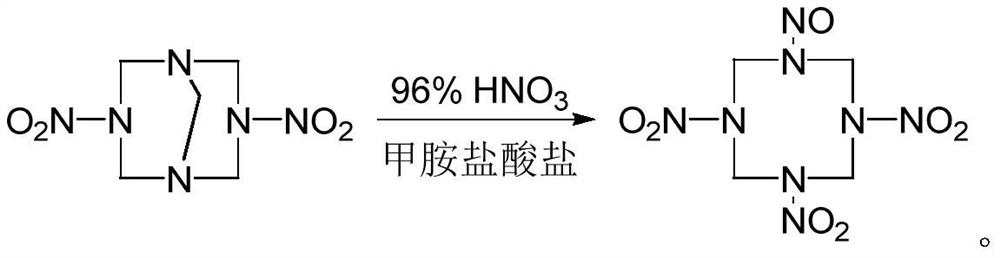
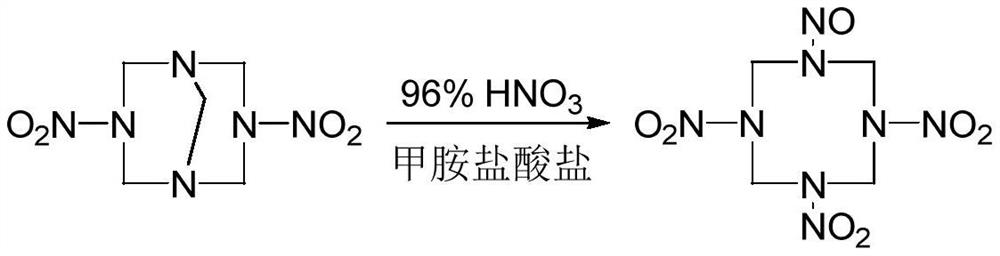
A kind of synthetic method of 1-nitroso-3,5,7-trinitro-1,3,5,7-tetraazacyclooctane
A tetraazacyclooctane and synthesis method technology, which is applied in the field of energetic material preparation, can solve problems such as low product yield, difficult separation and purification, waste acid discharge, etc., to reduce equipment requirements, reduce costs, and reduce pollution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The optimized synthesis experiment of 1-nitroso-3,5,7-trinitro-1,3,5,7-tetraazacyclooctane was carried out according to the following steps:
[0016] (1) At -25°C, add 0.78g of methylamine hydrochloride solid to 8.0mL of 96% HNO 3 After it dissolves, add 1g of DPT and react for 5min at the changed temperature.
[0017] (2) Slowly add 20mL of water dropwise, control the temperature of the system not to exceed 0°C, filter, wash with water, and dry to obtain 1-nitroso-3,5,7-trinitro-1,3,5,7-tetraaza Cyclooctane 1.0g, the yield is 78.5% based on DPT.
Embodiment 2
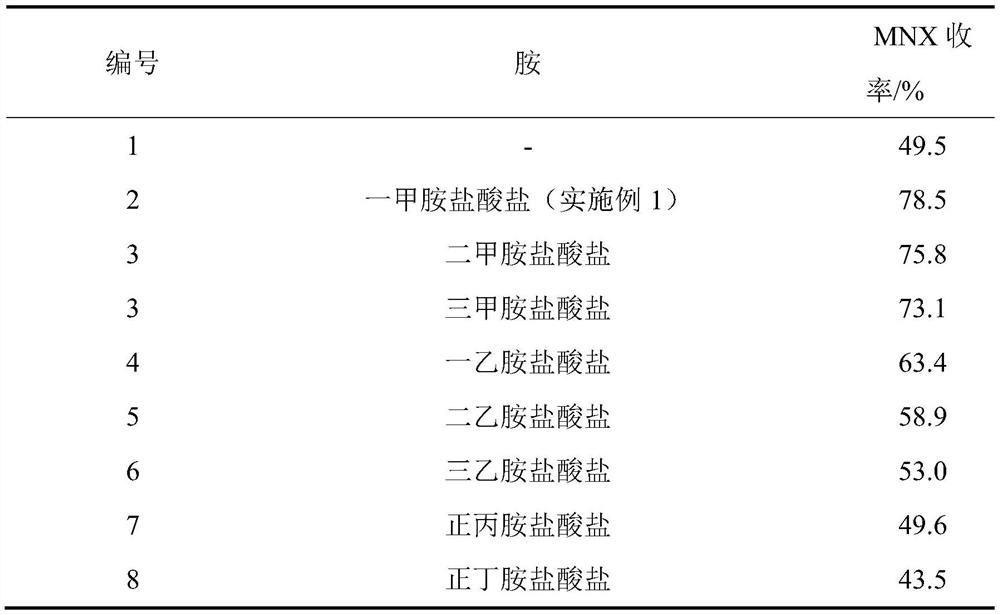
[0019] Other operating conditions and steps are the same as in Example 1, and the influence of the hydrochloride of different amines on the reaction is checked, and the results are shown in Table 1.
[0020] Table 1. Effect of hydrochloride salts of different amines on MNX yield
[0021]
[0022] As can be seen from the above-mentioned results, methylamine hydrochloride is the most favorable to this reaction, and even other hydrochlorides of its close structural amines are far inferior to it (embodiment 1) to the promoting effect of reaction.
Embodiment 3
[0024] Other operating conditions and steps are the same as in Example 1, and the impact of the amount of methylamine hydrochloride added on the reaction is checked, and the results are shown in Table 2.
[0025] Table 2. The impact of the amount of methylamine hydrochloride added on the yield of MNX.
[0026]
[0027]
[0028] As can be seen from the above results, the methylamine hydrochloride add-on is preferably 0.78g (embodiment 1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com