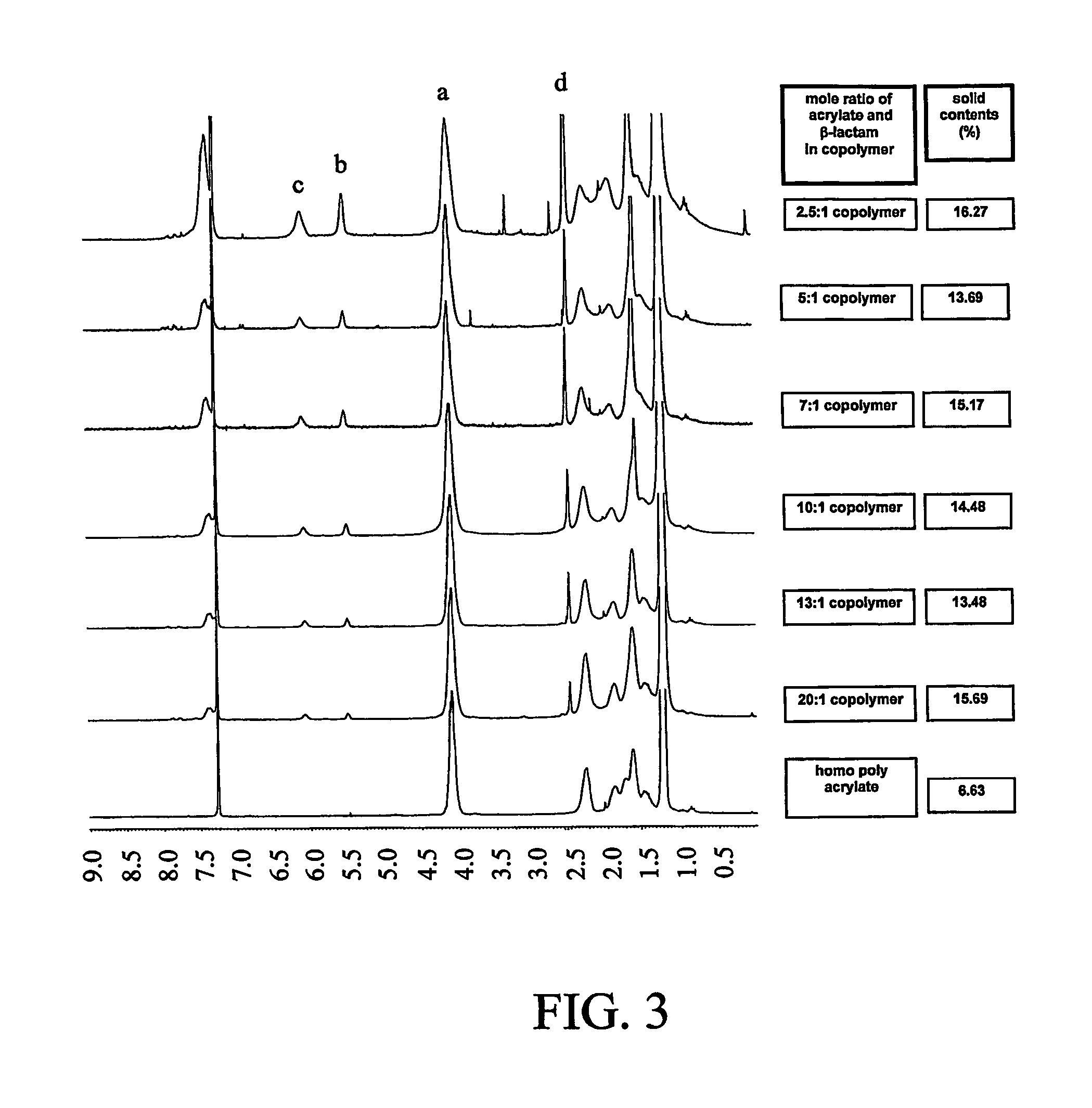
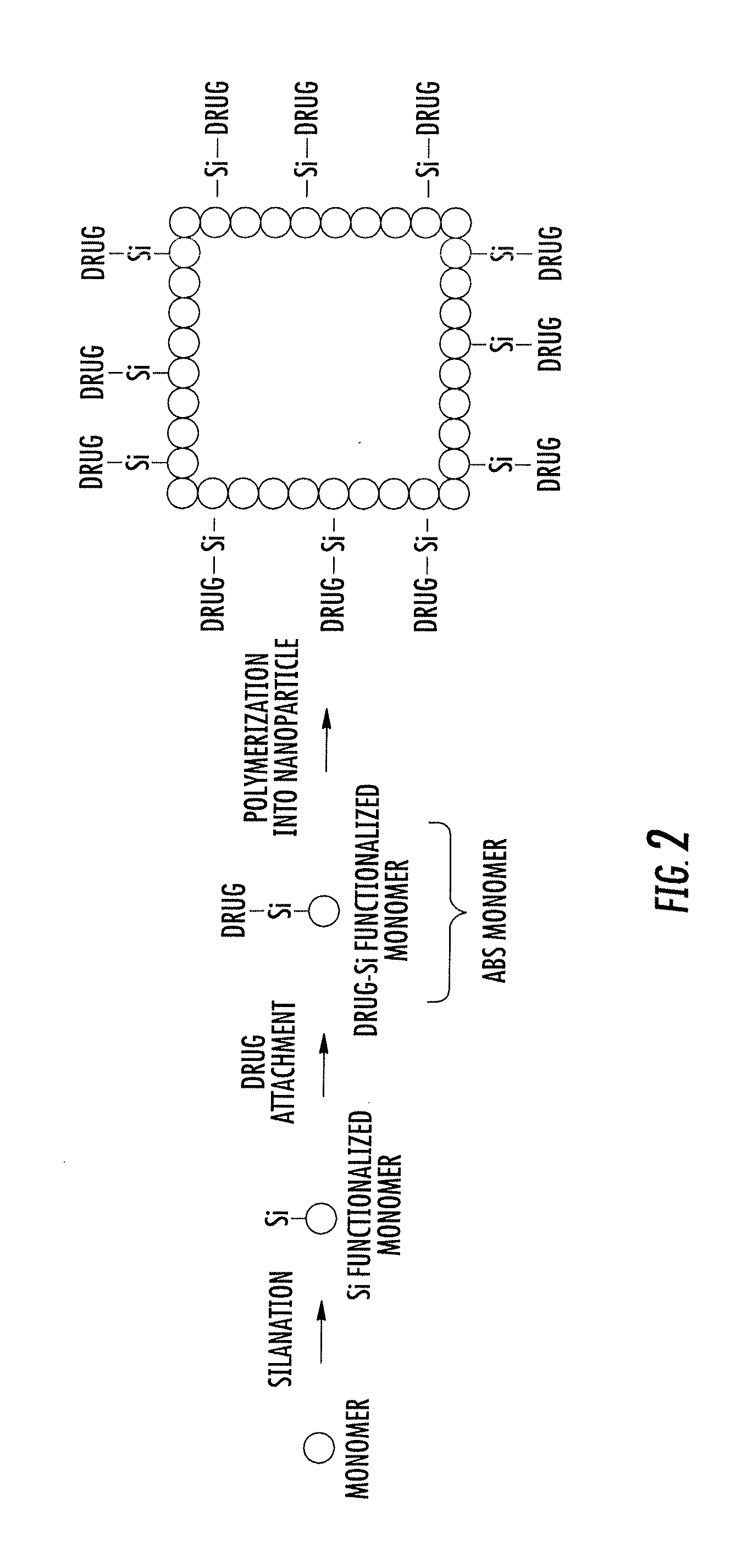
This invention relates to polymeric nanoparticles useful for drug delivery with target molecules bonded to the surface of the particles and having sizes of up to 1000 nm, preferably 1 nm to 400 nm, more preferably 1 nm to 200 nm, that are dispersed homogeneously in aqueous solution. The target drug / target substance is covalently bonded to the novel polymeric nanoparticles to secure them from outer intervention in vivo or cell culture in vitro until they are exposed at the target site within the cell. This invention also relates to microemulsion polymerization techniques useful for preparing the novel nanoparticles.
Owner:UNIV OF SOUTH FLORIDA
MULTIPLEX BARCODED PAIRED-END DITAG (mbPED) LIBRARY CONSTRUCTION FOR ULTRA HIGH THROUGHPUT SEQUENCING
Multiplex barcoded Paired-End Ditag (mbPED) library construction for ultra high throughput sequencing is disclosed. The mbPED library comprises multiple types of barcoded Paired-End Ditag (bPED) nucleic acid fragment constructs, each of which comprises a unique barcoded adaptor, a first tag, and a second tag linked to the first tag via the barcoded adaptor. The two tags are the 5′- and 3′-ends of a nucleic acid molecule from which they originate. The barcoded adaptor comprises a barcode, a first polynucleotide sequence comprising a first restriction enzyme (RE) recognition site, and a second polynucleotide sequence comprising a second RE recognition site and covalently linked to the first polynucleotide sequence via the barcode. The two REs lead to cleavage of a nucleic acid at a defined distance from their recognition sites. The length of the adaptor is set so that the bPED nucleic acid fragment fits one-step sequencing.
Owner:ACAD SINIC
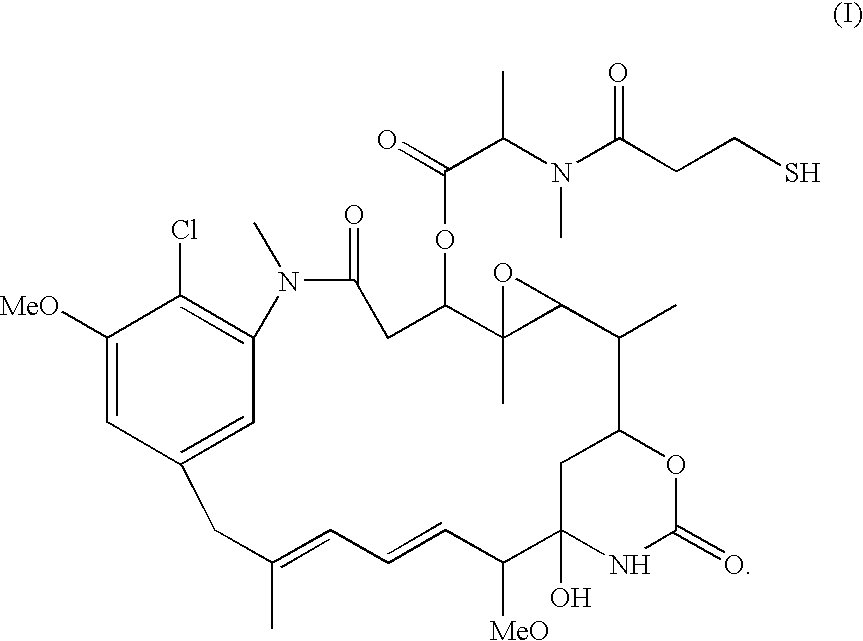
Antibody drug conjugates and methods
InactiveUS20070134243A1Prevent proliferationHybrid immunoglobulinsImmunoglobulins against cytokines/lymphokines/interferonsDiseaseAntiendomysial antibodies
The invention relates to antibody drug conjugate (ADC) compounds represented by Formula I: Ab-(L-D)p I where one or more 1,8 bis-naphthalimide drug moieties (D) having Formulas IIa and IIb are covalently linked by a linker (L) to an antibody (Ab). The invention also relates to pharmaceutical compositions comprising an effective amount of a Formula I ADC for treatment of hyperproliferative disorders and other disorders. The invention also relates to methods for killing or inhibiting the multiplication of a tumor cell or cancer cell including administering to a patient an effective amount of a Formula I ADC.
Owner:GENENTECH INC
Covalent tethering of functional groups to proteins and substrates therefor
ActiveUS20060024808A1Rapidly and efficiently loaded into and washedStable rateMethine/polymethine dyesBacteriaAmino acid substitutionTethering
A mutant hydrolase optionally fused to a protein of interest is provided. The mutant hydrolase is capable of forming a bond with a substrate for the corresponding nonmutant (wild-type) hydrolase which is more stable than the bond formed between the wild-type hydrolase and the substrate and has at least two amino acid substitutions relative to the wild-type hydrolase. Substrates for hydrolases comprising one or more functional groups are also provided, as well as methods of using the mutant hydrolase and the substrates of the invention. Also provided is a fusion protein capable of forming a stable bond with a substrate and cells which express the fusion protein.
Owner:PROMEGA CORP
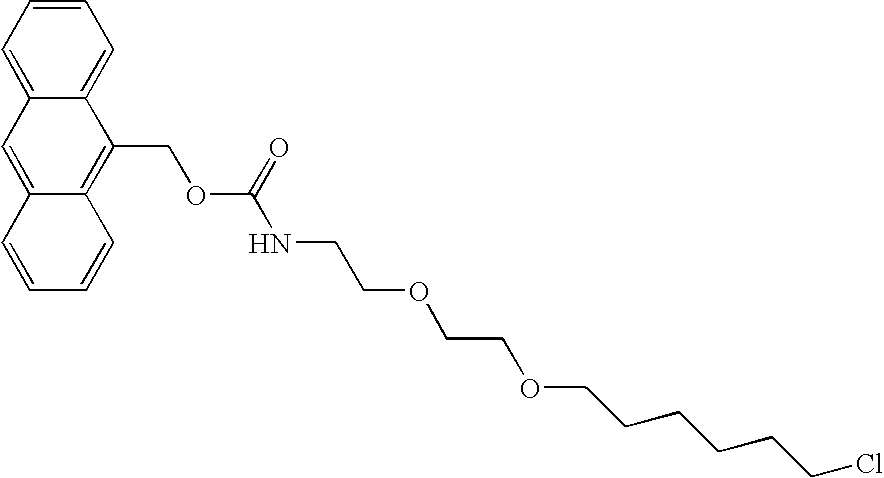
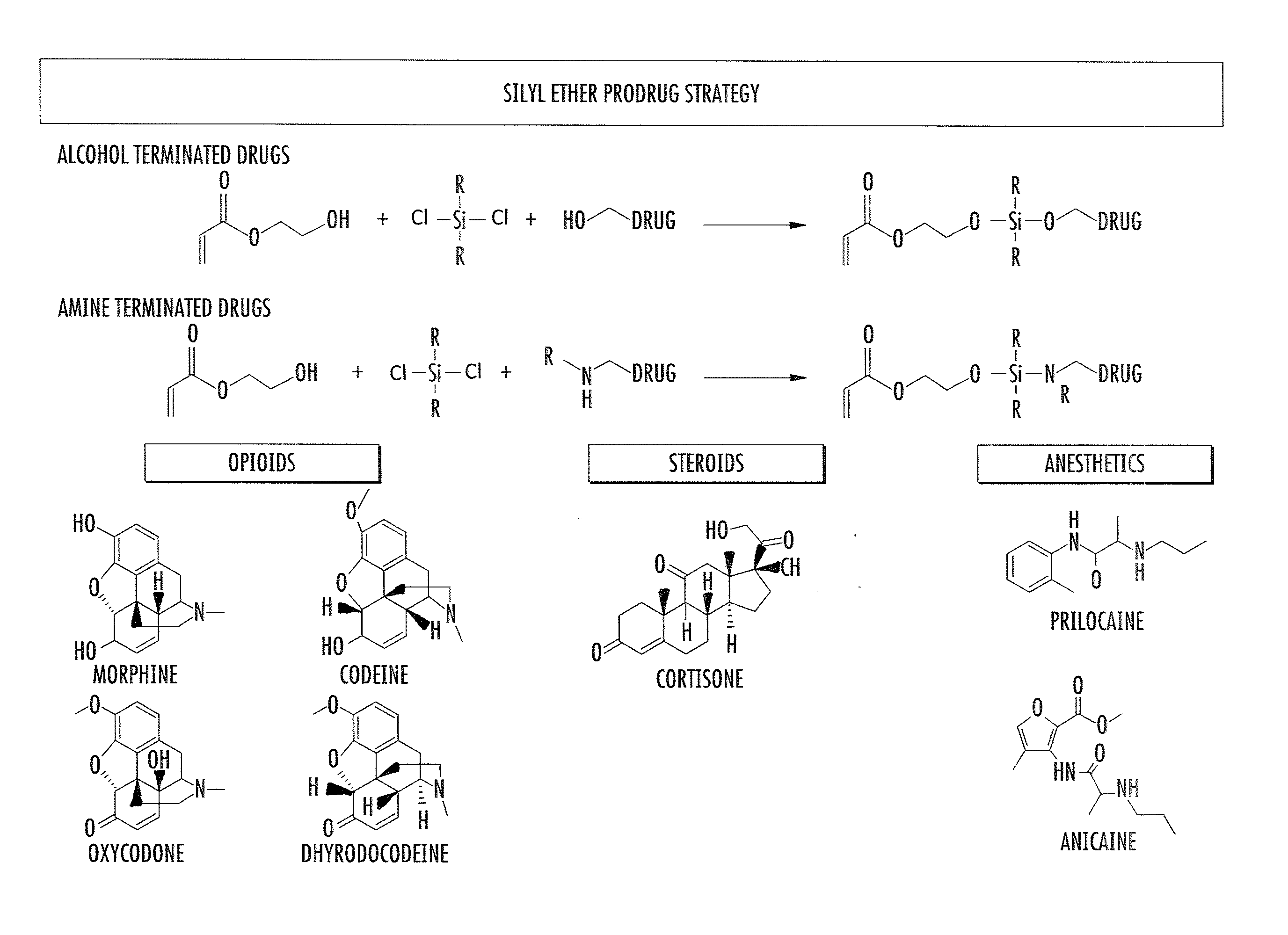
Asymmetric biofunctional silyl monomers and particles thereof as prodrugs and delivery vehicles for pharmaceutical, chemical and biological agents
Asymmetric bifunctional silyl (ABS) monomers comprising covalently linked pharmaceutical, chemical and biological agents are described. These agents can also be covalently bound via the silyl group to delivery vehicles for delivering the agents to desired targets or areas. Also described are delivery vehicles which contain ABS monomers comprising covalently linked agents and to vehicles that are covalently linked to the ABS monomers. The silyl modifications described herein can modify properties of the agents and vehicles, thereby providing desired solubility, stability, hydrophobicity and targeting.
Owner:DESIMONE JOSEPH M +4
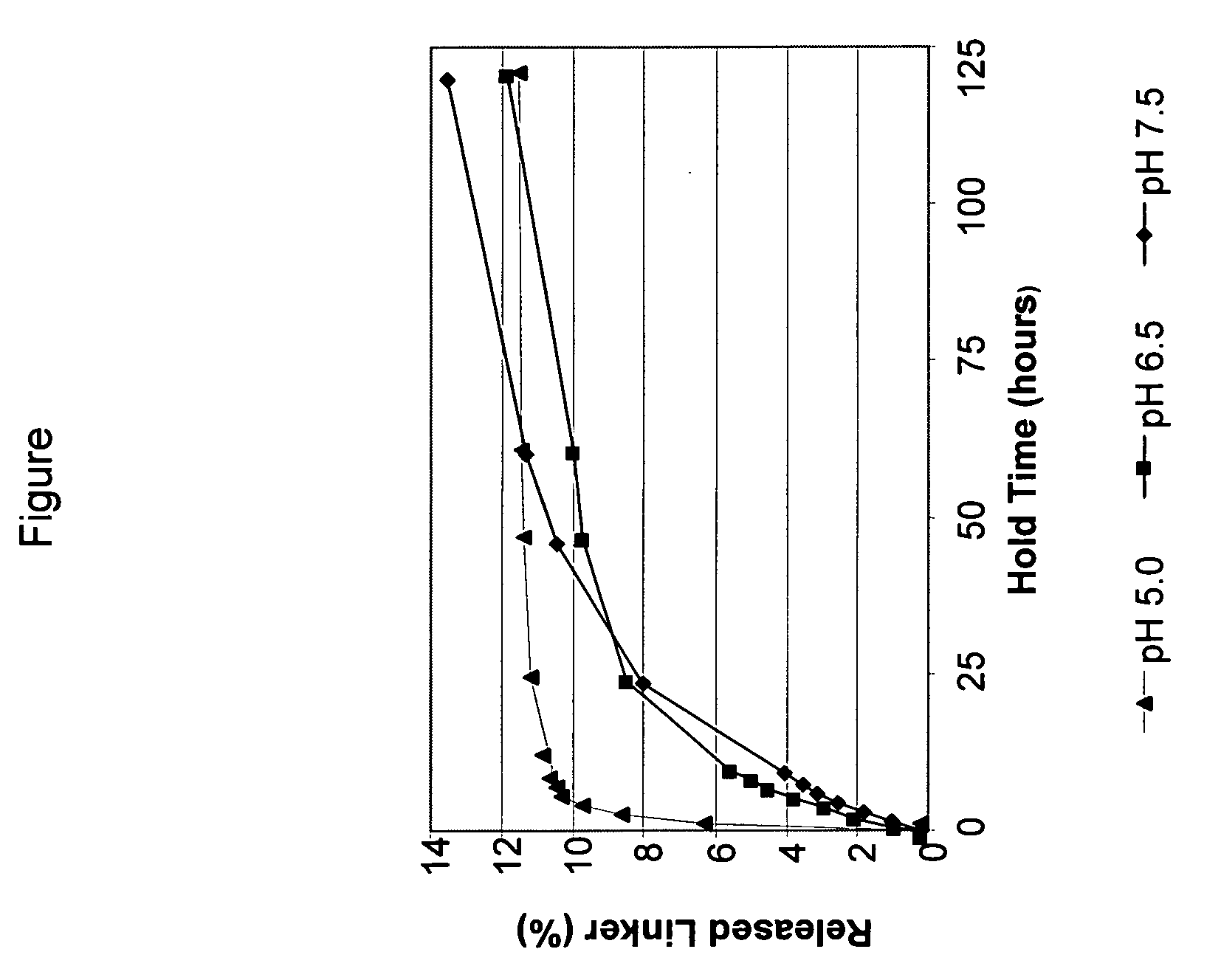
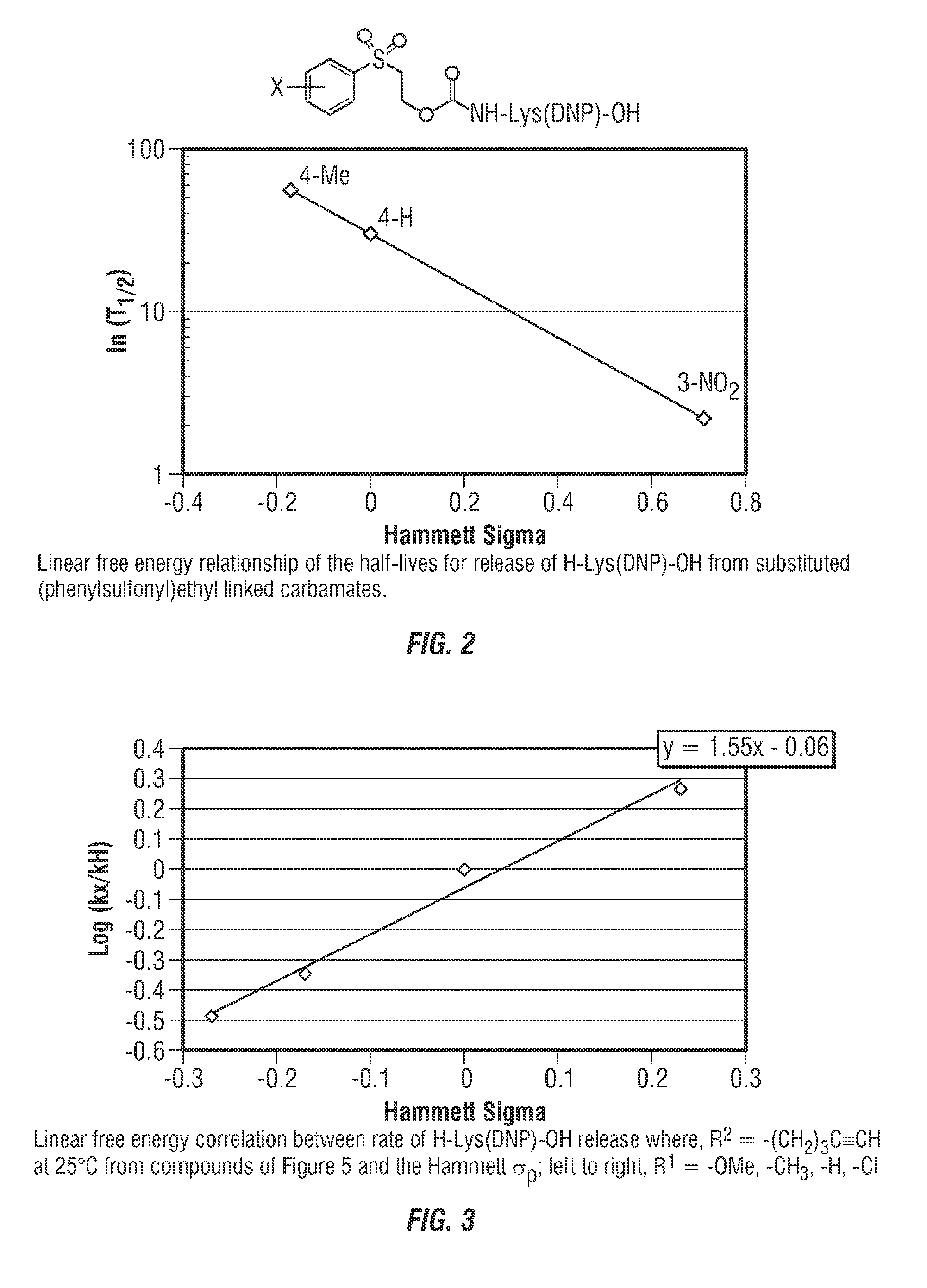
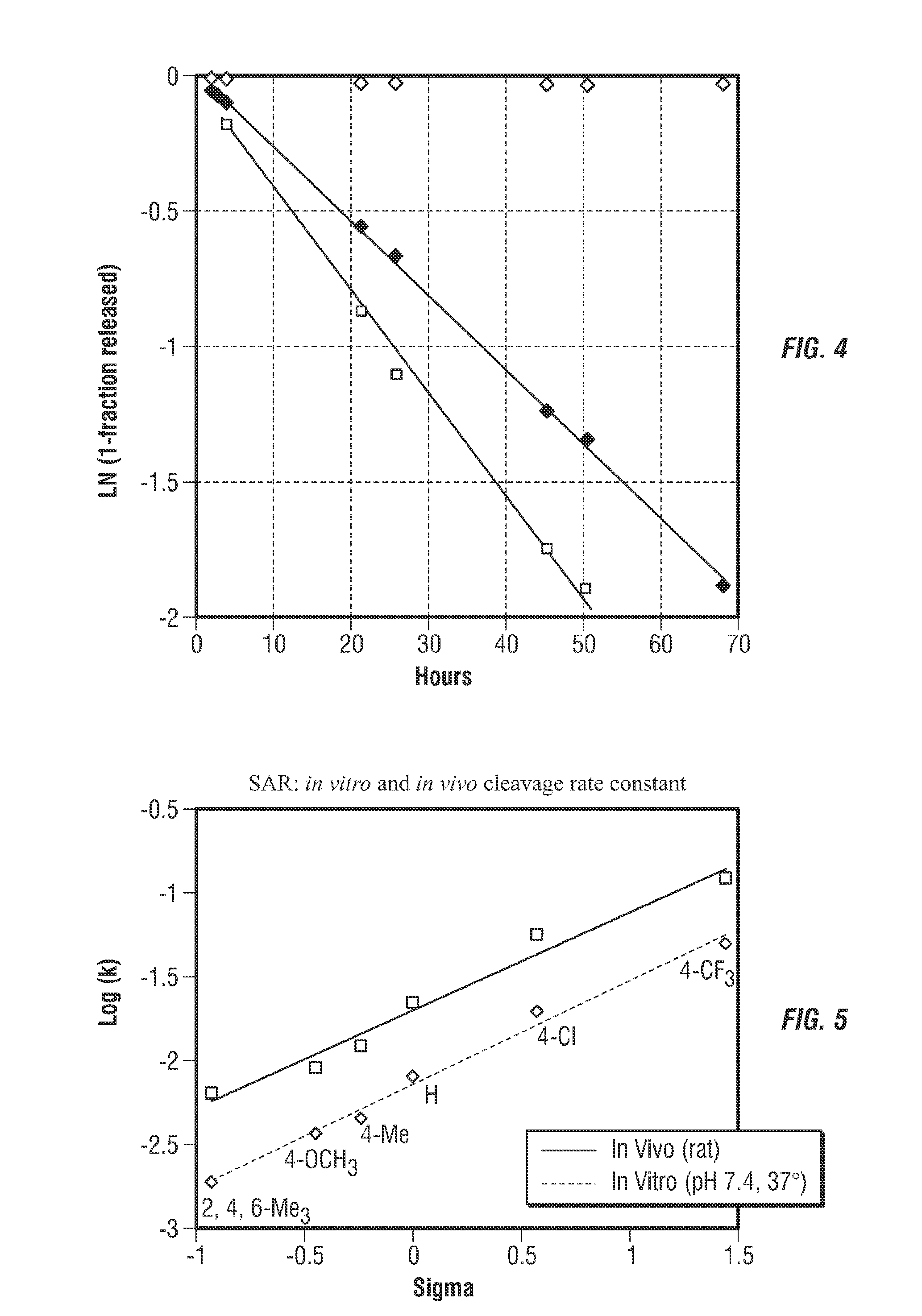
Controlled release from solid supports
The invention relates to solid supports useful in medical applications that provide controlled release of drugs, such as peptides, nucleic acids and small molecules. The drugs are covalently coupled to the solid support through a linkage that releases the drug or a prodrug through controlled beta elimination.
Owner:PROLYNX LLC
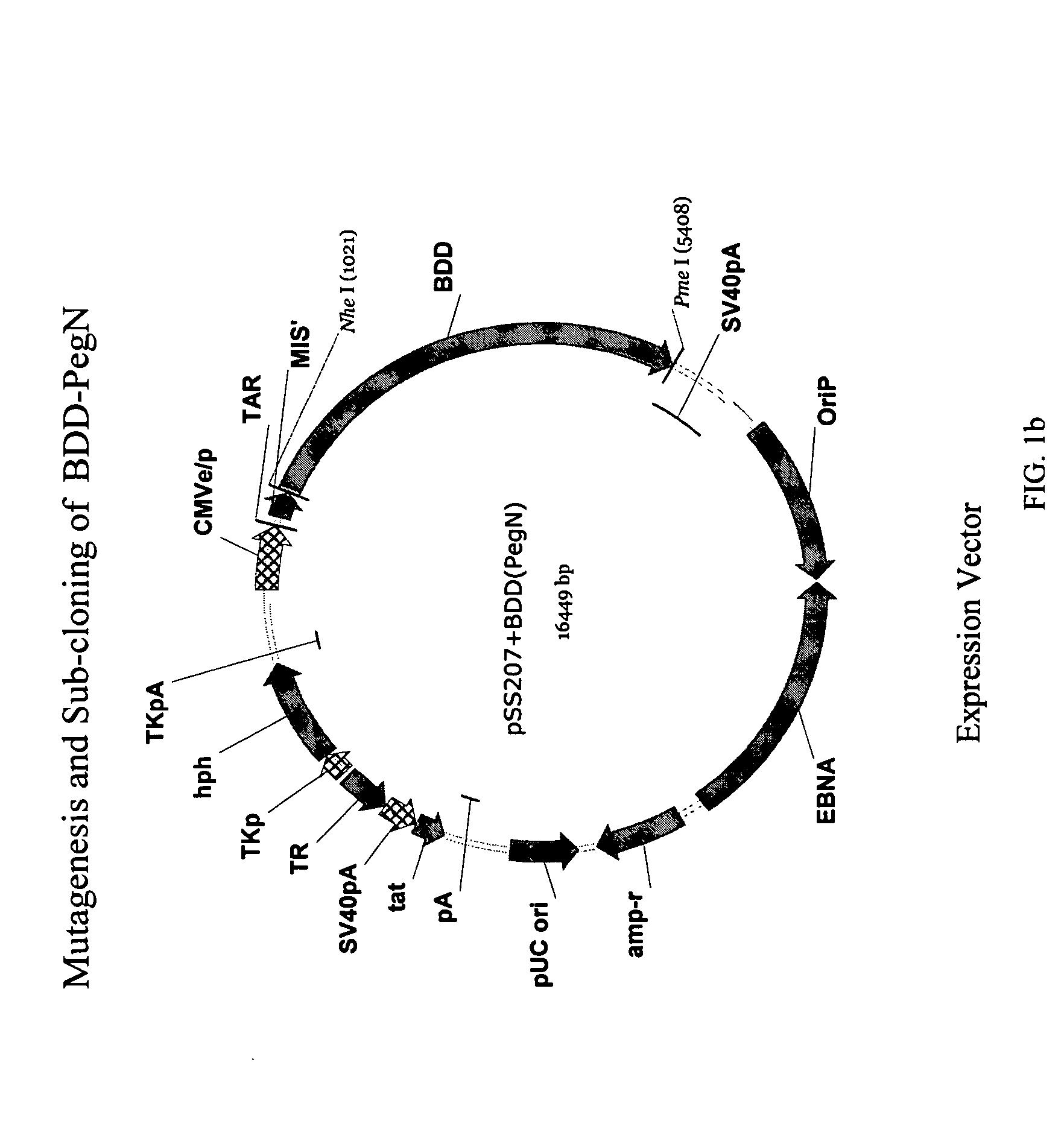
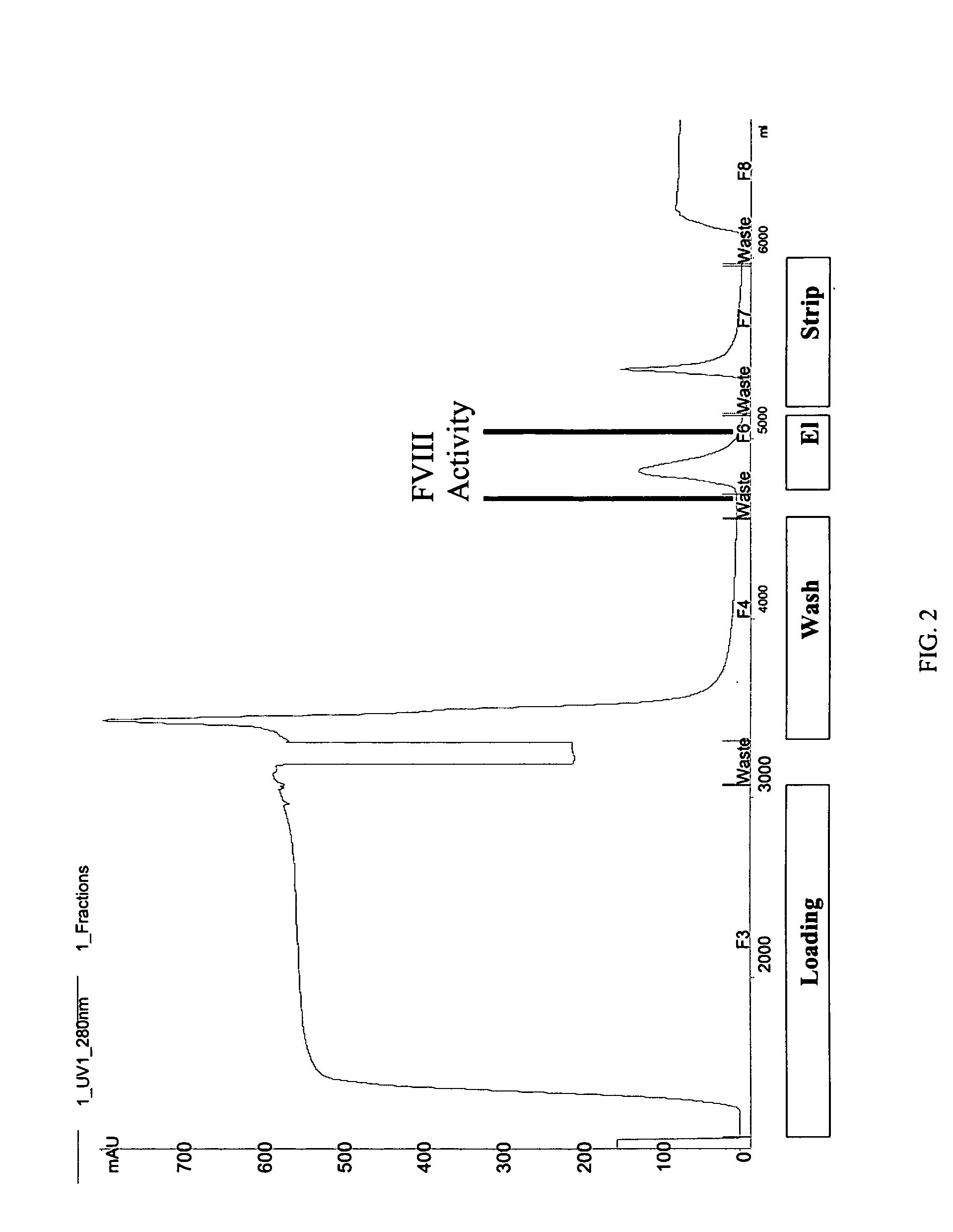
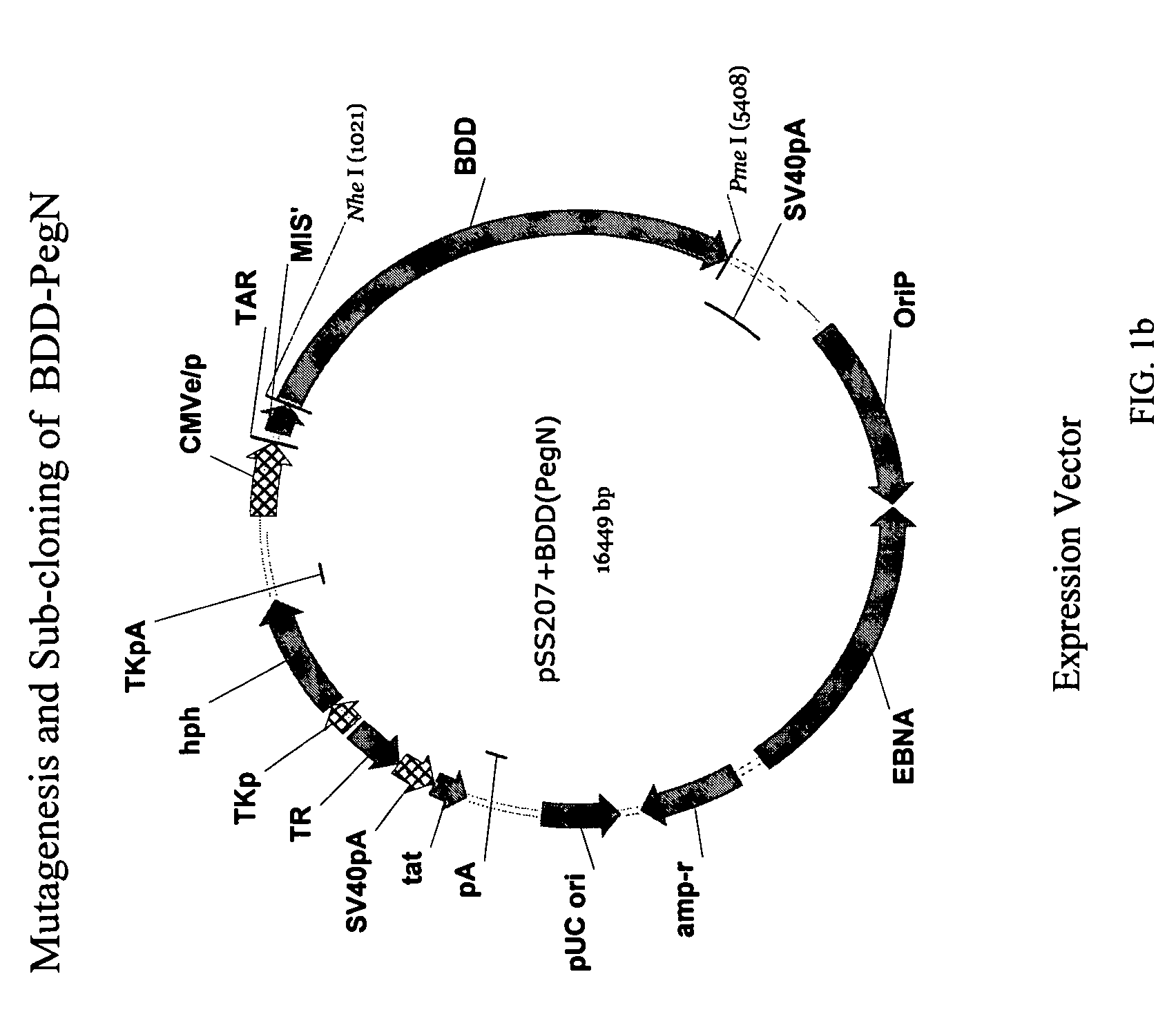
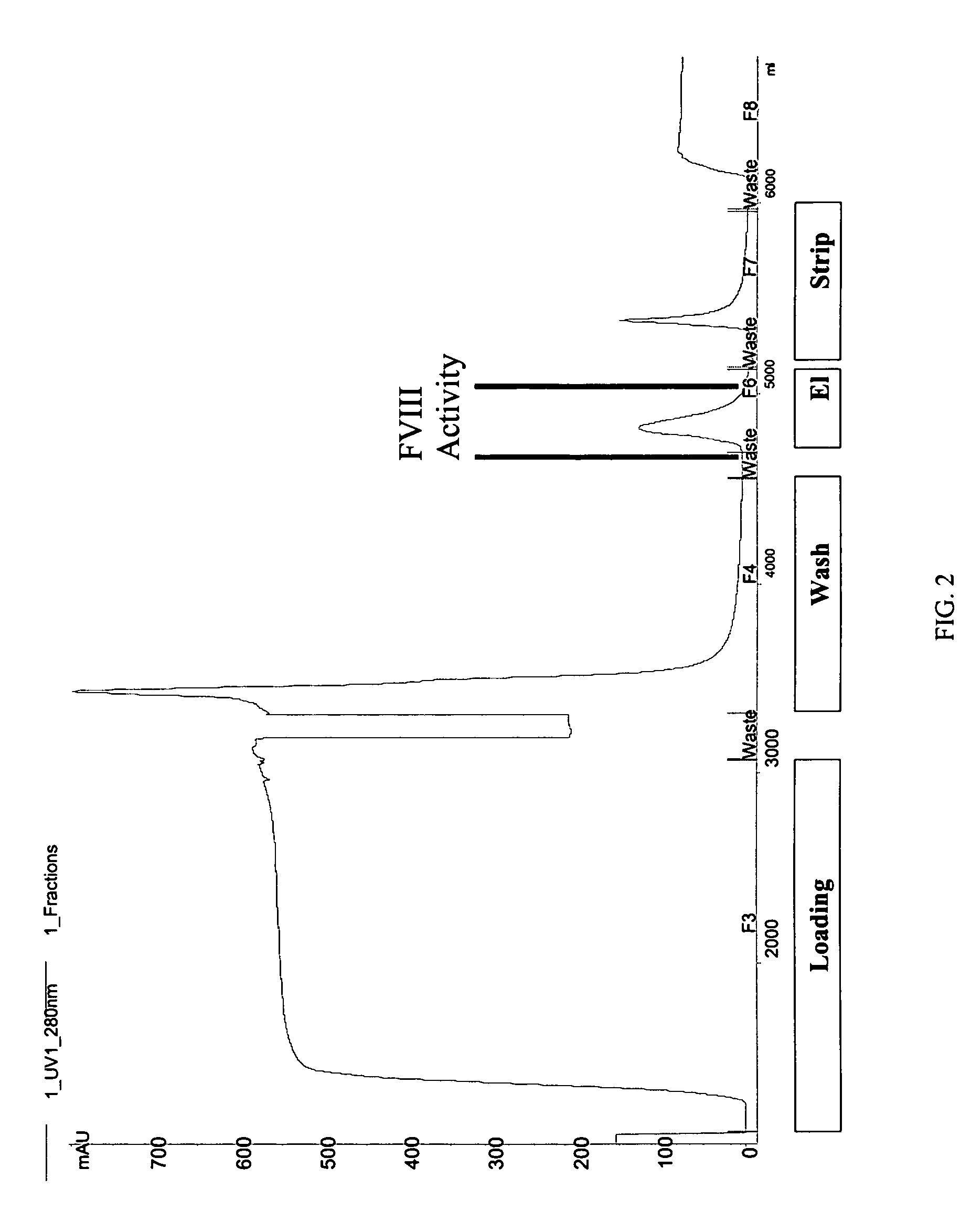
Site-directed modification of FVIII
ActiveUS20060115876A1Improve featuresImproved pharmacokinetic propertiesFactor VIIPeptide/protein ingredientsPolyethylene glycolMutant protein
This invention relates to Factor VIII muteins that are covalently bound, at a predefined site that is not an N-terminal amine, to one or more biocompatible polymers such as polyethylene glycol. The mutein conjugates retain FVIII procoagulant activity and have improved pharmacokinetic properties.
Owner:BAYER HEALTHCARE LLC
C-, S- and N-glycosylation of peptides
The present invention provides polypeptide conjugates wherein a modifying group such as a water-soluble polymer, a therapeutic agent or a biomolecule is covalently linked to the polypeptide through a glycosyl linking group. In one embodiment, the polypeptide includes a glycosylation consensus sequence, wherein glycosylation occurs at an aromatic amino acid residue, such as the C-2 or the N-1 position of a tryptophan side chain. Exemplary polypeptides of the invention are those in which the glycosylation consensus sequence has been introduced into the amino acid sequence of the polypeptide by mutation. In another aspect the invention provides polypeptide conjugates wherein the modifying group is covalently linked to the polypeptide via a glycosyl mimetic linking group. Also provided are methods of making and using as well as pharmaceutical compositions containing the polypeptide conjugates of the invention. Further provided are methods of treating, ameliorating or preventing diseases in mammals by administering an amount of a polypeptide conjugate of the invention sufficient to achieve the desired response.
Owner:NOVO NORDISK AS
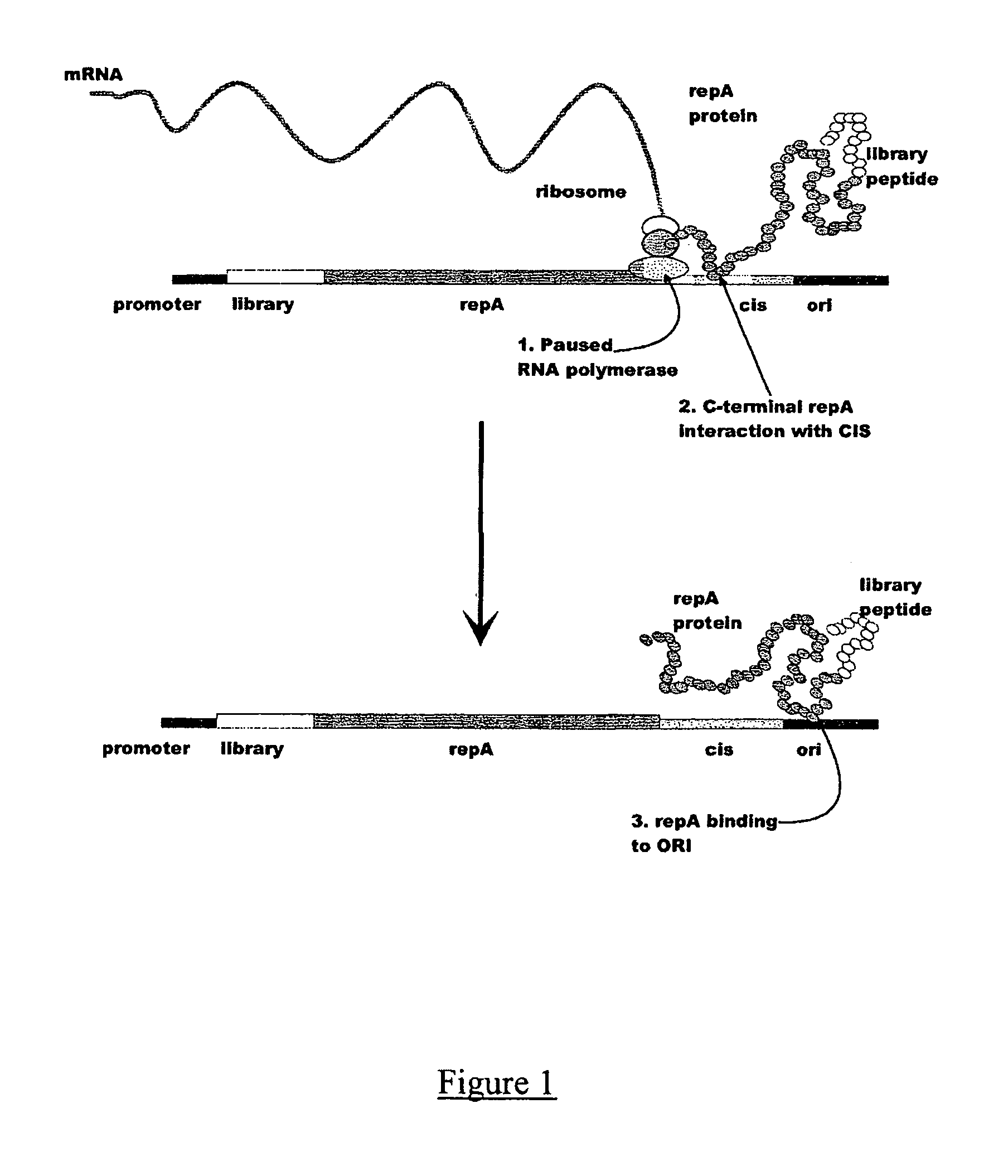
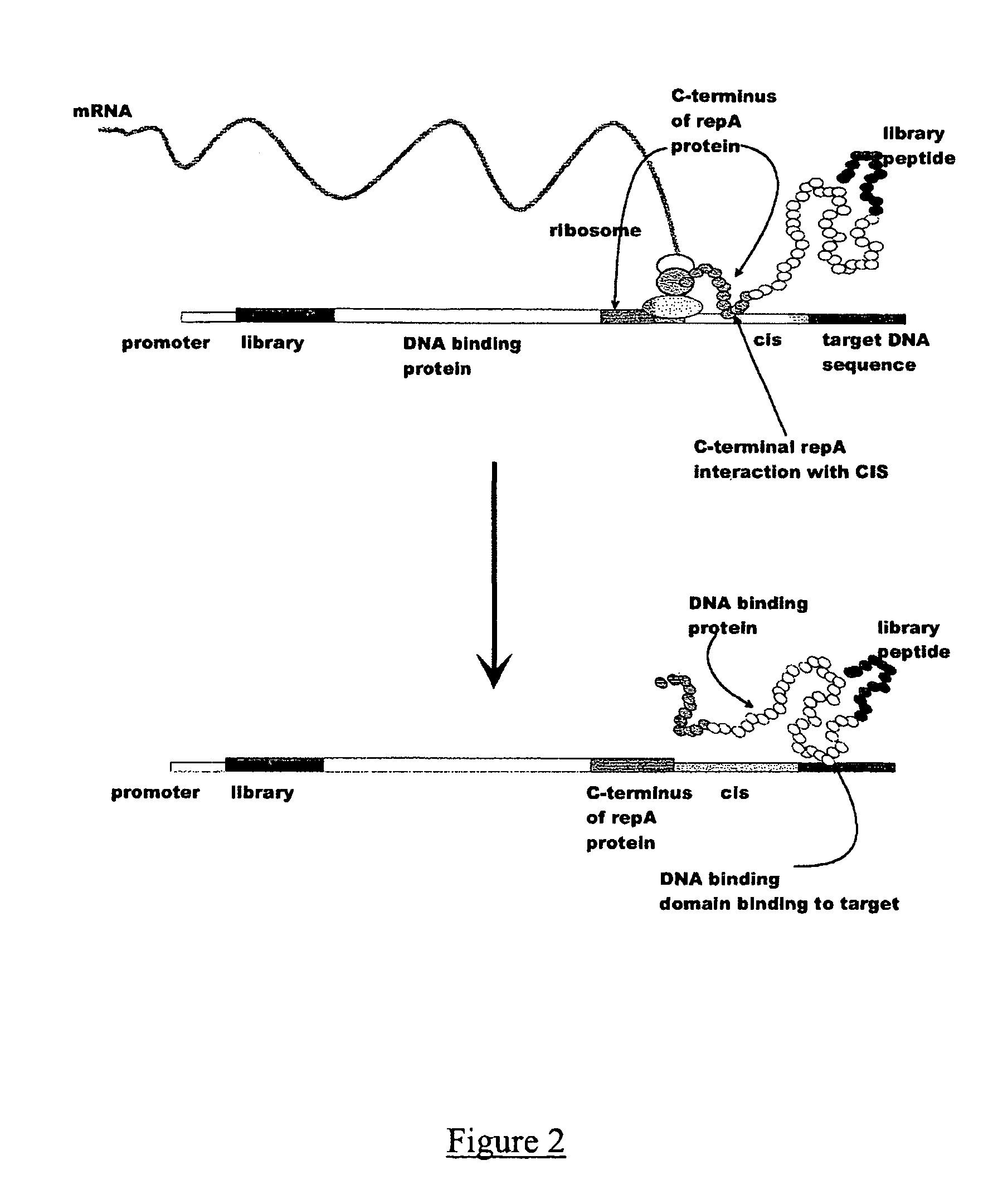
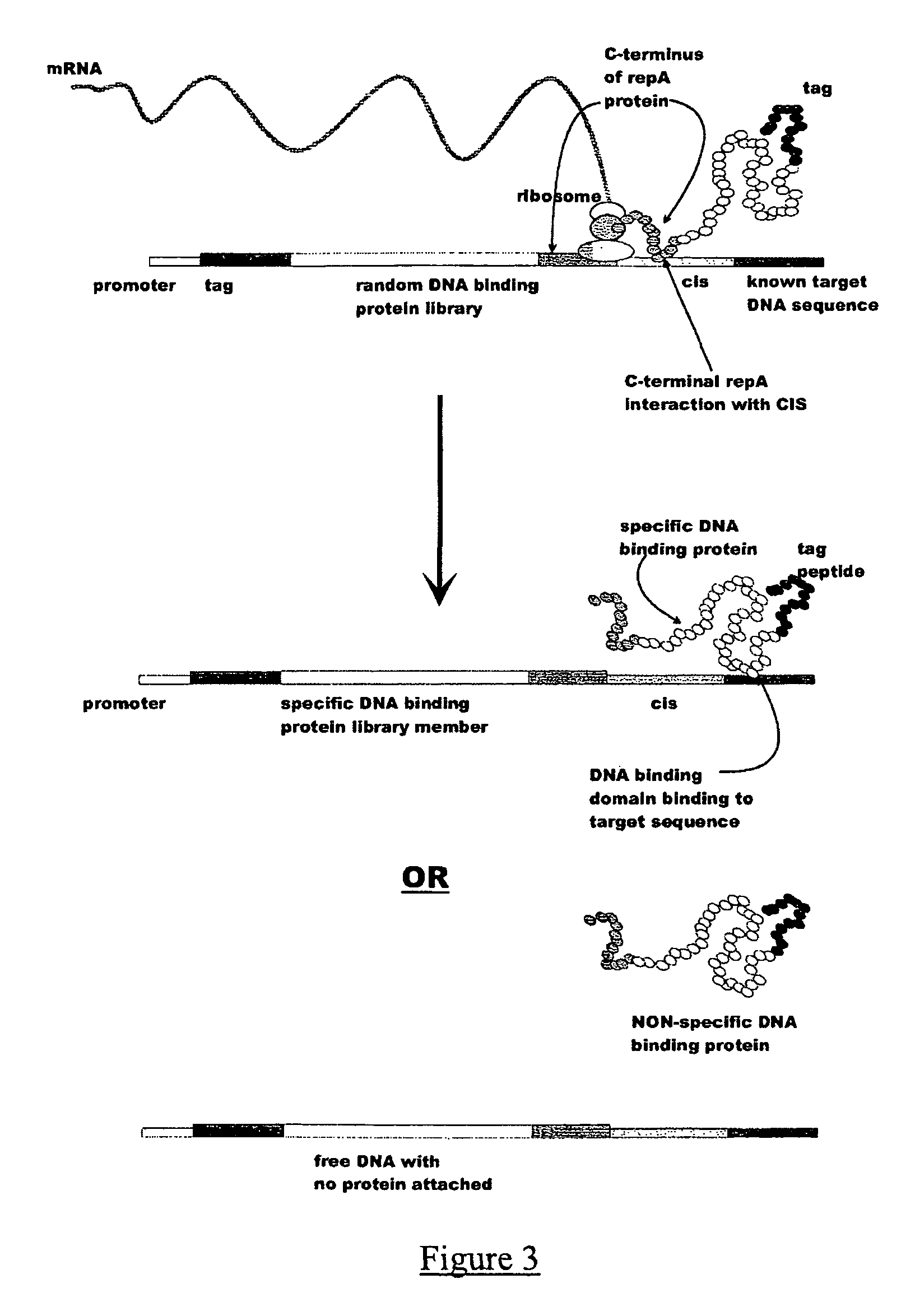
In vitro peptide expression library
ActiveUS7842476B2Fusion with DNA-binding domainMicrobiological testing/measurementExpression LibraryNucleotide
The invention provides a method for making in vitro peptide expression libraries, and for the isolation of nucleotide sequences encoding peptides of interest, wherein the peptides or proteins are specifically associated with the DNA encoding them through non-covalent protein:DNA binding. The method describes ways of making the library itself, DNA molecules encoding the library and uses of the expression library.
Owner:ISOGENICA LTD
Site-directed modification of FVIII
ActiveUS7632921B2Improve featuresImproved pharmacokinetic propertiesFactor VIIPeptide/protein ingredientsFactor VIII deficiencyPolyethylene glycol
Owner:BAYER HEALTHCARE LLC
Glycopegylated granulocyte colony stimulating factor
ActiveUS20070014759A1Improve pharmacokinetic propertyImproved pharmacokinetic propertiesPeptide/protein ingredientsFermentationPeptideSugar moiety
The present invention provides conjugates between Granulocyte Colony Stimulating Factor and PEG moieties. The conjugates are linked via an intact glycosyl linking group that is interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from both glycosylated and unglycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto either an amino acid or glycosyl residue on the peptide. Also provided are pharmaceutical formulations including the conjugates. Methods for preparing the conjugates are also within the scope of the invention.
Owner:NOVO NORDISK AS
Manufacturing process for the production of peptides grown in insect cell lines
InactiveUS20060246544A1Improve purityHigh concentrationHydrolasesPeptide/protein ingredientsPeptideGlycosyltransferase
The present invention provides a manufacturing method for the production of peptides that are grown in insect cell lines. The peptides are grown in insect cell cultures that are infected with baculovirus particles in a culture supplemented with a lipid mixture. The peptides are then isolated from the insect cell culture using a method that employs a tangential flow filtration cascade. The isolated peptides are glycopeptides having an insect specific glycosylation pattern. The glycopeptides may then be conjugated to a modifying group via linkage through a glycosyl linking group interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from glycosylated peptides by the action of a glycosyltransferase.
Owner:NOVO NORDISK AS
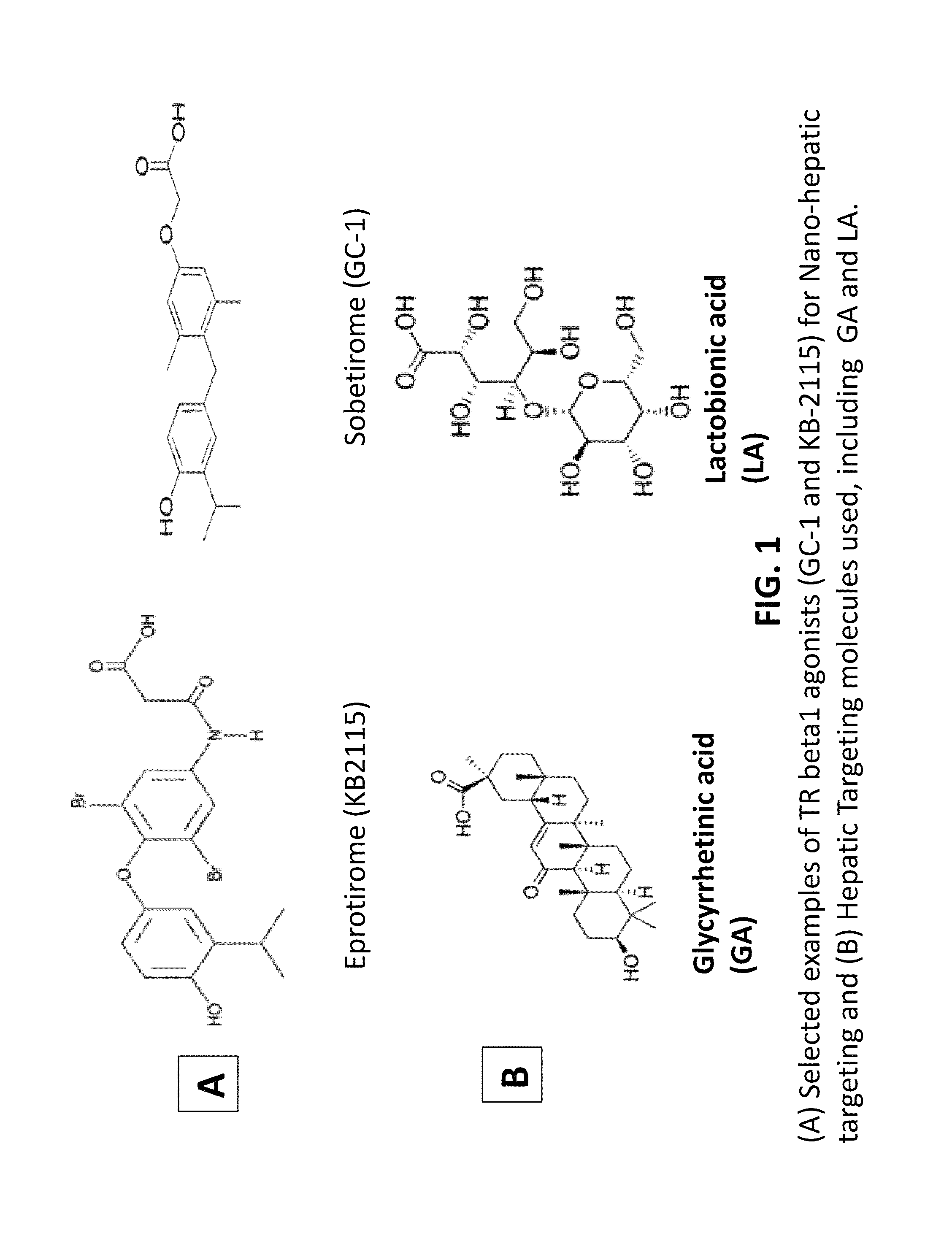
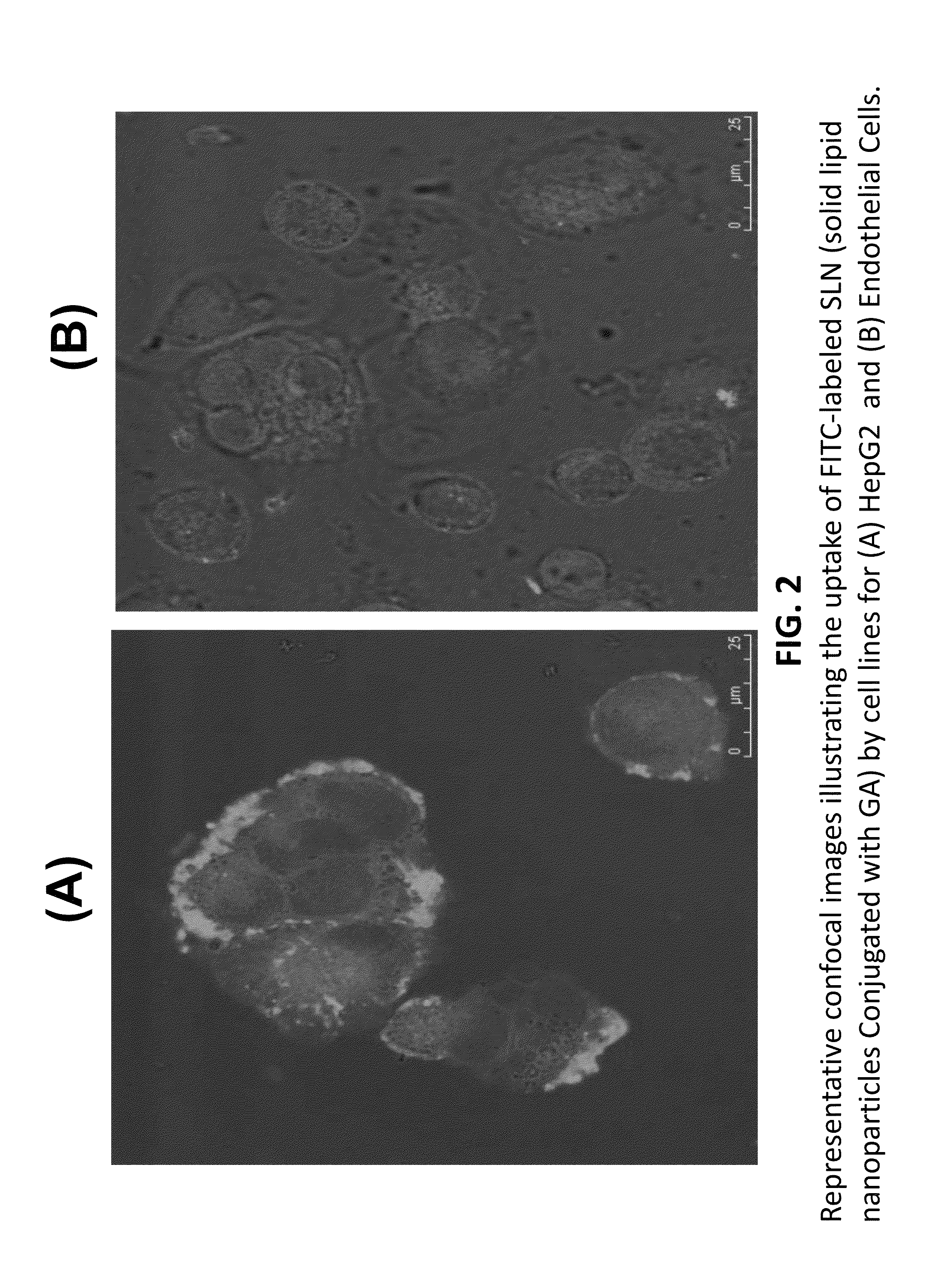
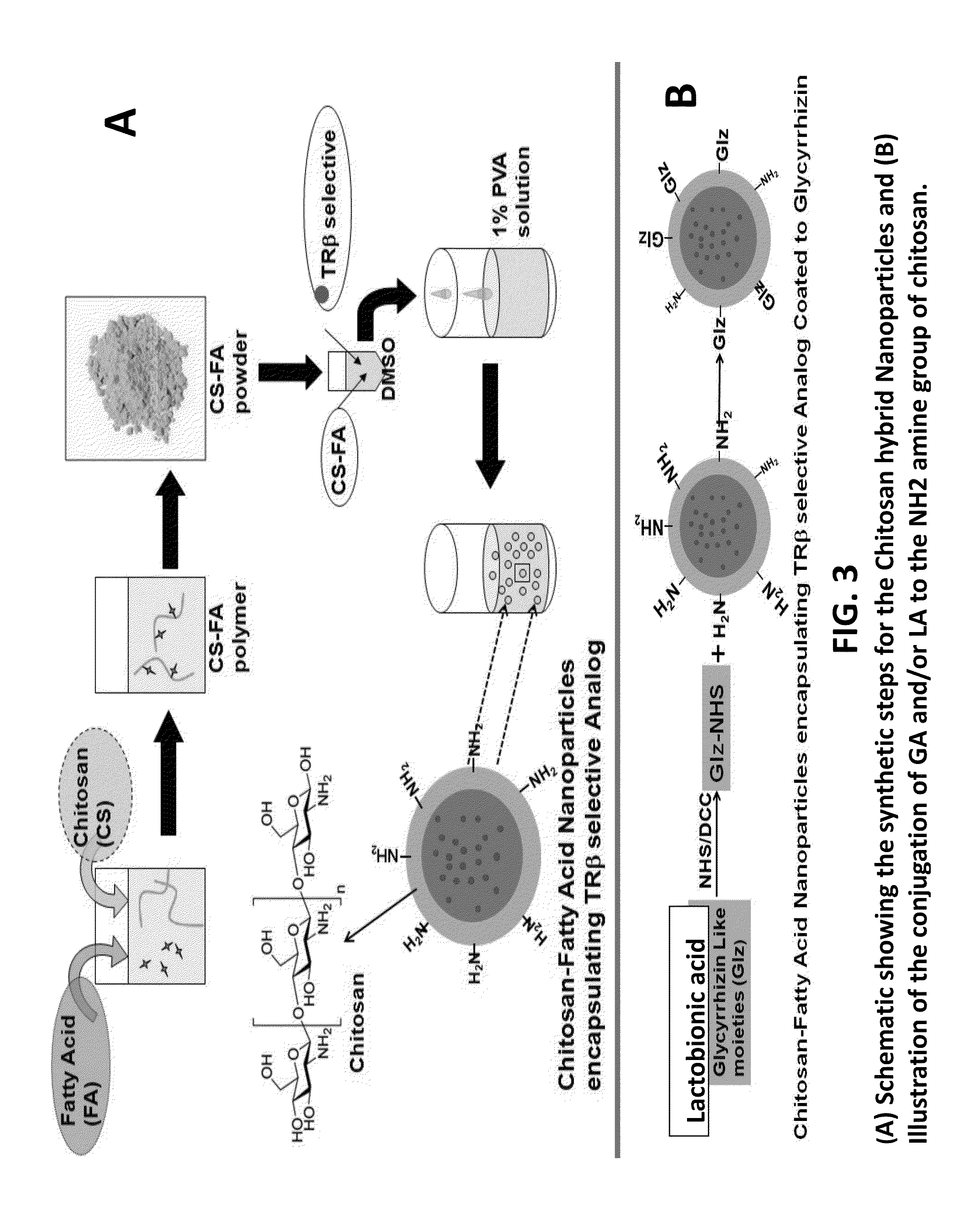
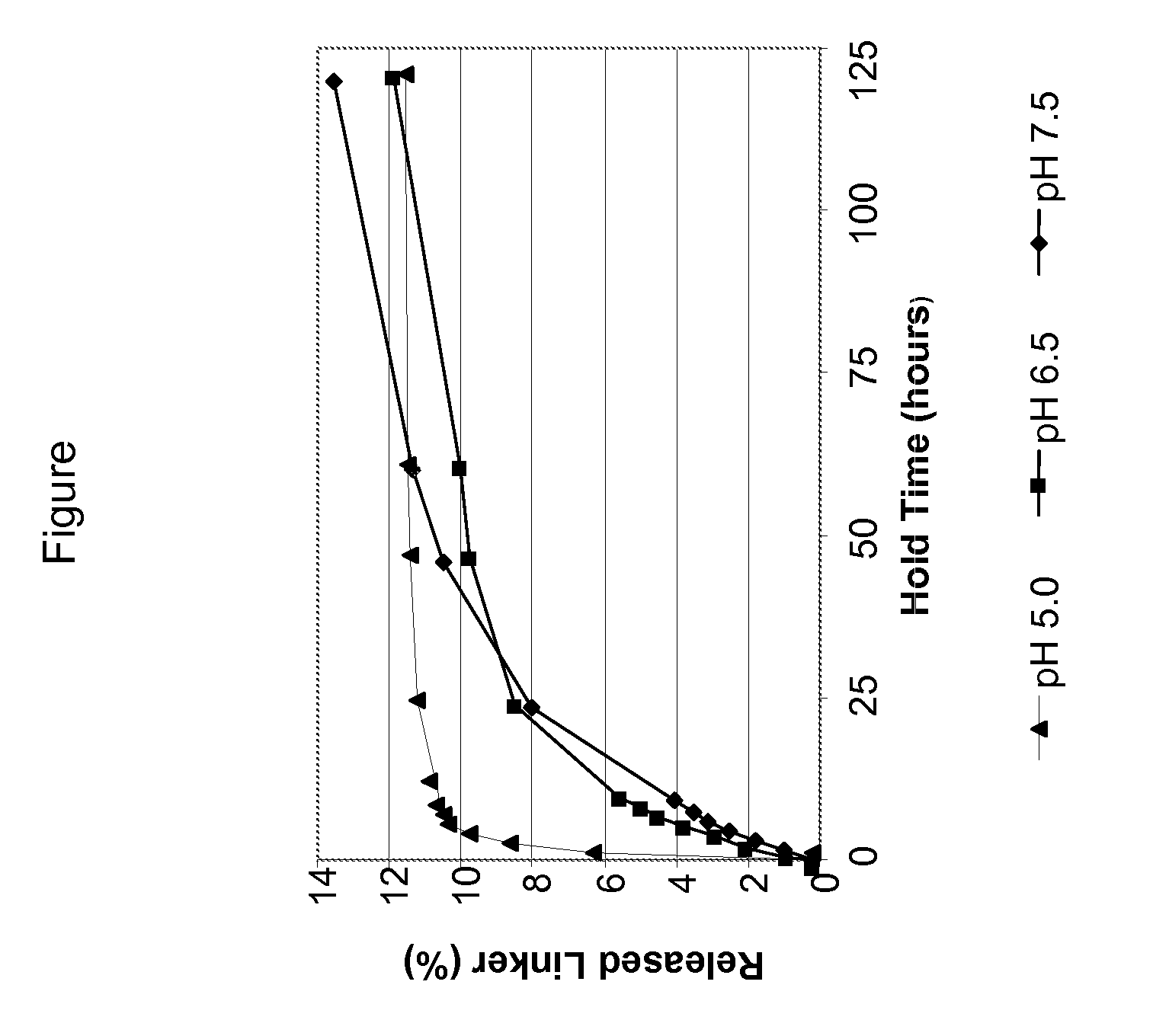
Nanoformulation and methods of use of thyroid receptor beta1 agonists for liver targeting
A composition and an associated method for hepatic targeted delivery of thyroid receptor beta1 (TRβ1) agonist to a liver of a subject. The composition includes hydrophobic nanoparticles, a liver targeting moiety exterior to each nanoparticle and covalently bonded to each nanoparticle, and at least one TRβ1 agonist encapsulated within each nanoparticle. The nanoparticles include chitosan hybrid nanoparticles, amine-modified PLGA nanoparticles, solid lipid nanoparticles, and combinations thereof. The liver targeting moiety includes Glycyrrhetinic acid (GA), Lactobionic acid (LA), or combinations thereof.
Owner:MOUSA SHAKER A
Process for preparing purified drug conjugates
InactiveUS20110166319A1High purityImprove stabilityPeptide/protein ingredientsMammal material medical ingredientsCombinatorial chemistryBiochemistry
The invention provides processes for preparing a cell-binding agent chemically coupled to a drug. A first process comprises covalently attaching a linker to a cell-binding agent, an optional purification step, conjugating a drug to the cell-binding agent, a subsequent purification step, and optional holding steps. A second process comprises covalently attaching a linker to a cell-binding agent, a purification step, conjugating a drug to the cell-binding agent, a subsequent purification step, holding steps, and optionally a tangential flow filtration (TFF) step.
Owner:IMMUNOGEN INC
Process for manufacturing conjugates of improved homogeneity
InactiveUS20120259100A1Organic active ingredientsAntibody ingredientsCombinatorial chemistryOrganic chemistry
The invention provides processes for manufacturing cell-binding agent-cytotoxic agent conjugates of improved homogeneity comprising performing the modification reaction at a lower temperature. The inventive processes comprise contacting a cell-binding agent with a bifunctional crosslinking reagent at a temperature of about 15° C. or less to covalently attach a linker to the cell-binding agent and thereby prepare a mixture comprising cell-binding agents having linkers bound thereto.
Owner:IMMUNOGEN INC
Conjugated of hydroxyalkyl starch and an active agent
InactiveUS20050063943A1Improved hydroxyalkyl starch-active ingredientUseful for clinical practiceAntibacterial agentsOrganic active ingredientsOrganic solventActive agent
The invention relates to compounds, comprising a conjugate of hydroxyalkyl starch (HAS) and an active agent, whereby the hydroxyalkyl starch is either directly covalently bonded to the active agent, or by means of a linker. The invention further relates to methods for the production of a covalent HAS-active agent conjugate, whereby HAS and an active agent are reacted in a reaction medium, characterised in that the reaction medium is water or a mixture of water and an organic solvent, comprising at least 10 wt. % water.
Owner:FRESENIUS KABI DEUT GMBH
Tetrazine-based bio-orthogonal coupling reagents and methods
ActiveUS20090023916A1Organic compound preparationCarboxylic compound preparationCell lysatesPhosphine
Coupling reactions, suitable for use in organic or aqueous media, are performed by contacting a 1,2,4,5-tetrazine with a dienophile. The dienophile may be covalently bonded to a protein, and the coupling reaction may be performed in biological media such as those containing cells or cell lysates. The reactions may be performed in the presence of primary amines, thiols, acetylenes, azides, phosphines, and products of Staudinger and / or Sharpless-Huisgen reactions Novel 3-substituted cyclopropene compounds and trans-cyclooctenes are exemplary dienophiles for these reactions.
Owner:UNIVERSITY OF DELAWARE
AC methods for the detection of nucleic acids
InactiveUS7056669B2Simple compositionBioreactor/fermenter combinationsBiological substance pretreatmentsOligomerBiological materials
The invention relates to nucleic acids covalently coupled to electrodes via conductive oligomers. More particularly, the invention is directed to the site-selective modification of nucleic acids with electron transfer moieties and electrodes to produce a new class of biomaterials, and to methods of making and using them.
Owner:ROCHE MOLECULAR SYST INC +1
Method and device for immunoassay using nucleotide conjugates
ActiveUS20100167301A1Bioreactor/fermenter combinationsBiological substance pretreatmentsFluorescenceNucleotide
A composition of matter for use in an immunoassay devices and method comprising a signal antibody, e.g., FAB fragment, covalently linked to a first nucleotide; and one or more signal elements, e.g., signal enzymes such as ALP or fluorescent dyes, each covalently linked to a second nucleotide, wherein the first nucleotide has one or more repeated sequences, and the second nucleotide is bound to one of the one or more repeated sequences on said first nucleotide, and wherein the ratio of the signal antibody to the signal element is controlled by the number of repeated sequences.
Owner:ABBOTT POINT CARE
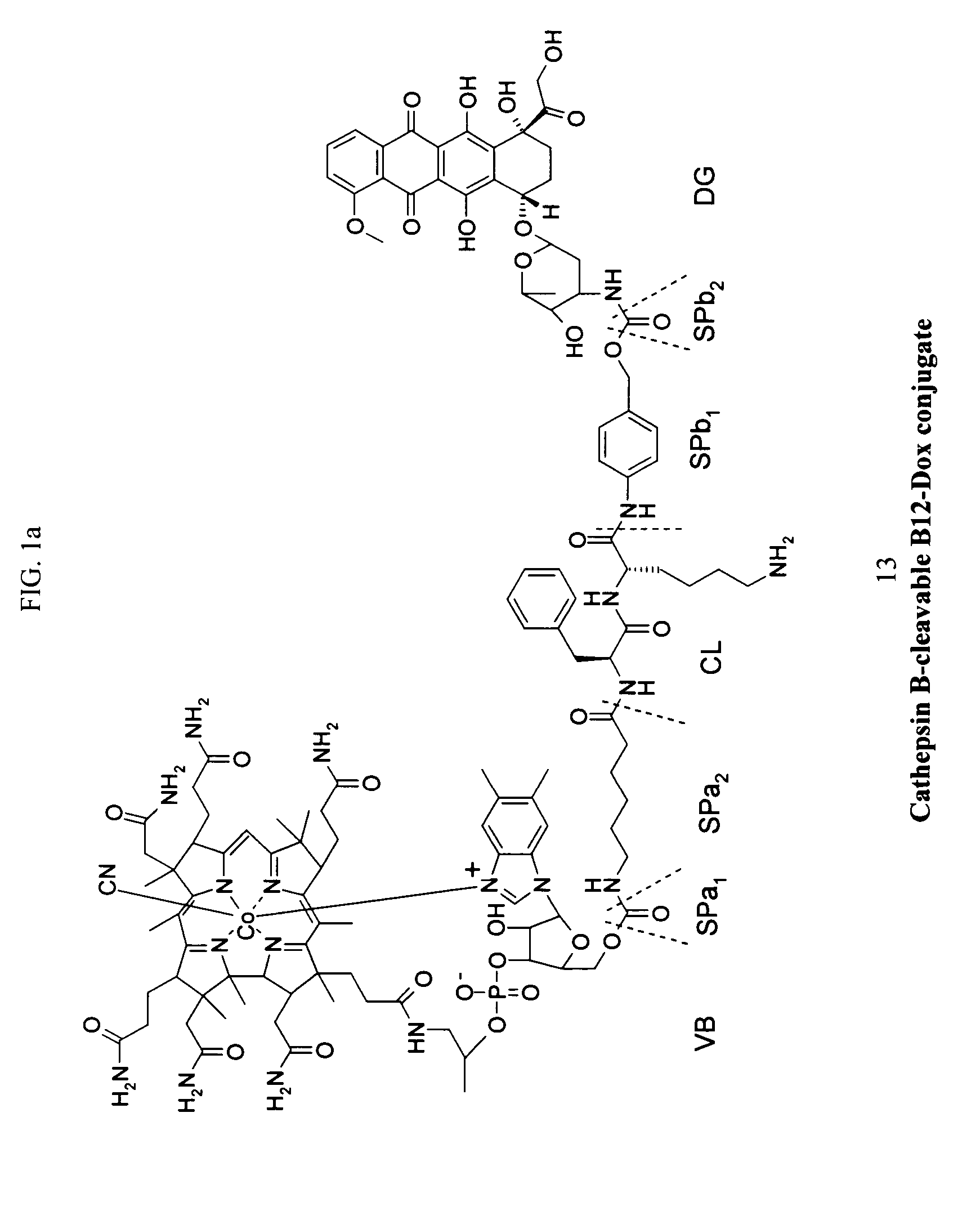
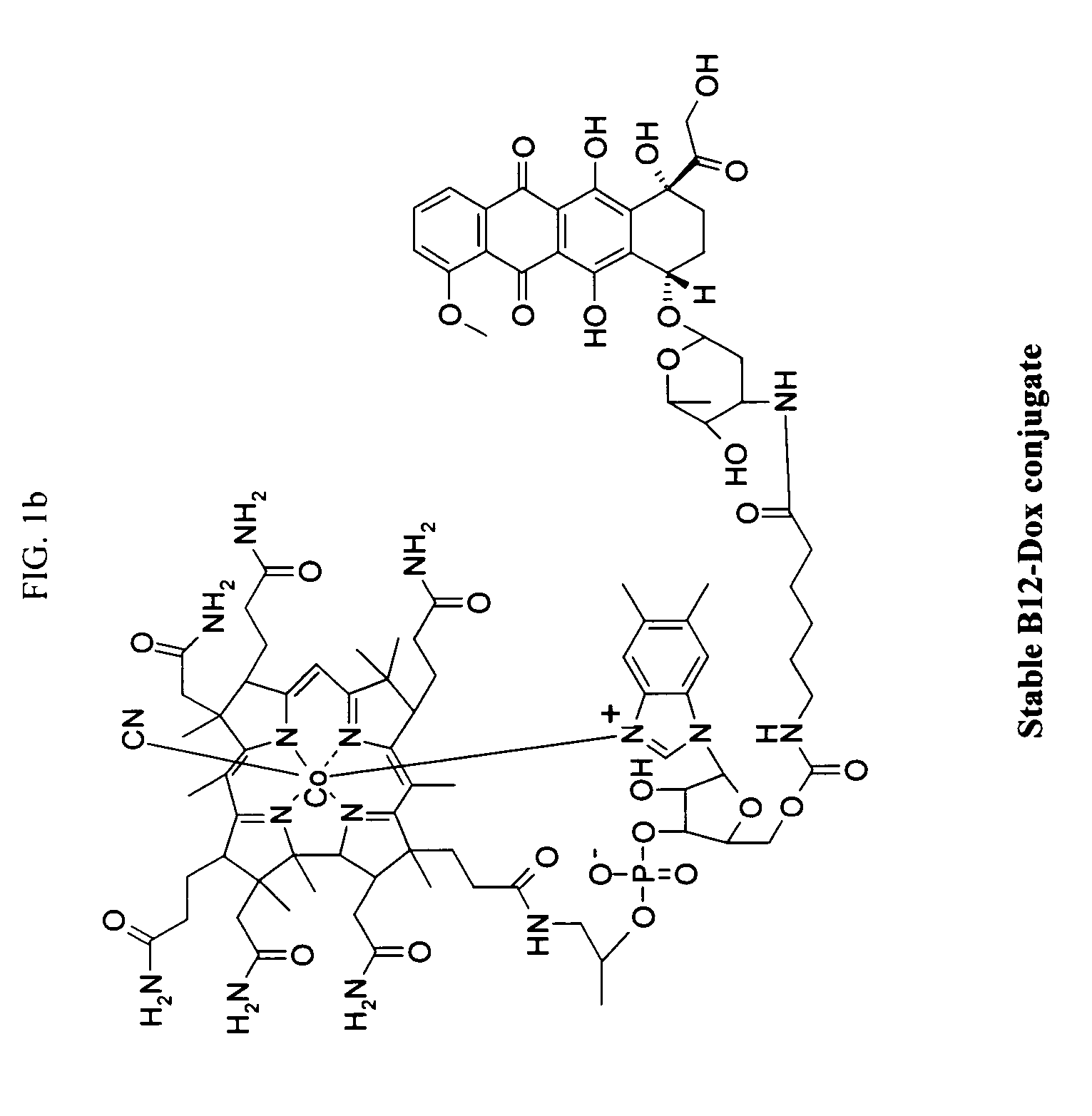
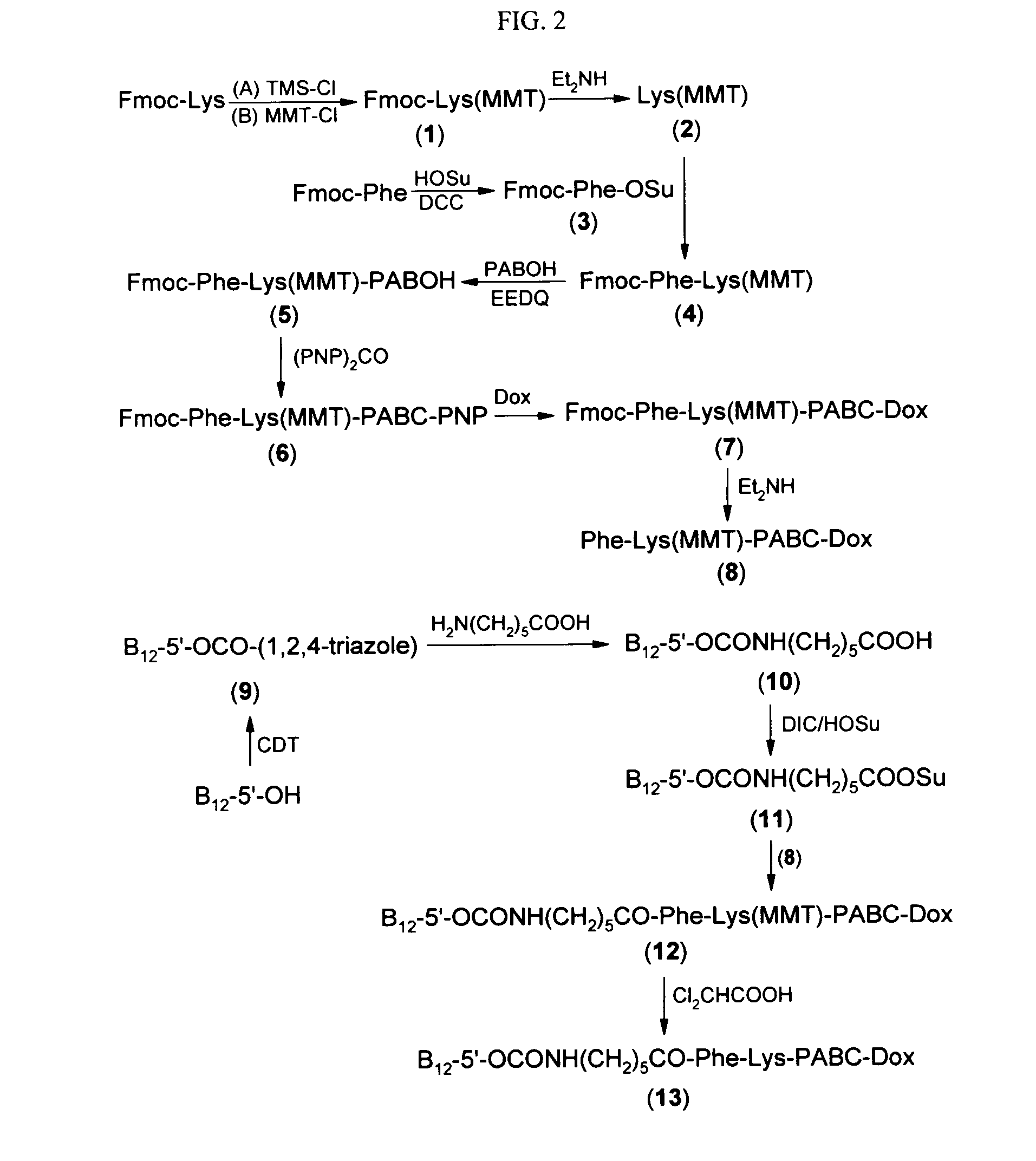
Cobalamin conjugates for anti-tumor therapy
The present invention provides a cobalamin-drug conjugate suitable for the treatment of tumor related diseases. Cobalamin is indirectly covalently bound to an anti-tumor drug via a cleavable linker and one or more optional spacers. Cobalamin is covalently bound to a first spacer or the cleavable linker via the 5′-OH of the cobalamin ribose ring. The drug is bound to a second spacer of the cleavable linker via an existing or added functional group on the drug. After administration, the conjugate forms a complex with transcobalamin (any of its isoforms). The complex then binds to a receptor on a cell membrane and is taken up into the cell. Once in the cell, an intracellular enzyme cleaves the conjugate thereby releasing the drug. Depending upon the structure of the conjugate, a particular class or type of intracellular enzyme affects the cleavage. Due to the high demand for cobalamin in growing cells, tumor cells typically take up a higher percentage of the conjugate than do normal non-growing cells. The conjugate of the invention advantageously provides a reduced systemic toxicity and enhanced efficacy as compared to a corresponding free drug.
Owner:INFLABLOC PHARMA
Process for preparing purified drug conjugates
ActiveUS20070048314A1High purityImprove stabilityPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsCell bindingOrganic chemistry
Owner:IMMUNOGEN INC
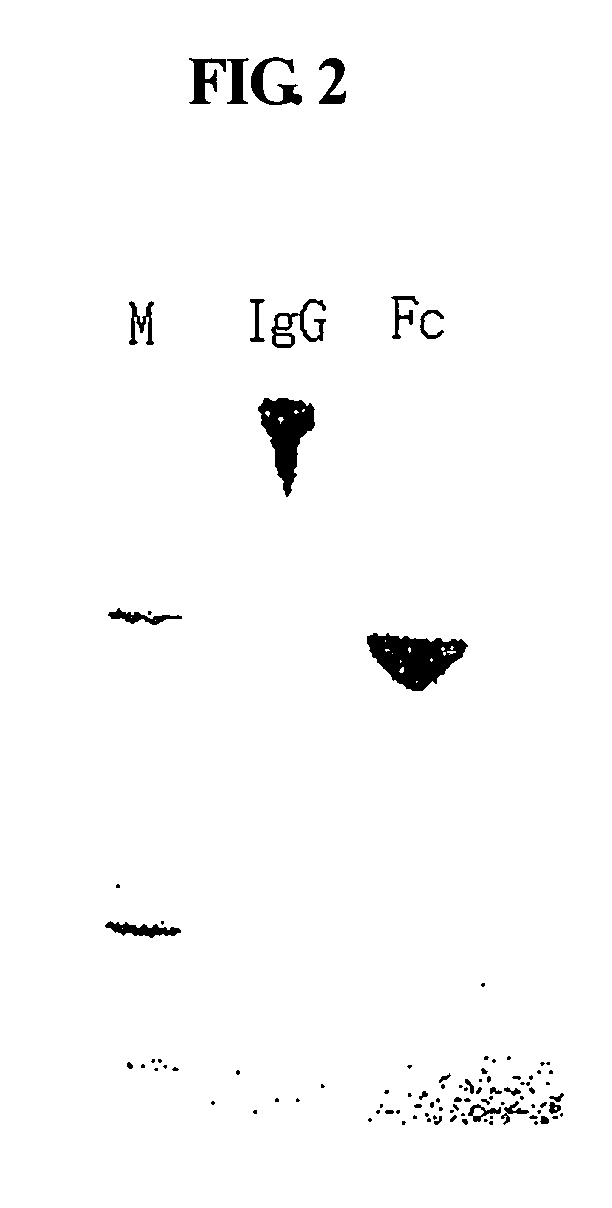
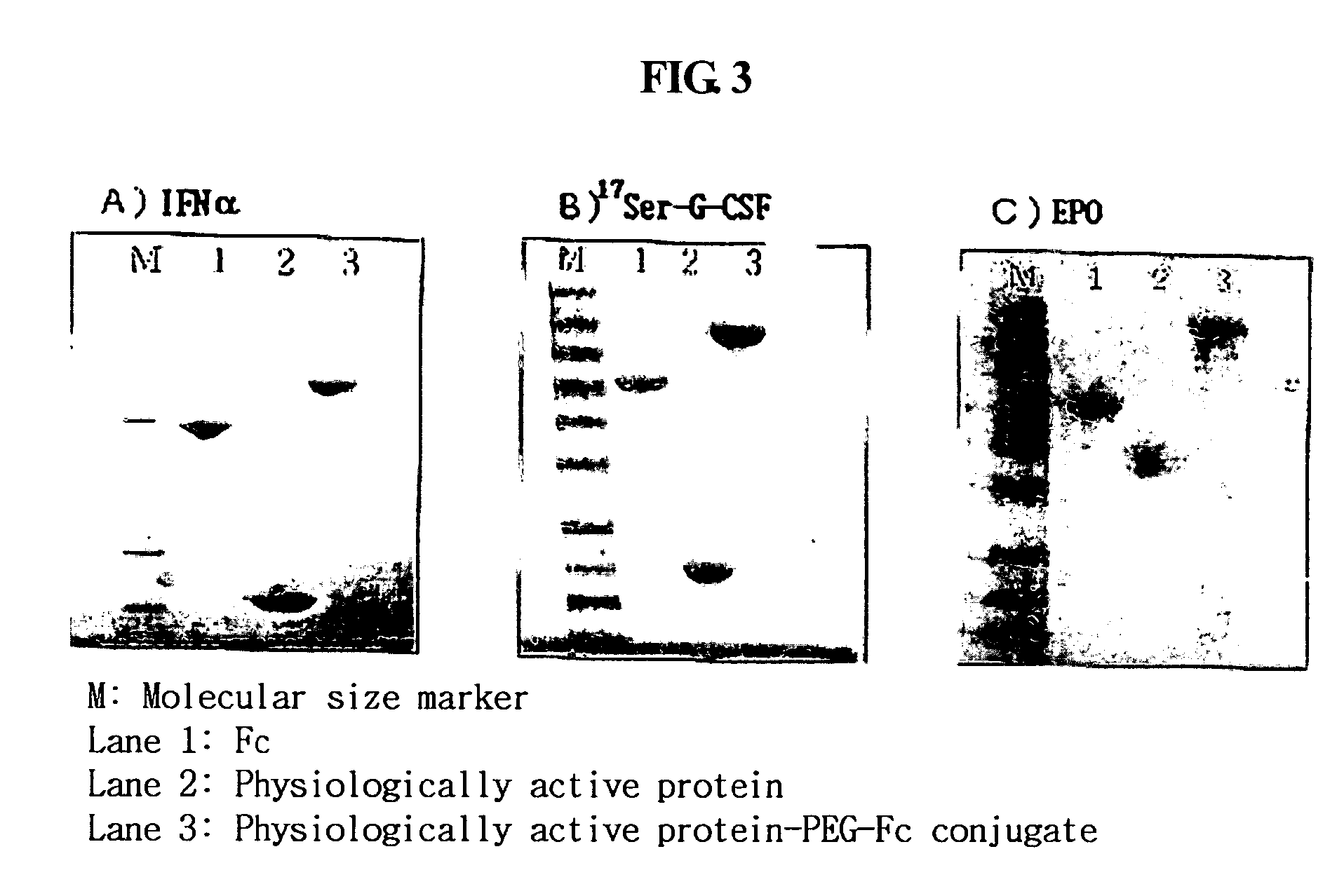
Protein complex using an immunoglobulin fragment and method for the preparation thereof
ActiveUS20060269553A1Improve stabilityExtended durationAntibody mimetics/scaffoldsImmunological disordersHalf-lifeImmunoglobulin Fc Fragments
Disclosed are a protein conjugate with improved in vivo duration and stability and the use thereof. The protein conjugate includes a physiologically active polypeptide, a non-peptide polymer and an immunoglobulin Fc fragment. Since the three components are covalently linked, the protein conjugate has extended in vivo duration and enhanced stability for the physiologically active polypeptide. The protein conjugate maintains the in vivo activity at relatively high levels and remarkably increases the serum half-life for the physiologically active polypeptide, with less risk of inducing undesirable immune responses. Thus, the protein conjugate is useful for developing long-acting formulations of various polypeptide drugs.
Owner:HANMI SCI CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com