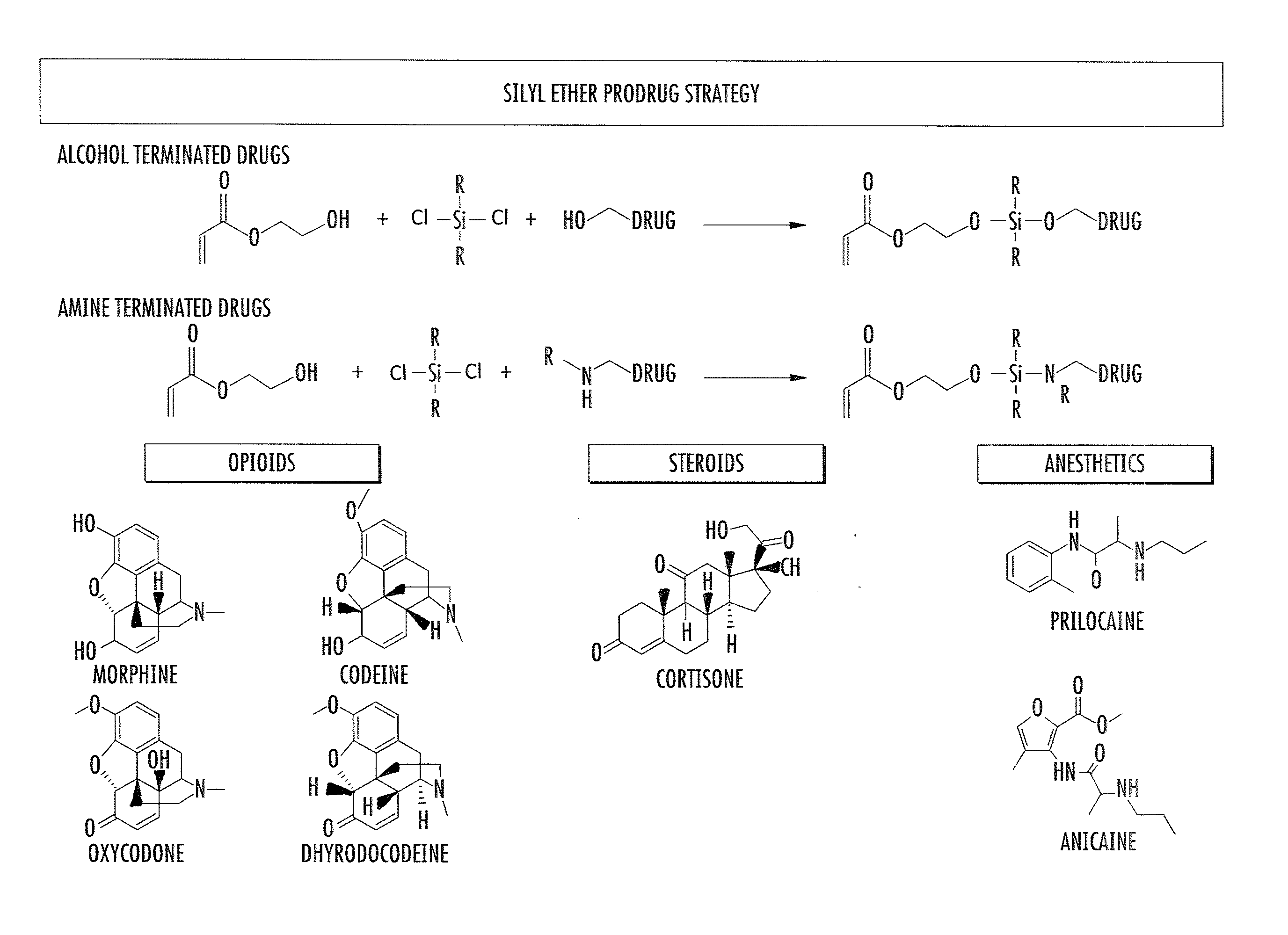
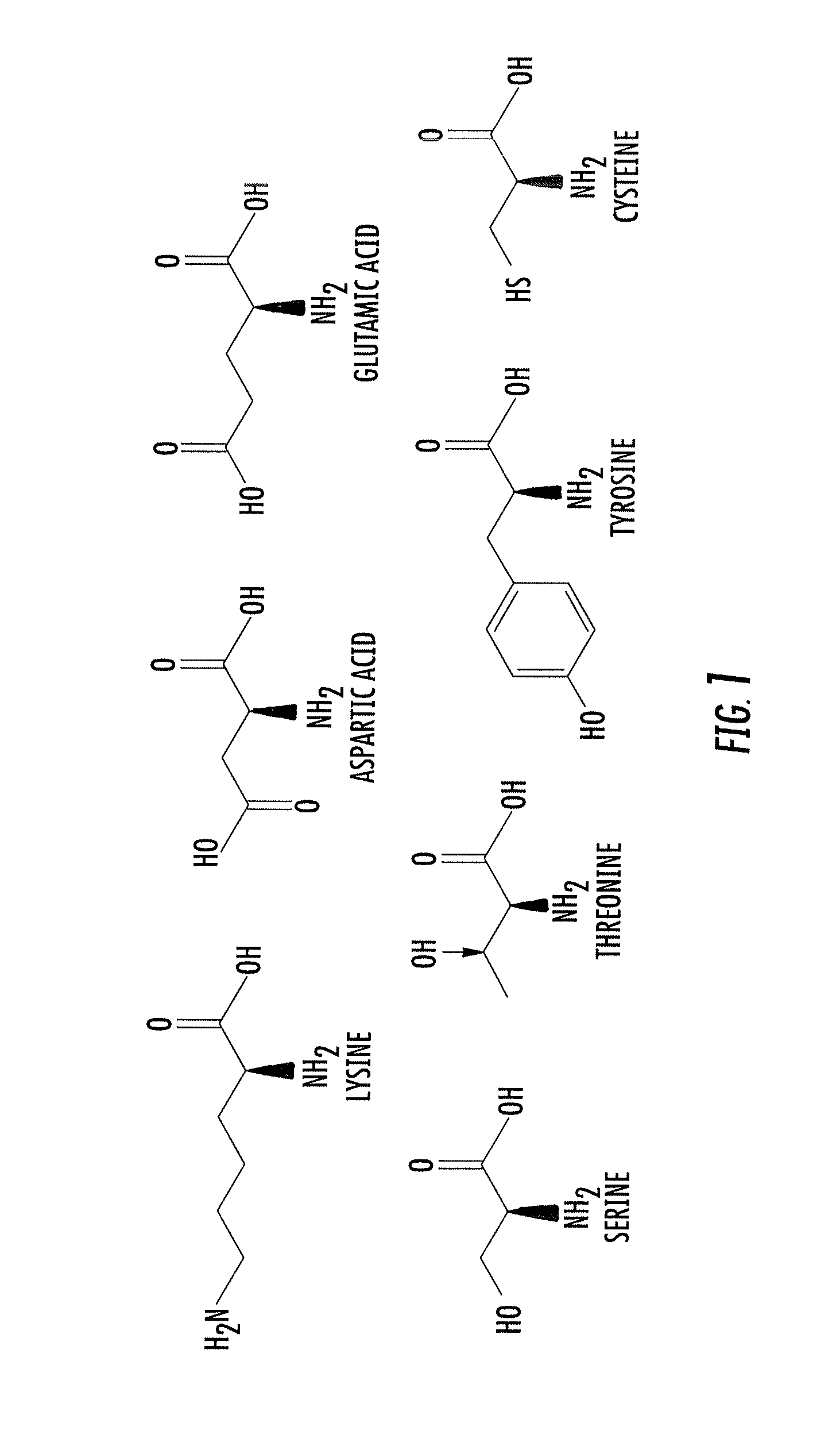
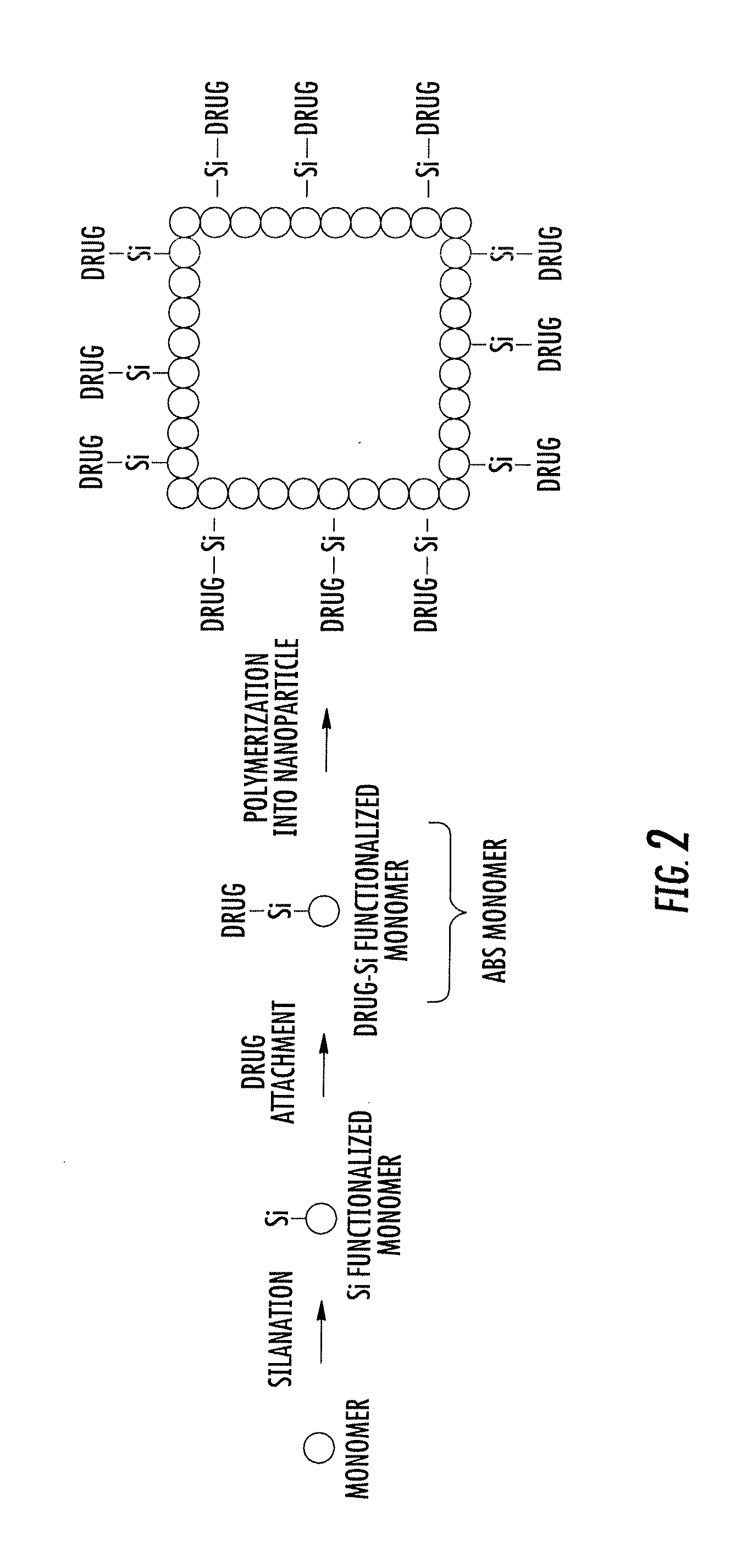
Asymmetric biofunctional silyl monomers and particles thereof as prodrugs and delivery vehicles for pharmaceutical, chemical and biological agents
a technology of biofunctional silyl monomers and prodrugs, applied in the direction of dna/rna fragmentation, silicon organic compounds, powder delivery, etc., can solve the problems of high toxicity to normal tissues, cellular delivery of various therapeutic compounds such as chemotherapeutic agents, and high restriction of the trafficking of many compounds into living cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
1. Reversible Lipidization of Particles
[0137]Chlorosilane lipids are used to “lipidize” the surface of a particle or liposome. Chemical modification by lipidization could improve oral bioavailability, minimize enzymatic degradation of the particle and / or its cargo, alter the circulation profiles of the nanoparticles, alter the biodistribution profile, alter the hydrophilic / hydrophobic character of the drug / cargo and / or change the charge of the drug until the particle or liposome contacts a pH range whereby the covalent Si linker is cleaved and the parent cargo / drug becomes available. The reversible nature of the attachment of the lipid allows for the loss of the lipid under controlled conditions. Once the particle has reached the target, e.g., the tumor, tissue, organ, cell of interest, etc., the lipid can be cleaved under slightly acidic conditions, that allows for better accessibility and easier release of the drug. In preferred embodiments, a drug as described elsewhere herein is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wt. % | aaaaa | aaaaa |
| wt. % | aaaaa | aaaaa |
| wt. % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com