Injectable parenteral medicinal preparation of temozolomide and preparation method thereof
A technology of external drug, temozolomide, which is applied in the field of parenteral pharmaceutical preparations, can solve the problems of patients doubting the quality of drugs, reducing patient compliance and acceptance, and achieve the convenience of clinical medication, reducing the time of thermal degradation and increasing stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Pharmaceutical preparation of the present invention is usually made through the following steps:
[0058] 1. Weigh the prescribed amount of stabilizer, wetting agent, excipient, buffer, stir and dissolve in at least one water-based diluent, the water-based diluent is about 90% of the prescribed amount, and the water temperature is controlled at 0-60°C .
[0059] 2. Weigh the prescribed amount of temozolomide, stir and dissolve in the above solution, measure the pH value of the solution after completely dissolving, and adjust the pH value of the solution with a pH regulator as required.
[0060] 3. Add the aqueous diluent to the final volume, and continue to stir the solution until the mixture is uniform.
[0061] 4. Filter sterilize the above solution.
Embodiment 2
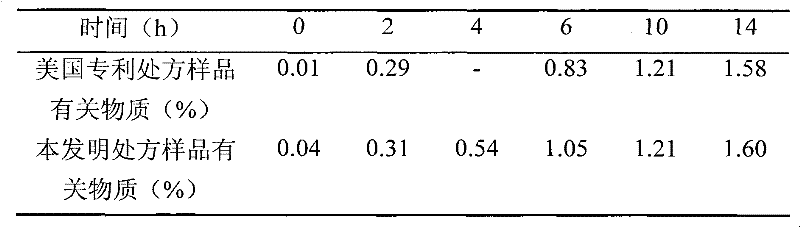
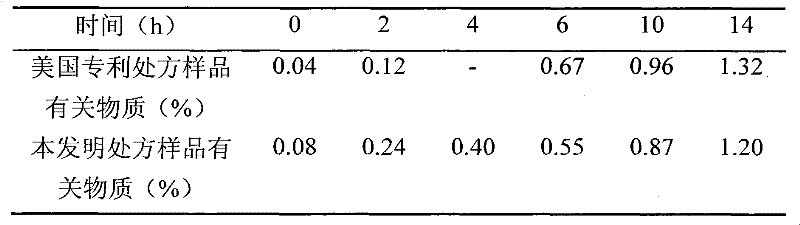
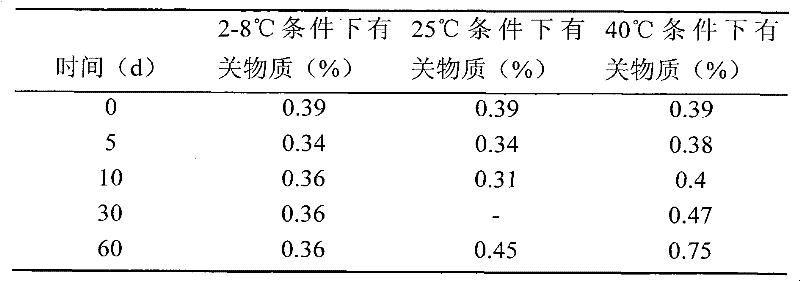
[0063] Weigh 3.00g polysorbate-80, 4.00g L-cysteine hydrochloride monohydrate, 25.00g mannitol, 9.90g acetic acid, 2.04g sodium acetate, add 900mL water for injection, and place in a water bath at 40°C Stir to dissolve, add 2.50g temozolomide (Jiangsu Hengrui Medicine Co., Ltd., the same source in the following examples), stir to dissolve, add water to 1000mL, filter with a 0.22 μm microporous membrane, sub-package, and freeze-dry to obtain temozolomide freeze-dried pink.
Embodiment 3
[0065] Weigh 3.00g polysorbate-80, 4.00g L-cysteine hydrochloride monohydrate, 15.00g mannitol, 2.35g sodium citrate dihydrate, 0.63g hydrochloric acid, add 900mL water for injection, in 40 Stir to dissolve in a water bath at ℃, add 2.50 g of temozolomide, stir to dissolve, add water for injection to 1000 mL, filter through a 0.22 μm microporous membrane, subpackage, and freeze-dry to obtain temozolomide freeze-dried powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com