Patents
Literature
135 results about "Azabicyclane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Anazocine (INN; also known as azabicyclane or CS-307) is an opioid analgesic of the morphan/benzomorphan family developed in the middle 1960s in the United States which was never marketed. It is listed by some sources as a teratogen.
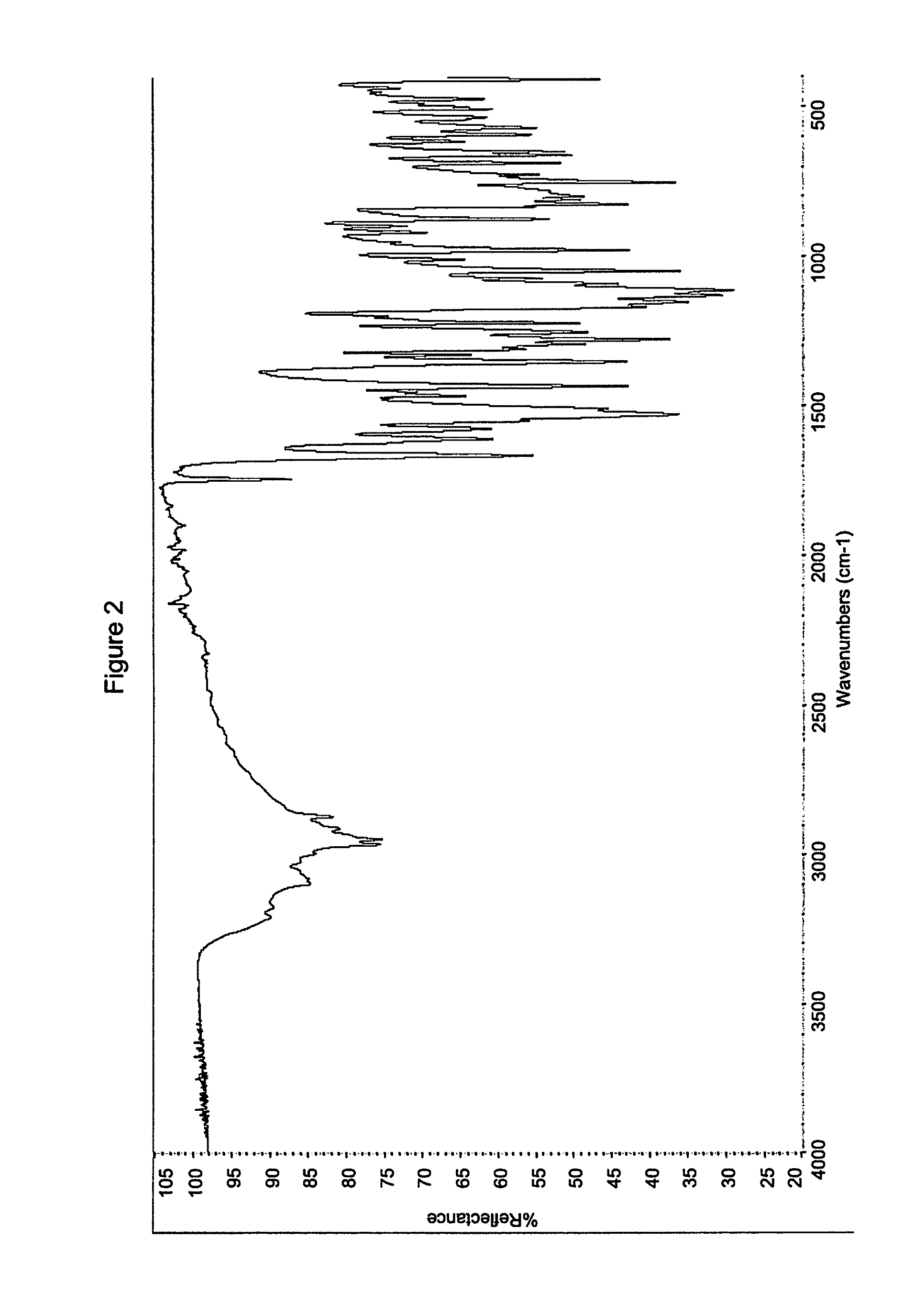
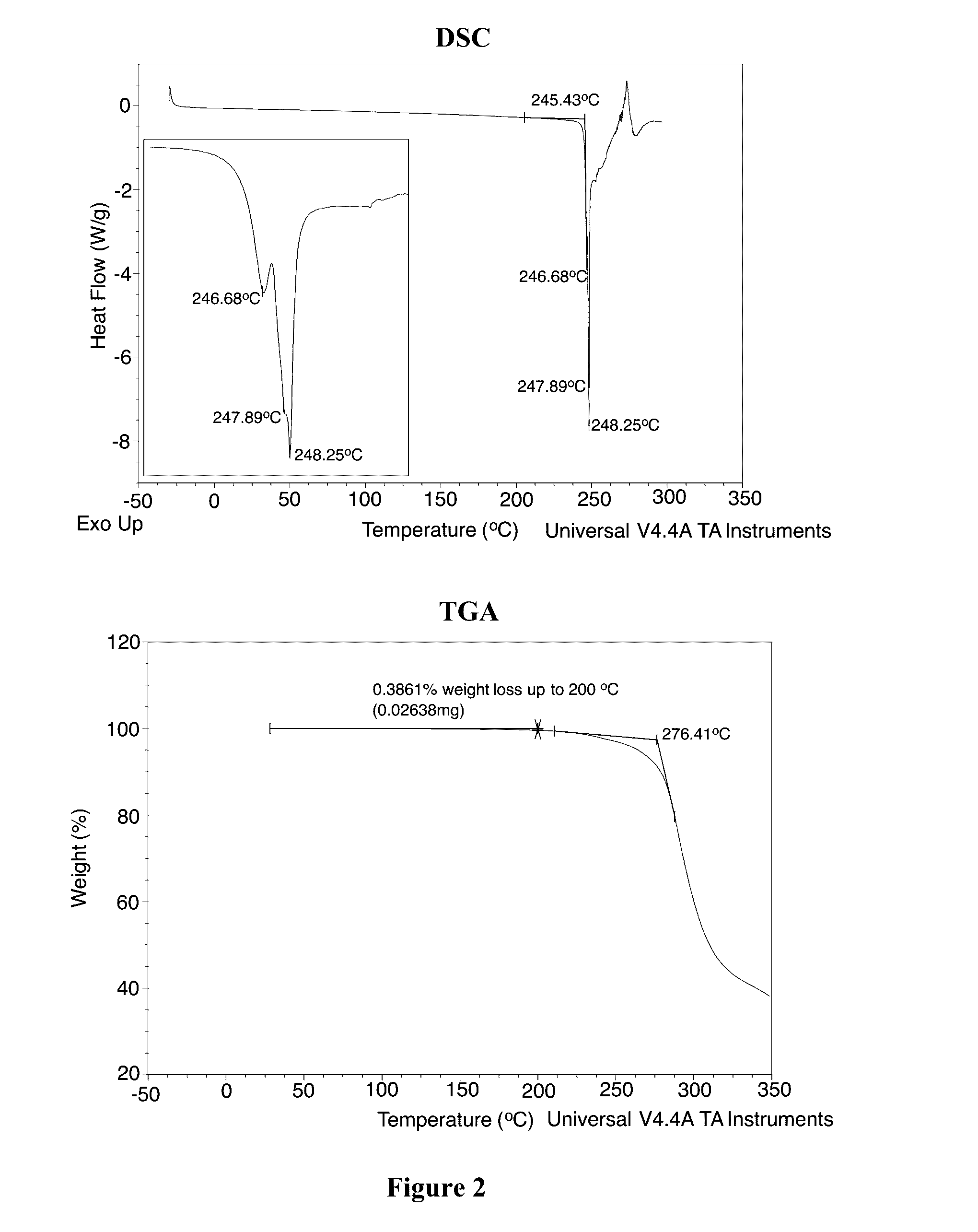
Solid Forms of N-(4-(7-Azabicyclo[2.2.1]Heptan-7-yl)-2-Trifluoromethyl)Phenyl)-4-Oxo-5-(Trifluoromethyl)-1,4-Dihydroquinoline-3-Carboxamide
The present invention relates to substantially crystalline and solid state forms of N-(4-(7-azabicyclo[2.2.1]heptan-7-yl)-2-(trifluoromethyl)phenyl)-4-oxo-5-(trifluoromethyl)-1,4-dihydroquinoline-3-carboxamide (Form A-HCl, Form B, Form B-HCl, or any combination of these forms), pharmaceutical compositions thereof, and methods of treatment therewith.
Owner:VERTEX PHARMA INC
Process for Preparing Modulators of Cystic Fibrosis Transmembrane Conductance Regulator
ActiveUS20110124869A1Senses disorderNervous disorderMedicineCystic fibrosis transmembrane conductance regulator
The present invention relates to processes for preparing solid state forms of N-(4-(7-azabicyclo[2.2.1]heptan-7-yl)-2-(trifluoromethyl)phenyl)-4-oxo-5-(trifluoromethyl)-1,4-dihydroquinoline-3-carboxamide, including Compound 1 Form A, Compound 1 Form A-HCl, Compound 1 Form B, and Compound 1 Form B-HCl, any combination of these forms, pharmaceutical compositions thereof, and methods of treatment therewith.
Owner:VERTEX PHARMA INC
Oral administration of [2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)alkyl] phosphonic acid and derivatives
InactiveUS20050142192A1Improve oral bioavailabilityBiocideSenses disorderTolerabilitySchizophreniform psychosis
Solid, pharmaceutical dosage forms of [2-(8,9-dioxo-2,6-diazabicyclo [5.2.0]non-1(7)-en-2-yl)alkyl]phosphonic acid and derivatives thereof are disclosed. In addition, methods of use are disclosed for the treatment, inter alia, of cerebral vascular disorders, anxiety disorders; mood disorders; schizophrenia; schizophreniform disorder; schizoaffective disorder; cognitive impairment; chronic neurodegenerative disorders; inflammatory diseases; fibromyalgia; complications from herpes zoster; prevention of tolerance to opiate analgesia; withdrawal symptoms from addictive drugs; and pain.
Owner:WYETH LLC
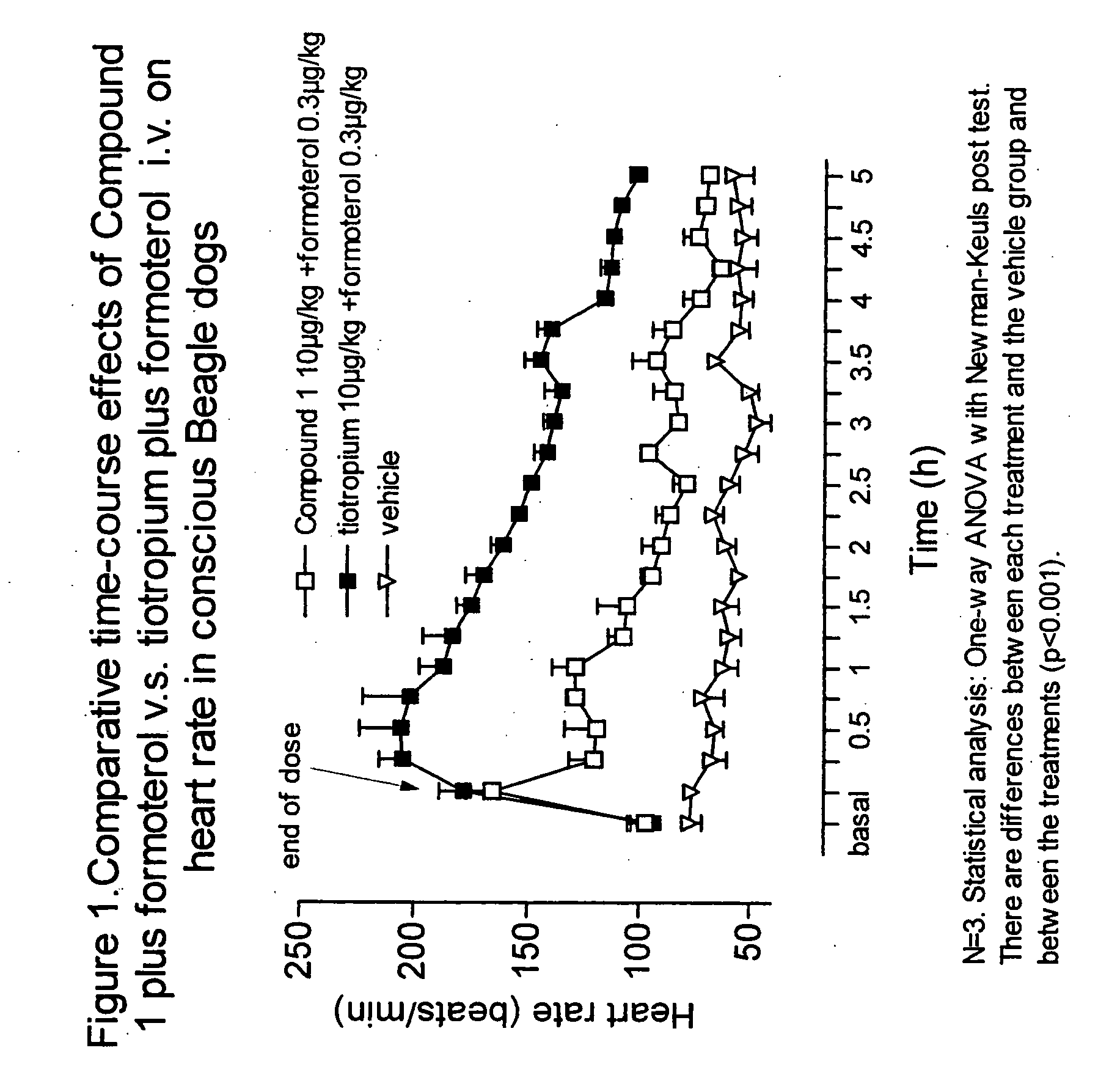
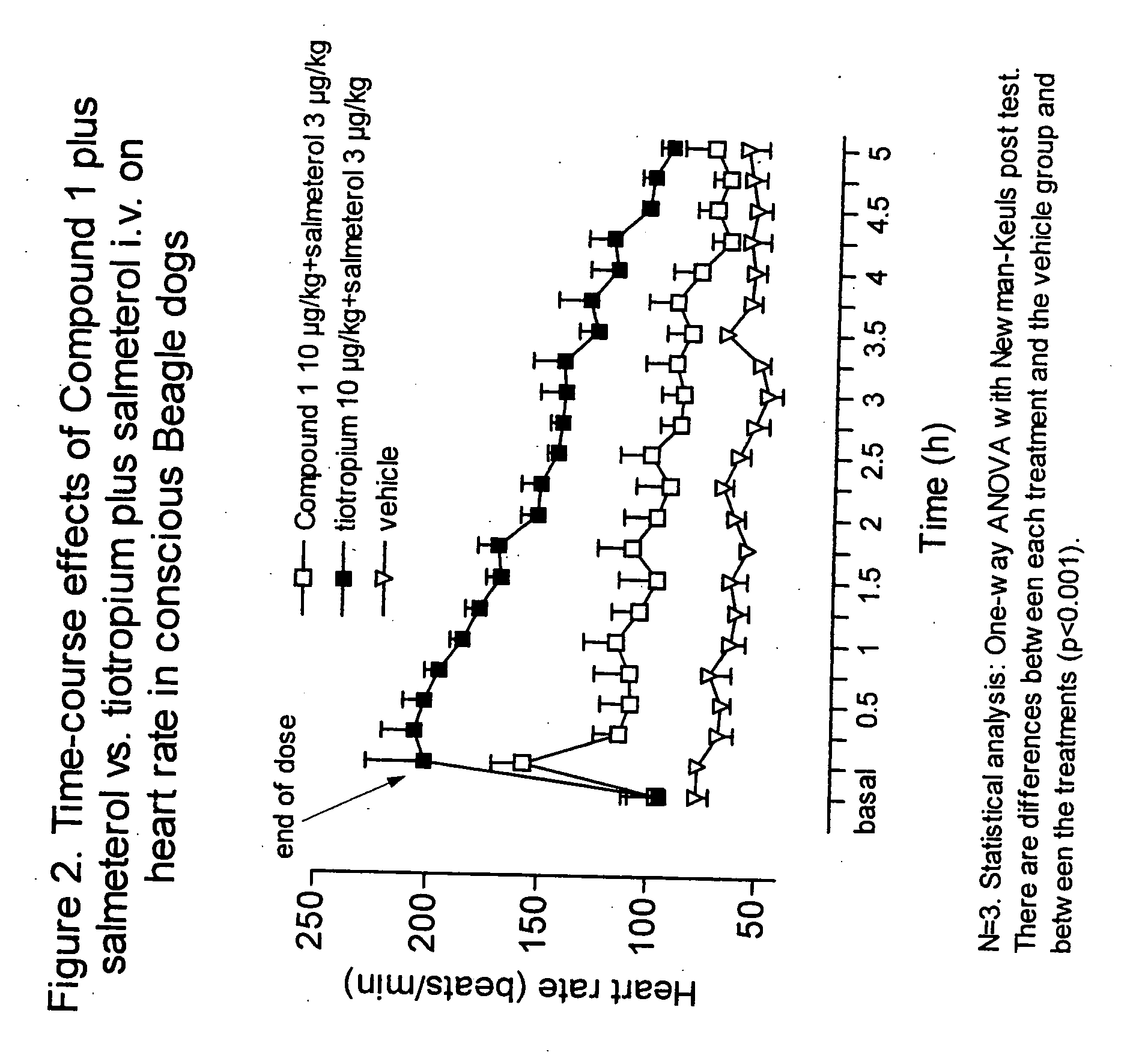
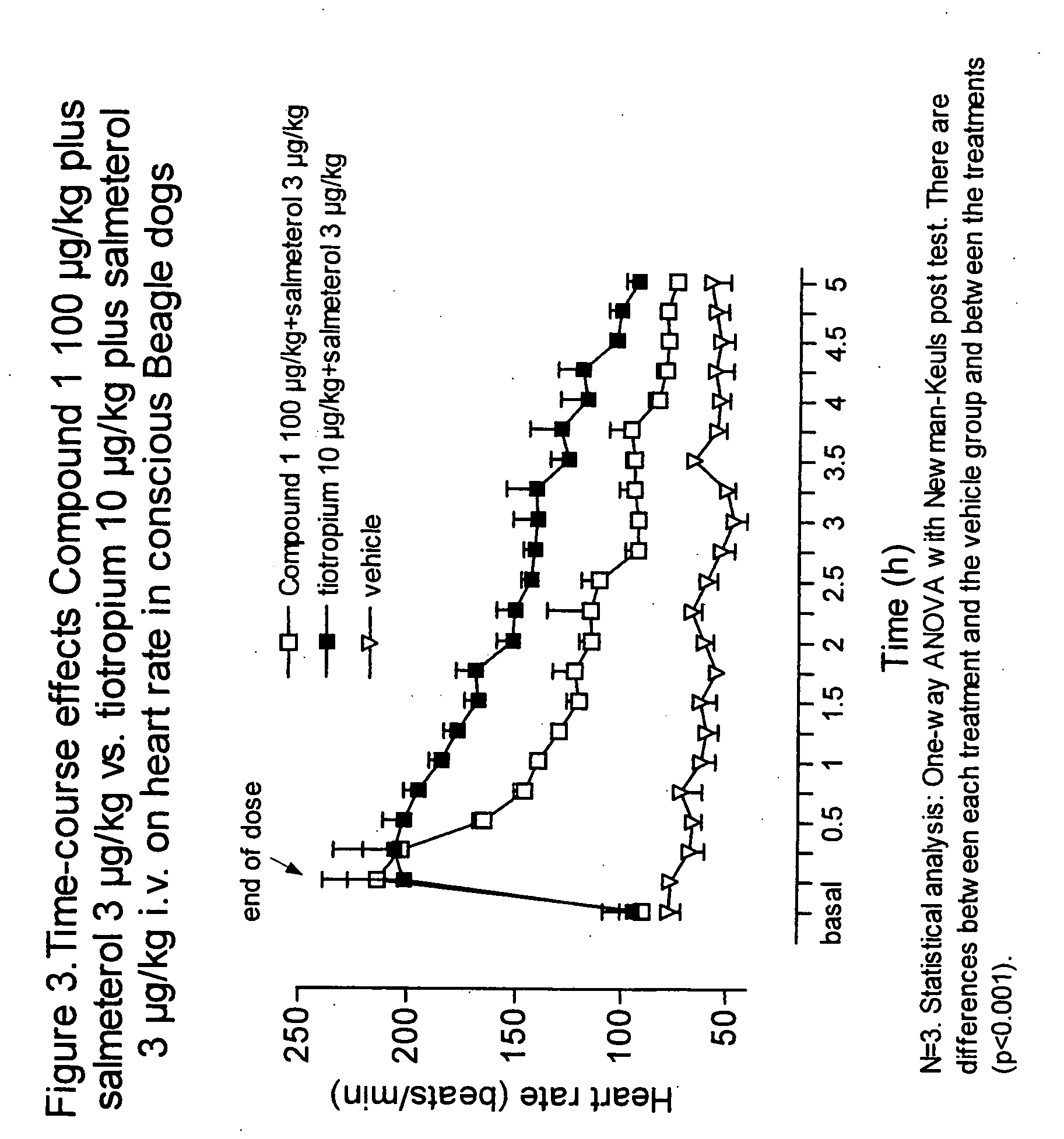
Combinations comprising antimuscarinic agents and beta-adrenergic agonists
Combinations comprising (a) a β2 agonist and (b) an antagonist of M3 muscarinic receptors which is 3(R)-(2-hydroxy-2,2-dithien-2-ylacetoxy)-1-(3-phenoxypropyl)-1-azoniabicyclo[2.2.2]octane, in the form of a salt having an anion X, which is a pharmaceutically acceptable anion of a mono or polyvalent acid are useful, e.g., for the treatment of respiratory disease, e.g., asthma or chronic obstructive pulmonary disease.
Owner:ALMIRALL
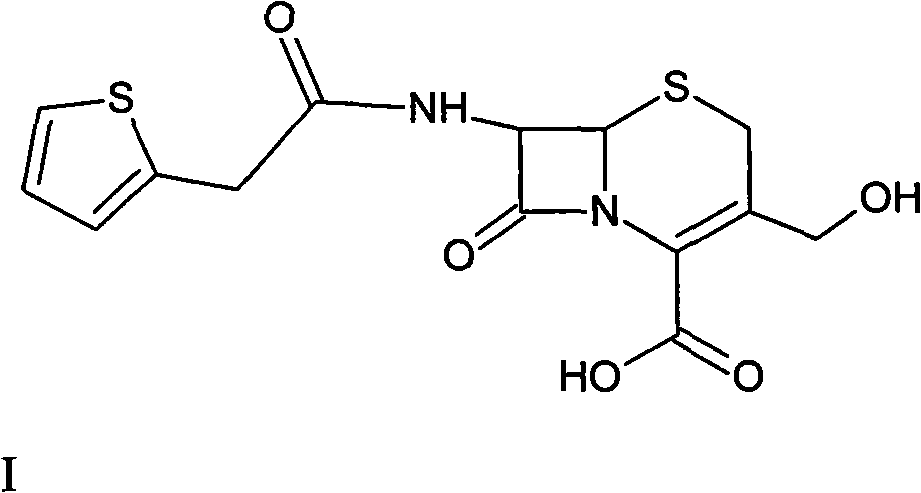
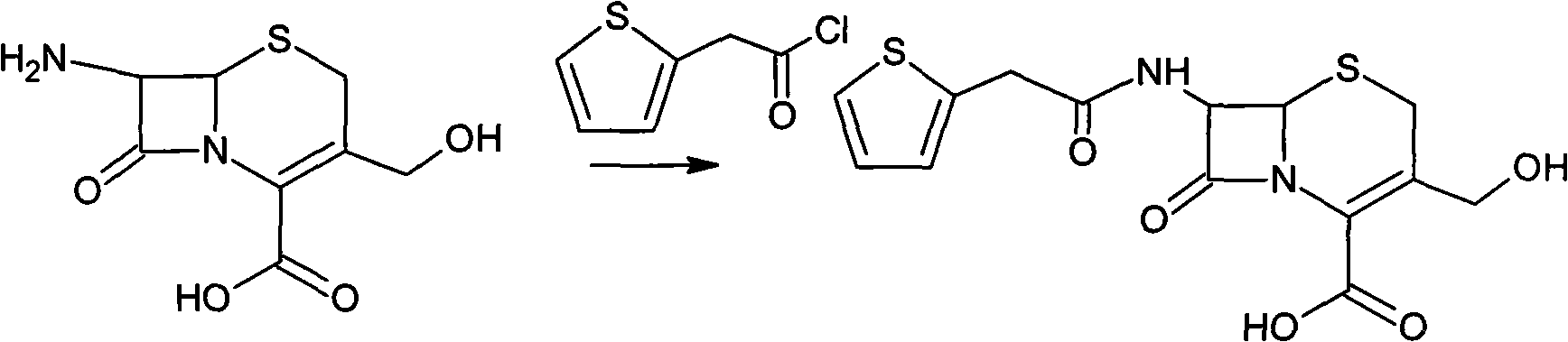
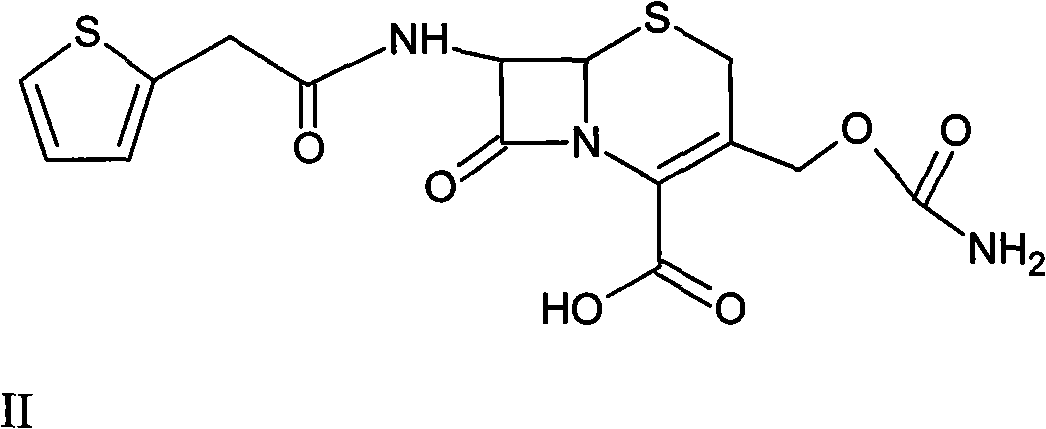
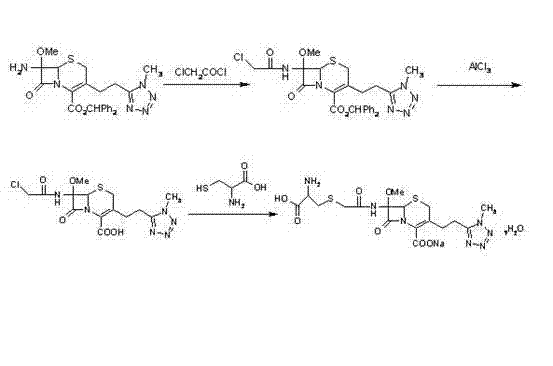
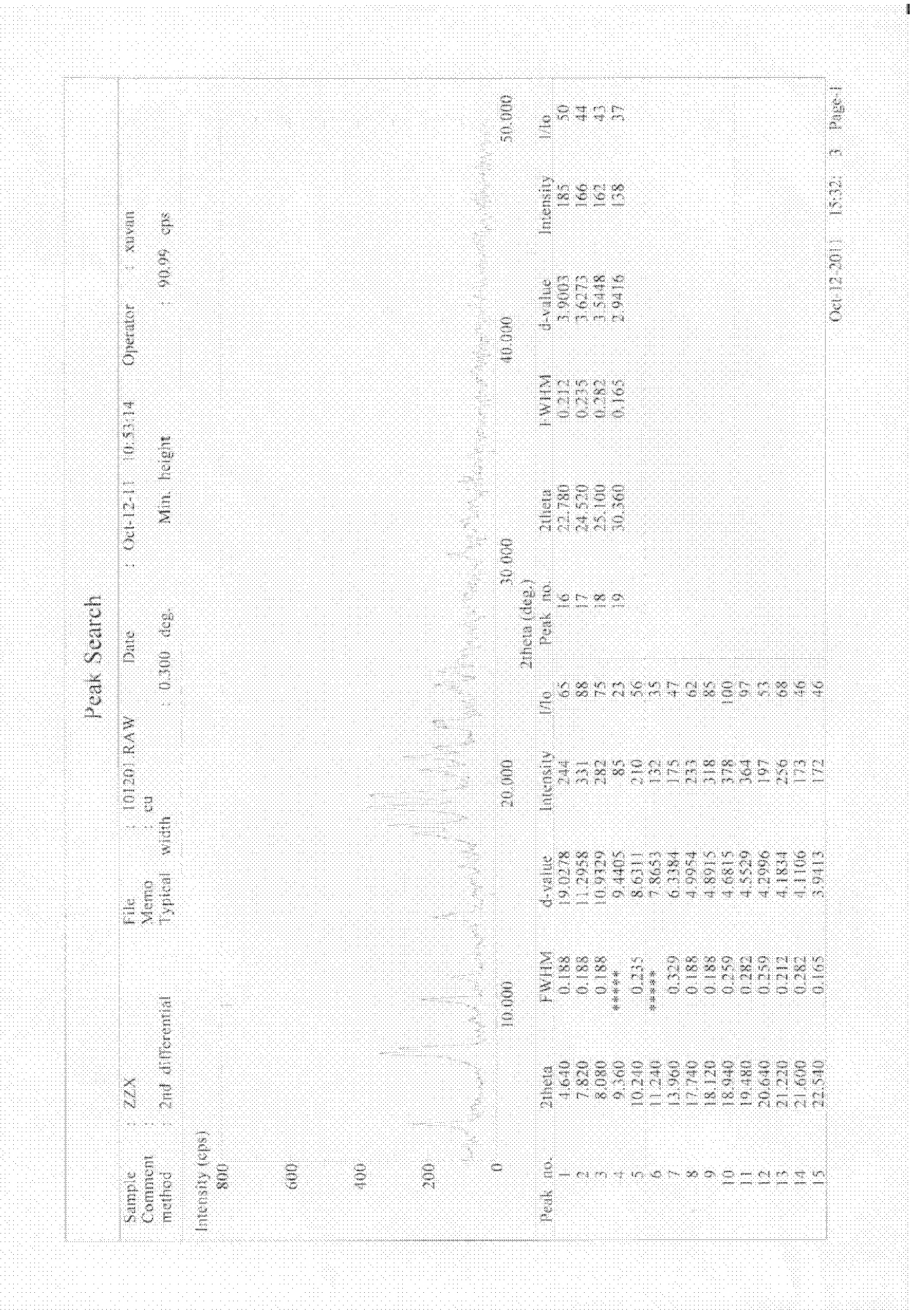
Method for preparing cefoxitin sodium
ActiveCN101613361ASimplify production stepsHigh yieldOrganic chemistryAntiinfectivesLithium methoxideOrganic solvent
The invention provides a method for preparing cefoxitin sodium, which comprises the following steps: 1, using deacetyl7-ACA as a raw material, and introducing thiopheneacetyls to obtain an deacetylthiophene acid I; 2, introducing carbamoymethoxyls to the positions 3 of molecules of deacetylthiophene acid I to obtain an intermediate of (6R, 7S)-3-carbamoymethoxyl-7-[2-(2-theiophene) acetamino]-8-oxo-5-thia-1-azabicyclo[4.2.0]octa-2-en-2-methanoic acid II; 3, reacting the intermediate II with lithium methoxide to introduce methoxyls into positions 7 of the molecules of the intermediate to obtain cefoxitin acid III; and 4, adding ethanol solution of a sodium salt into an organic solvent containing the cefoxitin acid and obtaining the cefoxitin sodium IV through suction filtration and drying.
Owner:哈药集团股份有限公司 +1
Derivatives of 3-Azabicyclo[3.1.0] Hexane as Dipeptidyl Peptidase-IV Inhibitors
InactiveUS20080300251A1Avoid chemical reactionsBiocideNervous disorderDipeptidyl peptidasePrediabetes
The present invention relates to novel 3-azabicyclo[3.1.0]hexane derivatives as dipeptidyl peptidase-IV inhibitors and the processes for the synthesis of the said compounds. This invention also relates to pharmacological compositions containing the compounds of the present invention, and methods of treating diabetes, especially type 2 diabetes, as well as prediabetes, diabetic dyslipidemia, metabolic acidosis, ketosis, satiety disorders, and obesity. These inhibitors can also be used to treat conditions manifested by a variety of metabolic, neurological, anti-inflammatory, and autoimmune disorders like inflammatory disease, multiple sclerosis, rheumatoid arthritis; viral, cancer and gastrointestinal disorders. The compounds of this invention can also be used for treatment of infertility arising due to polycystic ovary syndrome.
Owner:RANBAXY LAB LTD
8-azabicyclo[3.2.1]octane compounds as MU opioid receptor antagonists
Owner:THERAVANCE BIOPHARMA R&D IP LLC
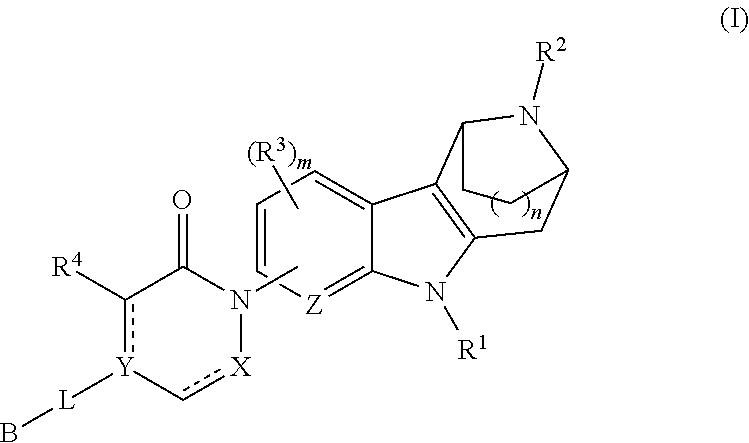
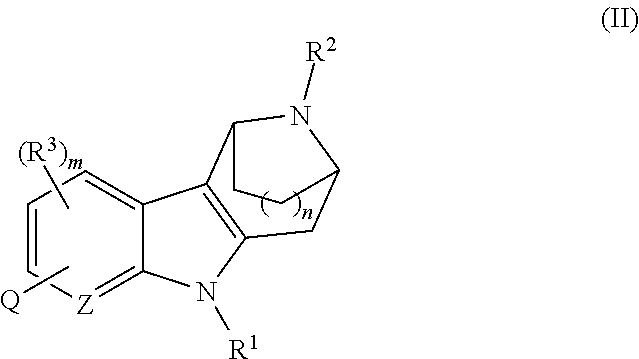
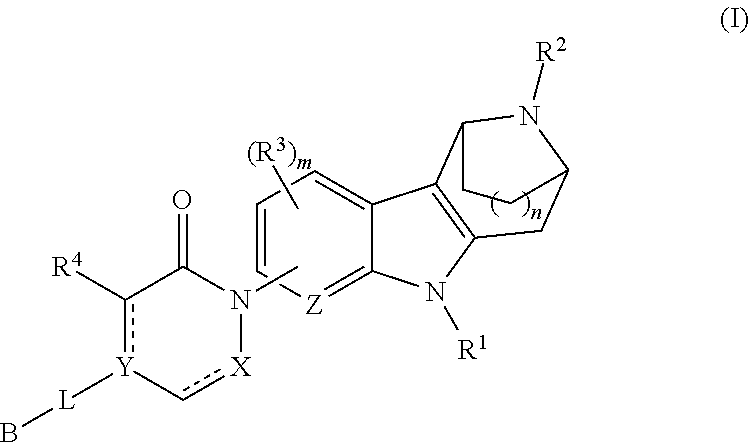
Azabicycloalkane-indole and azabicycloalkane-pyrrolo-pyridine mch-1 antagonists, methods of making, and use thereof
Novel MCH-1 receptor antagonists are disclosed. These compounds are used in the treatment of various disorders, including obesity, anxiety, depression, non-alcoholic fatty liver disease, and psychiatric disorders. Methods of making these compounds are also described in the present invention.
Owner:ALBANY MOLECULAR RESEARCH INC
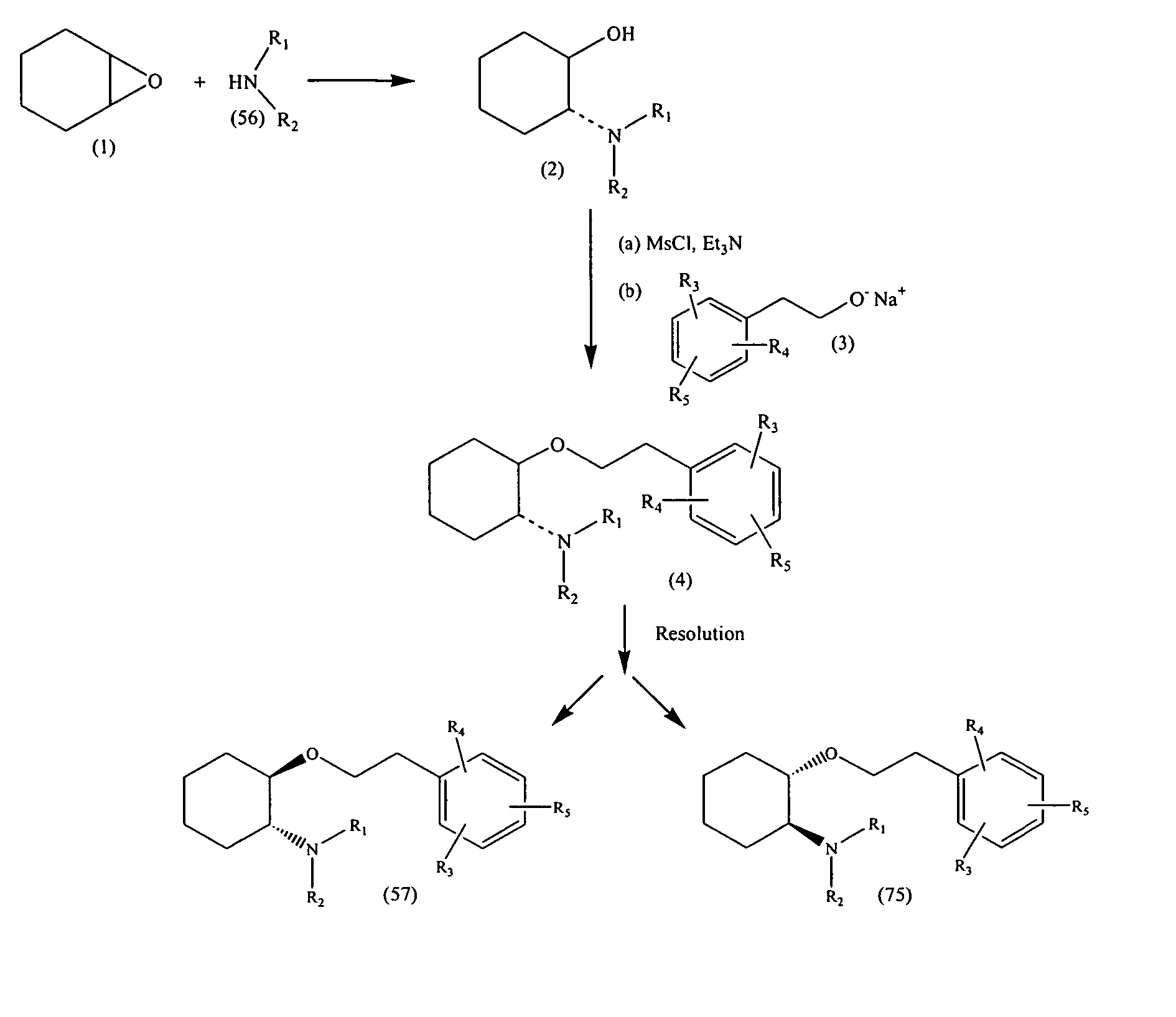
Methods and compositions for production, formulation and use of 1 aryl-3-azabicyclo[3.1.0]hexanes
The invention provides novel compositions and methods of making (−)-1-(3,4-dichlorophenyl)-3-azabicyclo[3.1.0]hexane and other 1-aryl-3-azabicyclo[3.1.0]hexanes, including synthetic methods that form novel intermediate compounds of the invention for producing)-(−)-1-(3,4-dichlorophenyl)-3-azabicyclo[3.1.0]hexane and other 1-aryl-3-azabicyclo[3.1.0]hexanes and pharmaceutically acceptable salts thereof.
Owner:DOV PHARMA
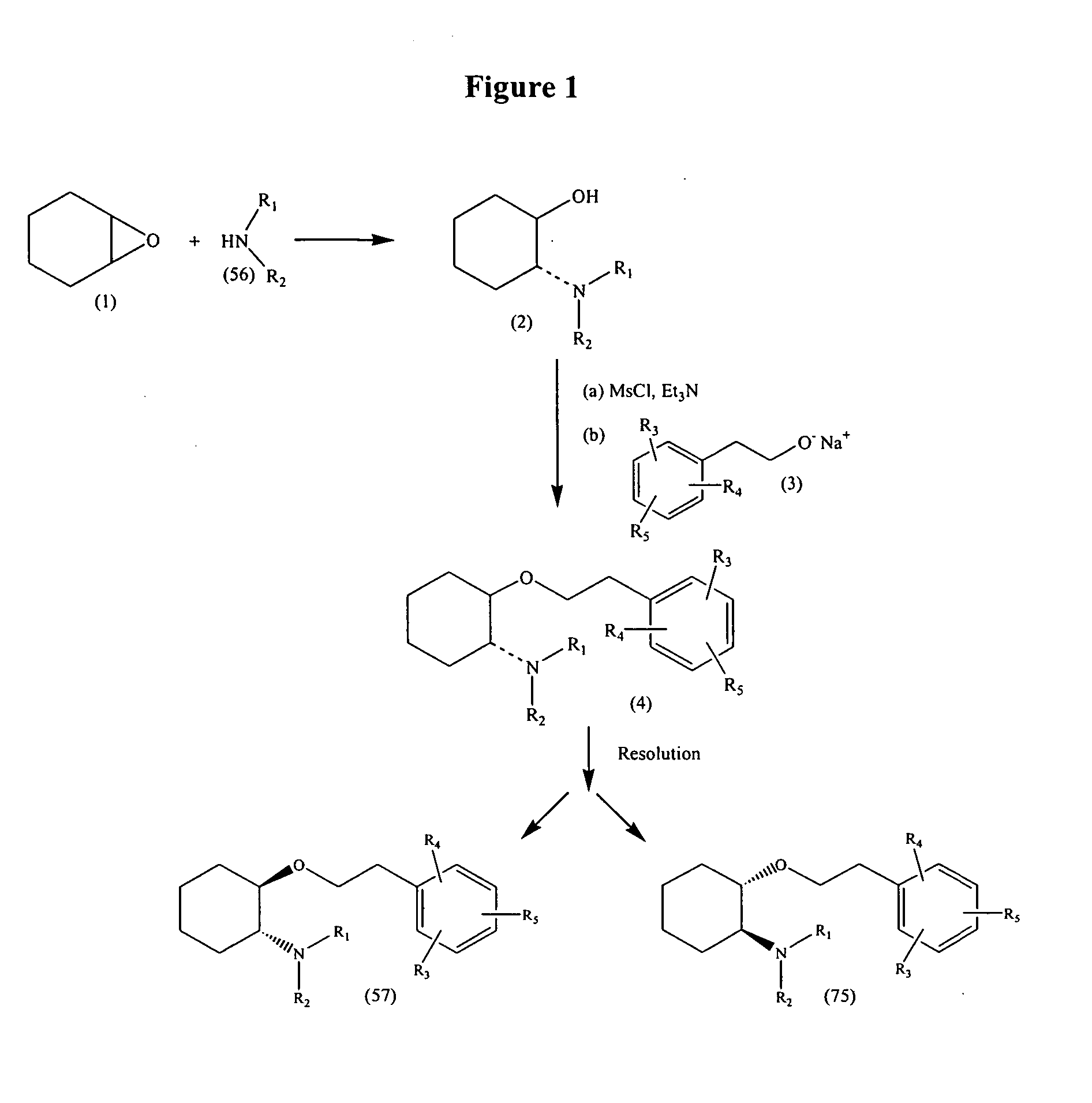
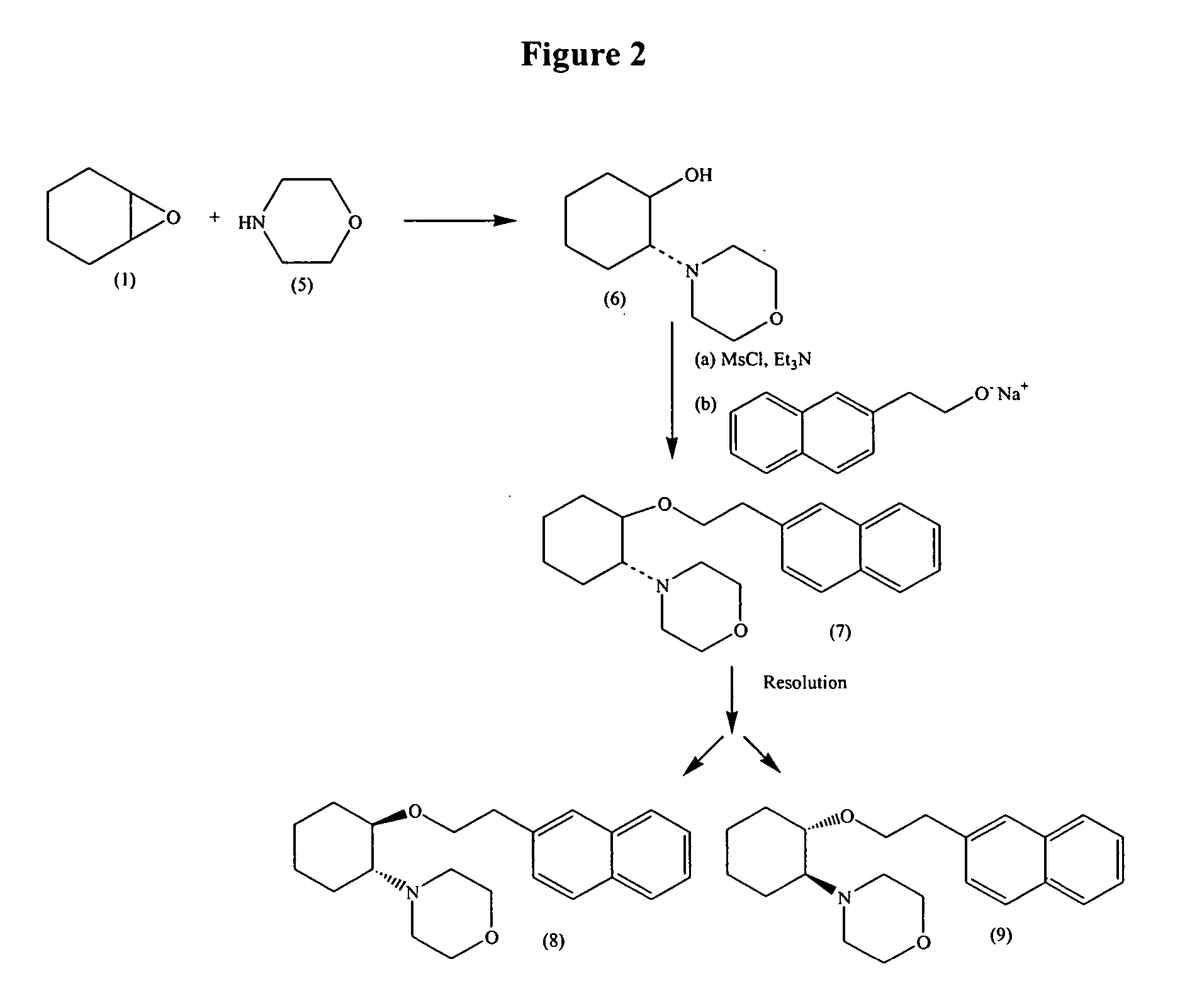
Synthetic process for trans-aminocyclohexyl ether compounds
InactiveUS20060094880A9Organic compound preparationHeterocyclic compound active ingredientsLeaving groupAcyl group
Owner:CARDIOME PHARMA CORP
8-azabicyclo[3.2.1]octyl-2-hydroxybenzamide compounds as mu opioid receptor antagonists
InactiveUS20090062333A1Reduce exerciseUseful in preparationBiocideNervous disorderOpioid receptorMu-opioid receptor activity
The invention provides 8-azabicyclo[3.2.1]octyl-2-hydroxybenzamide compounds of formula (I):wherein R2, R7, and m are defined in the specification, or a pharmaceutically-acceptable salt thereof, that are antagonists at the mu opioid receptor. The invention also provides pharmaceutical compositions comprising such compounds, methods of using such compounds to treat conditions associated with mu opioid receptor activity, and processes and intermediates useful for preparing such compounds.
Owner:THERAVANCE BIOPHARMA R&D IP LLC
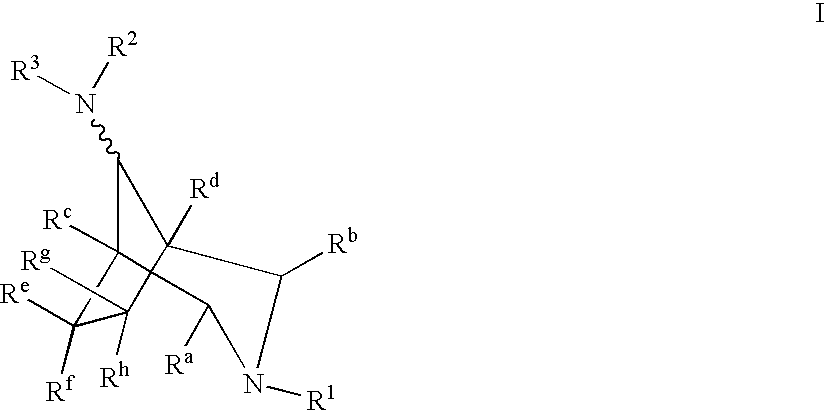
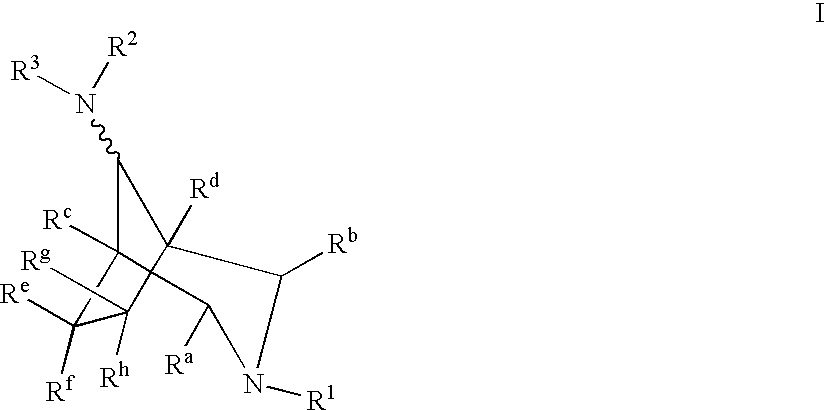
Azabicyclooctane derivatives useful in the treatment of cardiac arrhythmias
InactiveUS6559162B2Effective against cardiac arrhythmiaBiocideOrganic chemistryChemistryVentricular dysrhythmia
Owner:ASTRAZENECA AB
3-trifluoromethyl-5-tert-butoxycarbonyl-2,5-diheterobicyclo[2.2.1]heptane and preparation method thereof
InactiveCN102167700AImprove drug-like propertiesImprove medicinal propertiesOrganic chemistryTrifluoromethylSolubility
The invention discloses 3-trifluoromethyl-5-tert-butoxycarbonyl-2,5-diheterobicyclo[2.2.1]heptane and a preparation method thereof. The invention mainly solves the technical problems that when a methyl derivative is introduced to the 3-site of the 2,5-diheterobicyclo[2.2.1]heptane compound, the synthesis steps are complicated, the water solubility is poor and the total yield is low. The structural formula of the compounds is shown as below, wherein R is NH or O; when R is NH, the compound is 3-trifluoromethyl-5-tert-butoxycarbonyl-2,5-diazabicyclo[2.2.1]heptane; and when R is O, the compound is 3-trifluoromethyl-5-tert-butoxycarbonyl-2-oxa-5-azabicyclo[2.2.1]heptane. The obtained compounds are mainly used to effectively connect the pharmacophore unit.
Owner:上海药明康德新药开发有限公司 +1
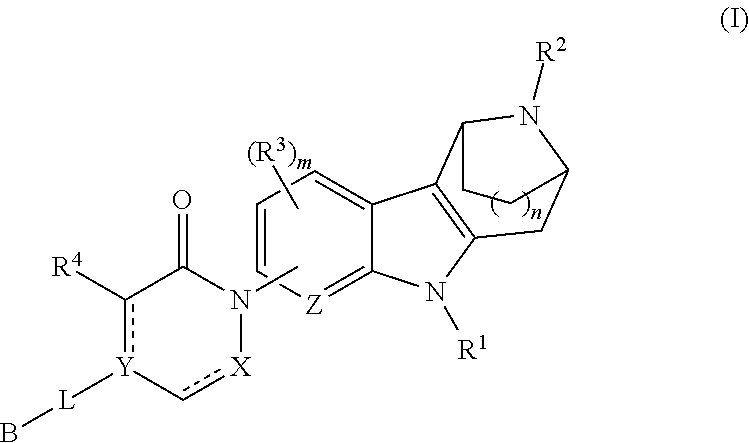
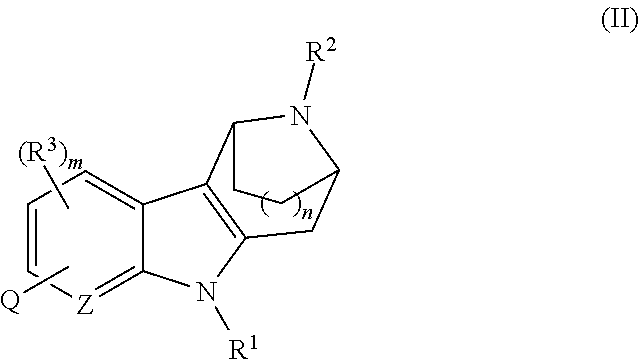
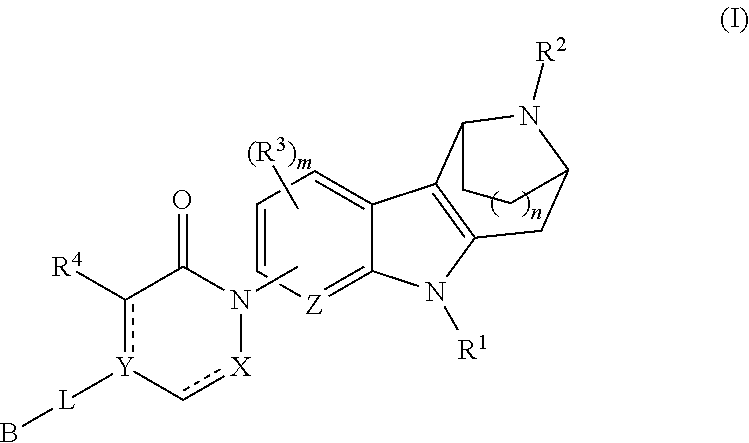
Azabicycloalkane-indole and azabicycloalkane-pyrrolo-pyridine mch-1 antagonists, methods of making, and use thereof
Novel MCH-1 receptor antagonists are disclosed. These compounds are used in the treatment of various disorders, including obesity, anxiety, depression, non-alcoholic fatty liver disease, and psychiatric disorders. Methods of making these compounds are also described in the present invention.
Owner:ALBANY MOLECULAR RESEARCH INC
8-azabicyclo[3.2.1]octane-8-carboxamide derivative
Disclosed is a compound represented by formula (1) or a pharmacologically acceptable salt thereof. (In the formula, A represents a group that is represented by formula (A-1); R1a and R1b may be the same or different and each independently represents a C1-6 alkyl group which may be substituted by one to three halogen atoms; m and n each independently represents an integer of 0-5; X1 represents a hydroxyl group or an aminocarbonyl group; Z1 represents a single bond or the like; and R2 represents an optionally substituted C1-6 alkyl group, an optionally substituted C6-10 aryl group or the like.)
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
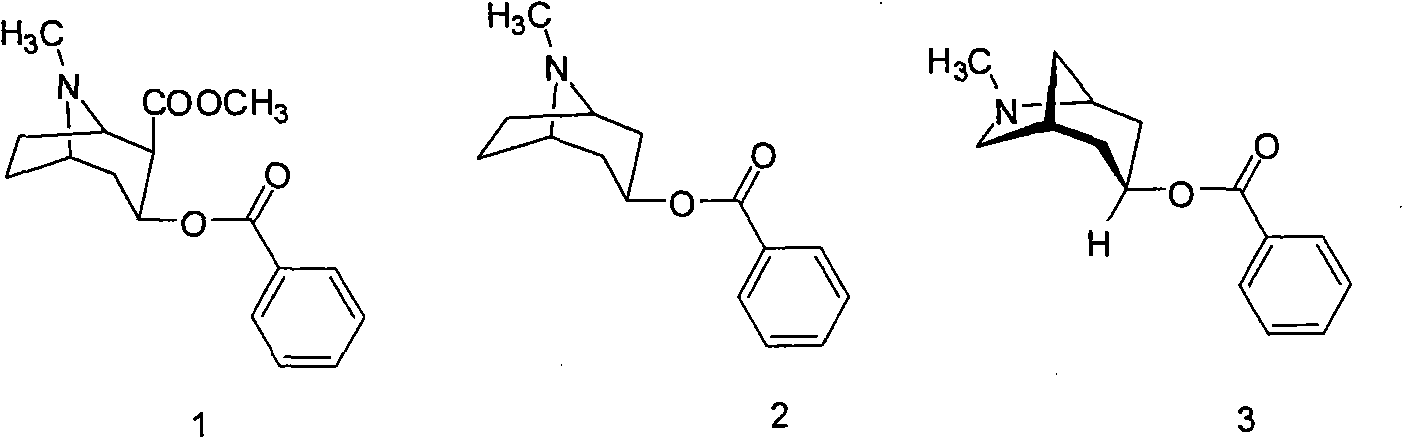
Method for preparing 2-hydroxyl-2,2-diphenyl acetic acid-3alpha-(8-azabicyclo[3,2,1]-3-octyl ester
The invention discloses a method for preparing 2-hydroxyl-2,2-diphenyl acetic acid-3alpha-(8-azabicyclo[3,2,1]-3-octyl ester which is a key intermediate of trospium chloride of an anticholinergic agent. The method comprises the following steps that: firstly, 2-hydroxyl-2,2-diphenyl acetic acid serving as a raw material is mixed with carbonyl imidazole for activation of acyl radicals; secondly, theresulting product reacts with tropine alcohol to form 2-hydroxyl-2,2-diphenyl acetic acid tropine ester; and finally, the resulting product of the previous step undergoes N formylation and acidulation reaction to obtain the 2-hydroxyl-2,2-diphenyl acetic acid-3alpha-(8-azabicyclo[3,2,1]-3-octyl ester. The method has the advantages of mild reaction conditions, little pollution and easy realizationof industrialized production.
Owner:北京迈劲医药科技有限公司
Synthesis method of 6, 6-dimethyl-3-azabicyclo [3.1. 0] hexane
ActiveCN114105859ASimple post-processingThree wastes less pollutionPeptidesAzabicyclaneCombinatorial chemistry
The invention relates to the technical field of drug intermediates, in particular to a synthesis method of 6, 6-dimethyl-3-azabicyclo [3.1. 0] hexane, 6, 6-dimethyl-3-oxazolo [3.1. 0] hexane-2-ketone is used as a raw material, the 6, 6-dimethyl-3-azabicyclo [3.1. 0] hexane is prepared through hydrolysis, oxidation, dehydration, ammonolysis cyclization and reduction, the 6, 6-dimethyl-3-azabicyclo [3.1. 0] hexane is used as a raw material, and the 6, 6-dimethyl-3-azabicyclo [3.1. 0] hexane is used as a raw material for preparing the 6, 6-dimethyl-3-azabicyclo [3.1. 0] hexane. The synthesis method comprises the following steps of: synthesizing 6, 6-dimethyl-3-oxazole ring [3.1. 0] hexane-2-ketone as a compound as shown in a formula, and 6, 6-dimethyl-3-azabicyclo [3.1. 0] hexane as a compound as shown in a formula in the specification according to a specific synthesis route as follows: synthesizing 6, 6-dimethyl-3-oxazole ring [3.1. 0] hexane-2-ketone as shown in a formula in the specification; the method has the advantages that starting materials are cheap and easy to obtain, the obtained key intermediate is cis-form and can be subjected to ring closing at room temperature, 200-DEG C high-temperature conditions are avoided, post-treatment of the intermediate is simple, pollution of three wastes is less, energy consumption is low, environment cost is low, and the method is suitable for industrial production.
Owner:NANJING CHEMPION BIOTECHNOLOGY CO LTD +1
Preparation method for novel NS5A inhibitor medicine
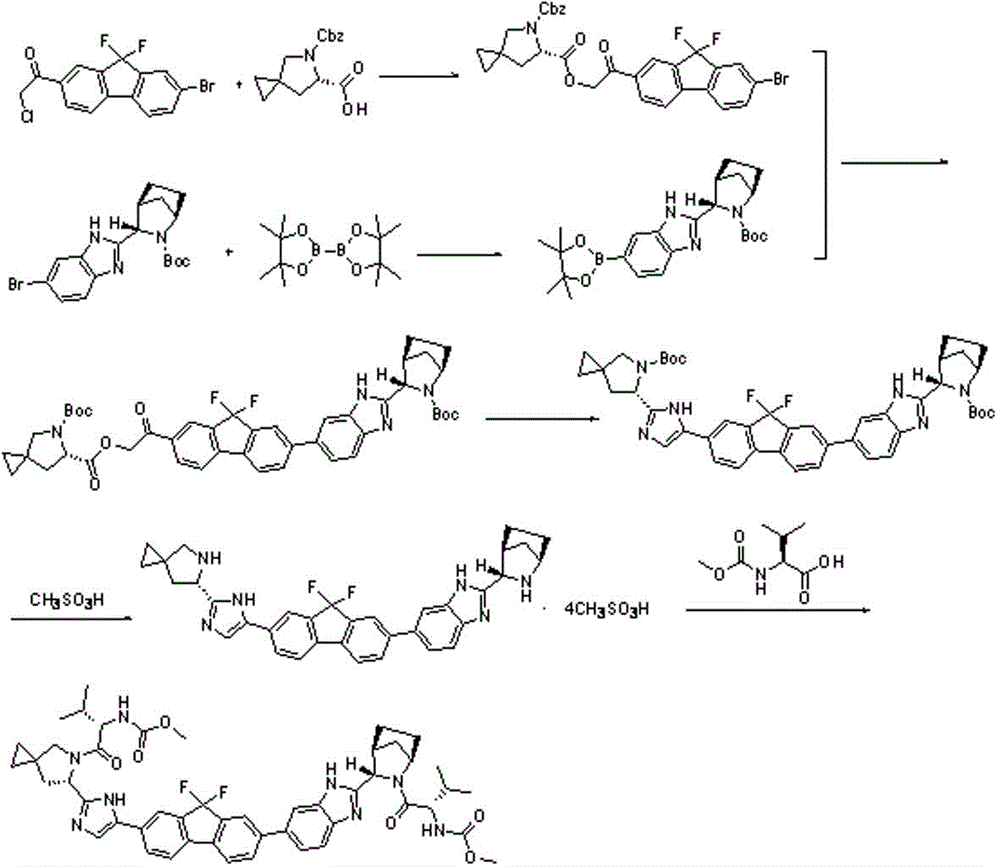
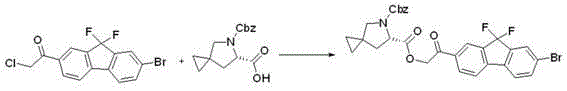
InactiveCN104926796AEasy to controlShort process routeOrganic chemistryBulk chemical productionPtru catalystAzabicyclane
The invention discloses a preparation method for novel NS5A inhibitor medicine. The preparation method includes the steps that step1, (1R, 3S, 4S)-3-(6-bromine-1H-benzimidazole-2-base)-2- azabicyalo (2.2.1) heptane-2-carboxylic acid tert-butyl ester serves as raw materials and reacts with bis (pinacolato) diboron under the action of a metal catalyst to obtain an intermediate L1; 1-(7-bromine-9,9-difluoro-9H-fluorene-2-base)-2-phenacyl chloride and (6S)-5-azaspiro (2.4) heptane-5,6-dicarboxylic acid 5-benzyl ester react under the action of a base to obtain an intermediate L2; the intermediate L1 and the intermediate L2 react under the action of a metal catalyst to obtain an intermediate L3; the intermediate L3 and an amine reagent react in a cyclization mode to obtain an intermediate L4; a protecting group is removed from the intermediate L4 under the action of methanesulfonic acid, so that an intermediate L5 in a mesylate form is obtained; the intermediate L5 and MOC valine react under the action of a condensing agent to obtain a Ledipasvir finished product. The process route is short, the preparation technology is simple, the intermediates are easy to control, and the reaction yield is high.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
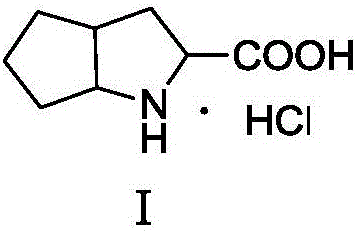
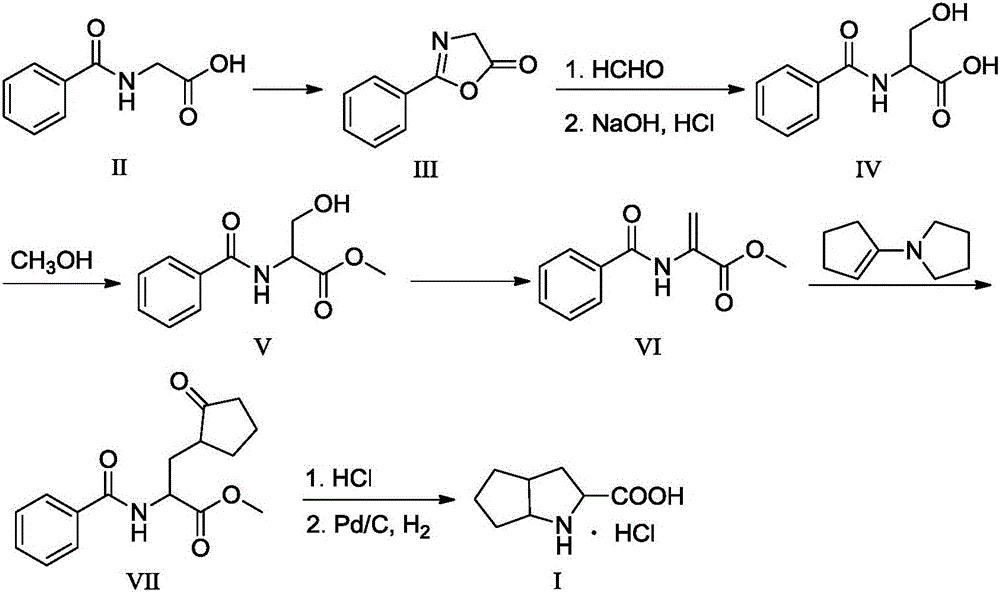
Purification method of 3-azabicyclo-octane hydrochloride
Belonging to the technical field of drug intermediate purification, the invention discloses a purification method of 3-azabicyclo-octane hydrochloride. The process includes: adding potassium borohydride and anhydrous zinc chloride into a mixed solvent of dried toluene and tetrahydrofuran at room temperature, mixing them, conducting nitrogen protection, inputting quantified 1, 2-cyclopentyl dicarboximide in batches, carrying out nitrogen protection and mixing, slow raising the temperature to 70-75DEG C, performing thermal preservation reaction for 4h, slowly raising the temperature to 95-105DEG C and performing thermal preservation reflux reaction for 10h to achieve complete reaction, distilling off some organic solvents, then implementing cooling to 30DEG C, slowly adding liquid alkali to pH of 13-14, conducting steam distillation till fraction pH of 7-8, extracting the fraction with an organic solvent for 3-5 times, acidifying the organic solvent layer with refined hydrochloric acid to pH of 1.5-2, carrying out heating backflow to remove water in the organic layer, and performing cooling suction filtration to obtain an azabicyclo solid crude product. The crude product is refined by a mixed solvent of alcohol and ester, thus obtaining the fine azabicyclo solid. The method provided in the invention has the characteristics that the production process is improved, the purity and yield of the target product are enhanced, and the production cost is reduced at the same time.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
A kind of raw material of cephalosporins and preparation method thereof
InactiveCN102260279ABeneficial technical effectQuality improvementAntibacterial agentsOrganic active ingredientsThio-Azabicyclane
The invention belongs to the technical field of medicine. The invention discloses a raw material of cephalosporins and a preparation method thereof. The raw material contains (6R,7S)-7-[2-[(S)-2-amino-2-carboxyethyl Thio]acetylamino]-7-methoxy-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-5-thia-1- Sodium azabicyclo[4.2.0]oct-2-ene-2-carboxylate heptahydrate, content greater than or equal to 99.6% less than 100%; containing 7-[2-[2-amino-2-carboxyethylthio] Acetylamino]-7-methylthio-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-5-thia-1-azabicyclo [4.2.0] Oct-2-ene-2-carboxylic acid or 7-[2-[2-amino-2-carboxyethylthio]acetamido]-7-methylthio-3-[[(1- Sodium methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate, The content is greater than 0 and less than or equal to 0.3%.
Owner:北京美迪康信医药科技有限公司
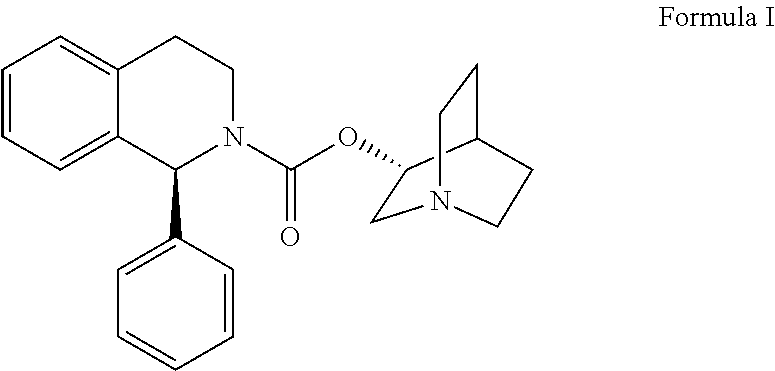
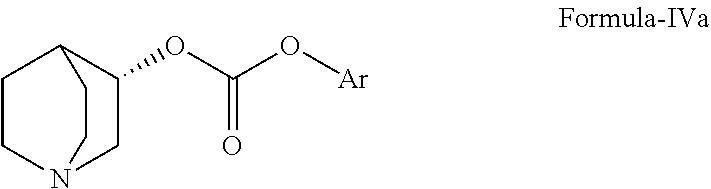
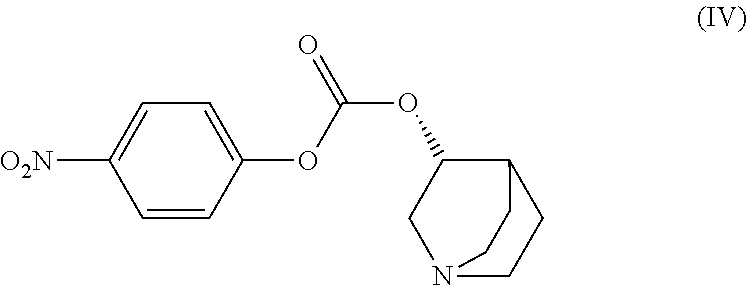
Method for the preparation of solifenacin and intermediate thereof
A method for the preparation of solifenacin by reacting quinuclidin-3-ol and bis(aryl) carbonate to form (3R)-1-azabicyclo[2.2.2]oct-3-yl 4-aryl carbonate of formula (IVa); and treating (3R)-1-azabicyclo[2.2.2]oct-3-yl 4-aryl carbonate of formula (IVa) with (1S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline of formula (V) in an inert atmosphere to form a Solifenacin base, which is converted into its pharmaceutically acceptable salts. The invention also provides a compound, (3R)-1-azabicyclo[2.2.2]oct-3-yl 4-aryl carbonate of formula (IVa), which is used as an intermediate for the preparation of Solifenacin base and a process for the preparation thereof.
Owner:MEGAFINE PHARMA (P) LTD
Process for preparing (1r,2s,5s)-n-[(1S)-3-amino-1-(cyclobutylmethyl)-2,3-dioxopropyl]-3-[(2S)-2-[[[(1,1-dimethylethyl)amino]-carbonyl]amino]-3,3-dimethyl-1-oxobutyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide
Owner:MERCK SHARP & DOHME CORP
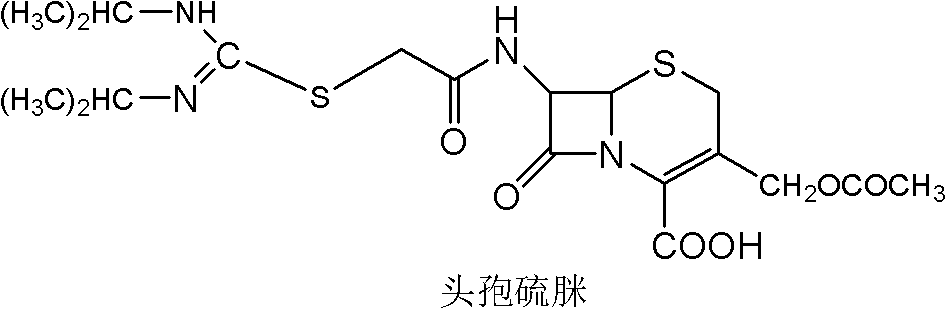
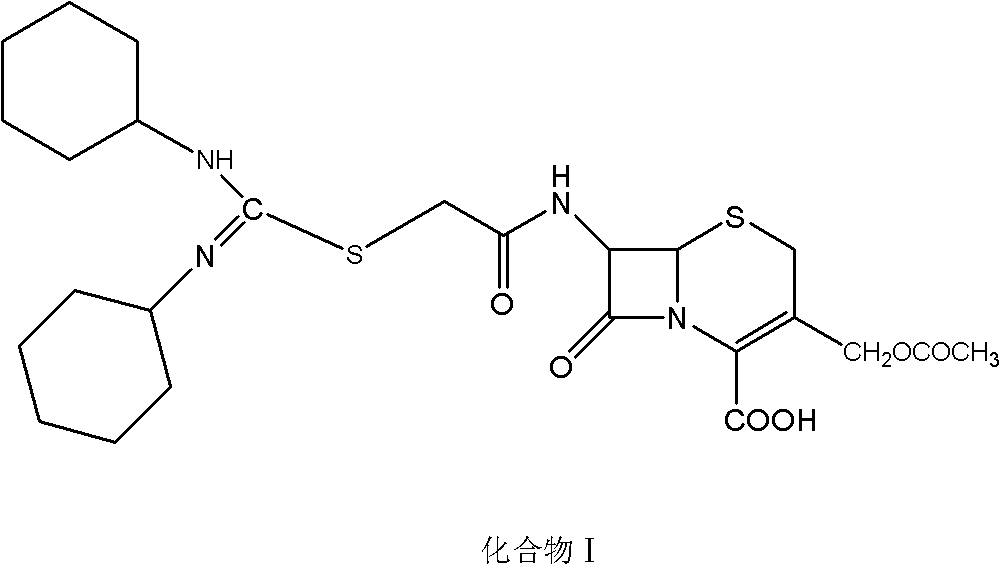
Cephalosporin compound, crystal thereof, and preparation method and application thereof
ActiveCN103102358AHigh purityHas antibacterial propertiesAntibacterial agentsOrganic active ingredientsAntimicrobial actionAntimicrobial drug
The invention relates to a (6R, 7R)-3-[(acetyloxy) methyl]-7-[alpha-(N, N'-dicyclohexyl amidine thio)-acetylamino]-8-oxo-5-thia-1-azabicyclo [4, 2, 0]-oct-2-ene-2-carboxylic acid, a crystal thereof, and a preparation method and application thereof. Under alkaline conditions, 7-ACA reacts with bromoacetyl bromide to obtain a 7-bromo acetamino-3-[(acetyloxy) methyl]-8-oxo-5-thia-1-azabicyclo [4.2.0] oct-2-ene-2 formic acid intermediate A; and the intermediate A reacts with N, N '- dicyclohexyl thiourea to obtain the compound provided by the invention. The compound is further refined to obtain the crystal with high purity and good stability of the compound provided by the invention. The compound and the crystal thereof have antibacterial effect on Staphylococcus aureus, Streptococcus pyogenes, Streptococcus pneumoniae and Enterococcus faecalis, and are potential antimicrobial drugs with promising application prospect.
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD
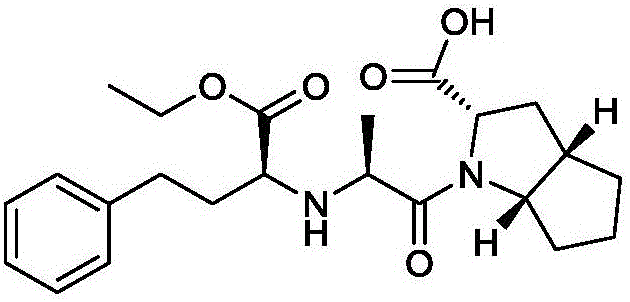
Synthesis method of ramipril key intermediate
ActiveCN106748966ASimple and fast operationMild reaction conditionsOrganic chemistrySynthesis methodsCarboxylic acid
The invention discloses a synthesis method of a ramipril key intermediate. The ramipril key intermediate is 2-azabicyalo [3.3.0] octane-3-carboxylic acid hydrochloride. The 2-azabicyalo [3.3.0] octane-3-carboxylic acid hydrochloride is obtained through sequential dehydration cyclization, formaldehyde condensation, hydrolysis, removal, Michael addition, cyclization and palladium-carbon catalytic hydrogenation reduction of N-benzoyl-glycine as a raw material. According to the synthesis method of the ramipril key intermediate, the required raw material and reagent are cheap and available, the yield is relatively high, the operation is simple, the cost is low and the method is suitable for industrial production.
Owner:ZHEJIANG UNIV OF TECH
Crystalline compounds
ActiveUS20160368871A1Easy to synthesizeOrganic active ingredientsNervous disorderCrystallographyHexane
The present invention relates to crystalline forms of (1R,5S)-1-(naphthalen-2-yl)-3-azabicyclo[3.1.0]hexane hydrochloride and compositions comprising the same and methods of making and using the same.
Owner:OTSUKA AMERICA PHARMA
Preparation and therapeutic applications of (2S,3R)-N-2-((3-pyridinyl)methyl)-1-azabicyclo[2.2.2]OCT-3-yl)-3,5-difluorobenzamide
The present invention relates to compounds that bind to and modulate the activity of neuronal nicotinic acetylcholine receptors, to processes for preparing these compounds, to pharmaceutical compositions containing these compounds, and to methods of using these compounds for treating a wide variety of conditions and disorders, including those associated with dysfunction of the central nervous system (CNS).
Owner:ATTENUA INC
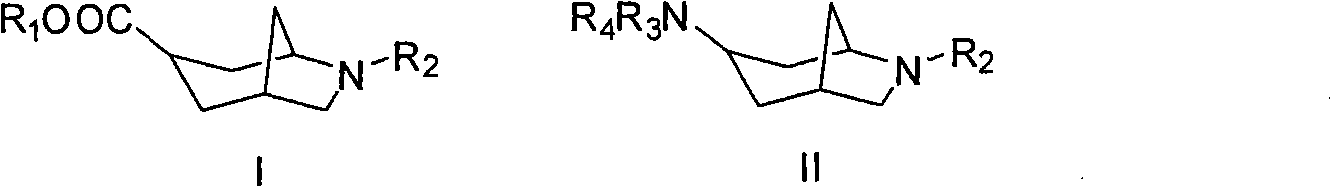
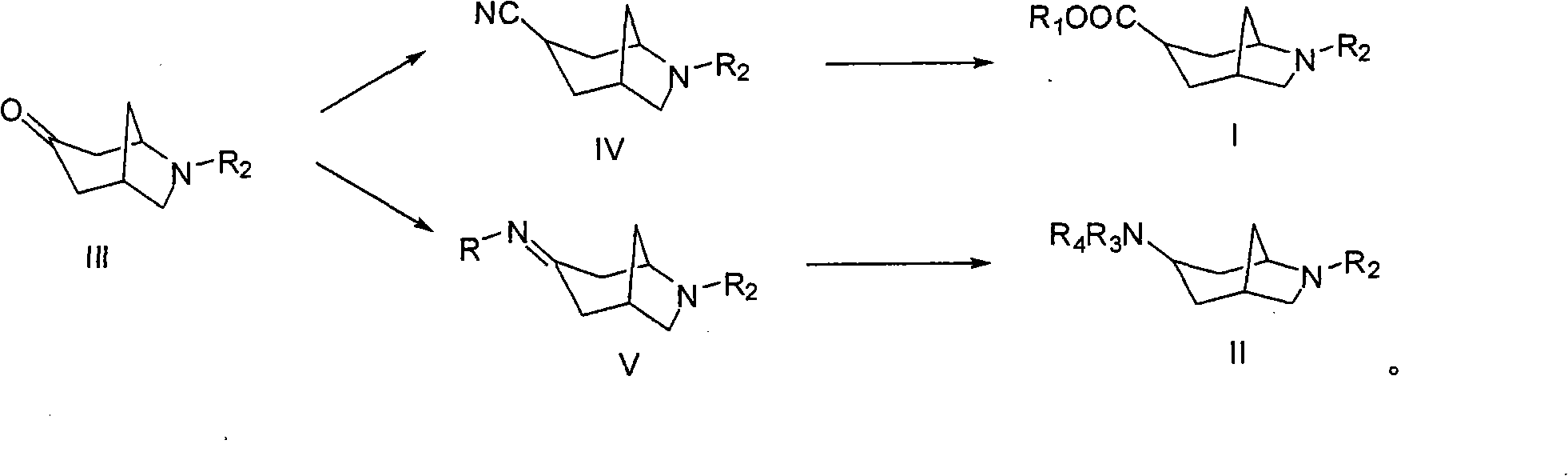
6-azabicyclo (3.2.1) nonane-3-substituted derivative and preparation method thereof
The invention relates to a 6-azabicyclo (3.2.1) nonane-3-substituted derivative and a preparation method thereof, which mainly solve the technical problems that the extension of the prior endocyclic compound with 6-azabicyclo (3.23.1) nonane structure in a space structure is limited and the water solubility of the compound is poor. The reaction formula of the 6-azabicyclo (3.2.1) nonane-3-substituted derivative is shown as above. 3-nitrile-6-azabicyclo (3.2.1) nonane compound IV is obtained by adopting 6-azabicyclo (3.2.1) nonane-3-ketone III as raw materials through nitrile addition, and then the compound IV is reacted with ethanol under acid condition to generate ester compound I; or the compound III is reacted with oxyammonia, aminos, hydrazines, amides, sulfonamides, hydrazides or sulfonyl hydrazides to generate compound V, and the compound V is reduced to generate compound II.
Owner:无锡药明康德新药开发股份有限公司 +1
Derivatives of [2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)alkyl]phosphonic acid and methods of making them
Compounds of formula (I) or pharmaceutically acceptable salts thereof are provided: wherein: A is alkylenyl of 1 to 4 carbon atoms, or alkenylenyl of 2 to 4 carbon atoms; R1 and R2 are, independently, hydrogen or a C5 to C7 aryl optionally substituted with 1 to 2 substituents, independently, selected from the group consisting of —C(O)R3, halogen, cyano, nitro, hydroxyl, C1-C6 alkyl, and C1-C6 alkoxy, with the proviso that at least one of R1 and R2 is not hydrogen; R3 is, independently, hydrogen, —OR4, alkyl, aryl, or heteroaryl; R4 is hydrogen, alkyl, aryl, or heteroaryl; R5 and R6 are, independently, hydrogen, alkyl, hydroxyl, alkoxy, or C5 to C7 aryl; wherein any R3 to R6 group having an aryl or heteroaryl moiety can optionally be substituted on the aryl or heteroaryl moiety with 1 to about 5 substituents, independently, selected from the group consisting of halogen, cyano, nitro, hydroxyl, C1-C6 alkyl, and C1-C6 alkoxy. Methods of making these compounds as well as methods using the compounds for treating a variety of conditions are also disclosed.
Owner:WYETH
5-amino-2-azabicyclo [2.2.1] heptane-3-carboxyl acid derivatives, and preparation thereof
InactiveCN101463000AGood water solubilityChange physiological activityOrganic chemistryBulk chemical productionSolubilityHalohydrocarbon
The invention relates to a 5-amino-2-diazabicyclo (2.2.1) heptane-3-carboxylic acid derivative and a preparing method thereof, solving the technical problems of poor water solubility and low bioavailability of the existing diazabicyclo structure. The chemical structural formula of the 5-amino-2-diazabicyclo (2.2.1) heptane-3-carboxylic acid derivative is shown in the formula above. (1R, 3S, 4S)-2boc-5-carbonyl-2-diazabicyclo (2.2.1) heptane-3-carboxylic ester (II) is used as raw material for obtaining non-optically pure 5-amino-2-diazabicyclo (2.2.1) heptane-3- carboxylic ester (III) by reduction amination reaction. Carbobenzoxy is added for the protection to obtain 5-benzyloxycarbonylamino-2-diazabicyclo (2.2.1) heptane-3-carboxylic ester (IV) which is condensated into acid amide with amine after resolution of chiral column, removing of protecting groups, hydrolysis and alkylation or arylation reaction and the acid amide reacts with alcohol or halohydrocarbon to form ester series to obtain a final product.
Owner:上海药明康德新药开发有限公司
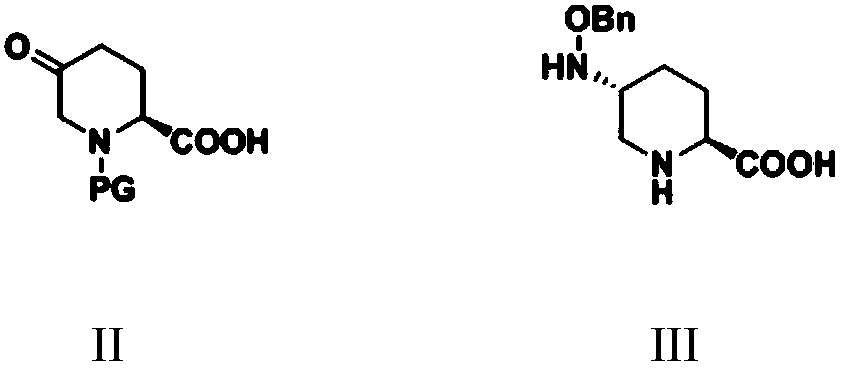
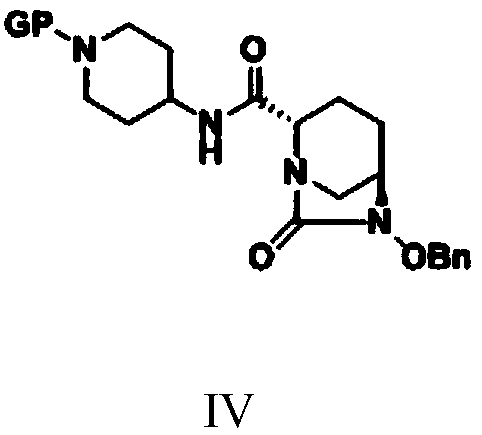
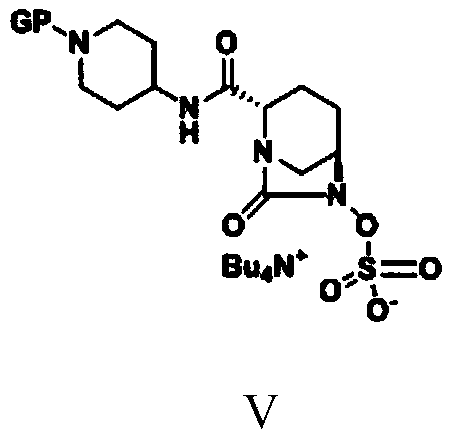
Simple and convenient preparation method of relebactam
ActiveCN111072660AReduce productionImprove economyOrganic chemistryBulk chemical productionRelebactamAzabicyclane
The invention discloses a simple and convenient preparation method of relebactam. According to the method, a key intermediate (2S,5R)-5-benzyloxyamino piperidine-2-formic acid is prepared by using (S)-N-protective group-5-oxo-2-piperidinecarboxylic acid or a salt form thereof as an initial raw material; the (2S,5R)-5-benzyloxyamino piperidine-2-formic acid and phosgene, solid phosgene or diphosgene are subjected to acylating chlorination, cyclic ureation, and reaction with 1-protective group-4-aminopiperidine to obtain (2S,5R)-6-benzyloxy-N-(1-protective group-4-yl)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-formamide; and debenzylation, sulfonyl oxidation, ammonium salt formation and deprotection are performed to prepare relebactam. According to the invention, the raw materials are cheap, easyto obtain and low in cost, the technological process is safe, simple and convenient to operate, small in wastewater and waste salt yield and environmentally friendly, the reaction atom economy is high, the reaction selectivity of each step is high, the purity and the yield are high, and industrial production is facilitated.
Owner:XINFA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Solid Forms of N-(4-(7-Azabicyclo[2.2.1]Heptan-7-yl)-2-Trifluoromethyl)Phenyl)-4-Oxo-5-(Trifluoromethyl)-1,4-Dihydroquinoline-3-Carboxamide Solid Forms of N-(4-(7-Azabicyclo[2.2.1]Heptan-7-yl)-2-Trifluoromethyl)Phenyl)-4-Oxo-5-(Trifluoromethyl)-1,4-Dihydroquinoline-3-Carboxamide](https://images-eureka.patsnap.com/patent_img/d237cee5-8ac9-42c9-8ded-2c772e6da88c/US20110123449A1-20110526-D00001.png)
![Solid Forms of N-(4-(7-Azabicyclo[2.2.1]Heptan-7-yl)-2-Trifluoromethyl)Phenyl)-4-Oxo-5-(Trifluoromethyl)-1,4-Dihydroquinoline-3-Carboxamide Solid Forms of N-(4-(7-Azabicyclo[2.2.1]Heptan-7-yl)-2-Trifluoromethyl)Phenyl)-4-Oxo-5-(Trifluoromethyl)-1,4-Dihydroquinoline-3-Carboxamide](https://images-eureka.patsnap.com/patent_img/d237cee5-8ac9-42c9-8ded-2c772e6da88c/US20110123449A1-20110526-D00002.png)
![Solid Forms of N-(4-(7-Azabicyclo[2.2.1]Heptan-7-yl)-2-Trifluoromethyl)Phenyl)-4-Oxo-5-(Trifluoromethyl)-1,4-Dihydroquinoline-3-Carboxamide Solid Forms of N-(4-(7-Azabicyclo[2.2.1]Heptan-7-yl)-2-Trifluoromethyl)Phenyl)-4-Oxo-5-(Trifluoromethyl)-1,4-Dihydroquinoline-3-Carboxamide](https://images-eureka.patsnap.com/patent_img/d237cee5-8ac9-42c9-8ded-2c772e6da88c/US20110123449A1-20110526-D00003.png)
![Oral administration of [2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)alkyl] phosphonic acid and derivatives Oral administration of [2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)alkyl] phosphonic acid and derivatives](https://images-eureka.patsnap.com/patent_img/83f30278-e678-491f-b6c6-374e15a6e507/US20050142192A1-20050630-D00000.png)
![Oral administration of [2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)alkyl] phosphonic acid and derivatives Oral administration of [2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)alkyl] phosphonic acid and derivatives](https://images-eureka.patsnap.com/patent_img/83f30278-e678-491f-b6c6-374e15a6e507/US20050142192A1-20050630-D00001.png)
![Oral administration of [2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)alkyl] phosphonic acid and derivatives Oral administration of [2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)alkyl] phosphonic acid and derivatives](https://images-eureka.patsnap.com/patent_img/83f30278-e678-491f-b6c6-374e15a6e507/US20050142192A1-20050630-D00002.png)
![Derivatives of 3-Azabicyclo[3.1.0] Hexane as Dipeptidyl Peptidase-IV Inhibitors Derivatives of 3-Azabicyclo[3.1.0] Hexane as Dipeptidyl Peptidase-IV Inhibitors](https://images-eureka.patsnap.com/patent_img/b07ea871-988e-445e-b196-080eb11a636d/US20080300251A1-20081204-C00001.png)
![Derivatives of 3-Azabicyclo[3.1.0] Hexane as Dipeptidyl Peptidase-IV Inhibitors Derivatives of 3-Azabicyclo[3.1.0] Hexane as Dipeptidyl Peptidase-IV Inhibitors](https://images-eureka.patsnap.com/patent_img/b07ea871-988e-445e-b196-080eb11a636d/US20080300251A1-20081204-C00002.png)
![Derivatives of 3-Azabicyclo[3.1.0] Hexane as Dipeptidyl Peptidase-IV Inhibitors Derivatives of 3-Azabicyclo[3.1.0] Hexane as Dipeptidyl Peptidase-IV Inhibitors](https://images-eureka.patsnap.com/patent_img/b07ea871-988e-445e-b196-080eb11a636d/US20080300251A1-20081204-C00003.png)
![8-azabicyclo[3.2.1]octane compounds as MU opioid receptor antagonists 8-azabicyclo[3.2.1]octane compounds as MU opioid receptor antagonists](https://images-eureka.patsnap.com/patent_img/43cf5f19-9dc4-434c-b9d8-21b418be5f30/US08263618-20120911-D00001.png)
![8-azabicyclo[3.2.1]octane compounds as MU opioid receptor antagonists 8-azabicyclo[3.2.1]octane compounds as MU opioid receptor antagonists](https://images-eureka.patsnap.com/patent_img/43cf5f19-9dc4-434c-b9d8-21b418be5f30/US08263618-20120911-D00002.png)
![8-azabicyclo[3.2.1]octane compounds as MU opioid receptor antagonists 8-azabicyclo[3.2.1]octane compounds as MU opioid receptor antagonists](https://images-eureka.patsnap.com/patent_img/43cf5f19-9dc4-434c-b9d8-21b418be5f30/US08263618-20120911-C00001.png)
![Methods and compositions for production, formulation and use of 1 aryl-3-azabicyclo[3.1.0]hexanes Methods and compositions for production, formulation and use of 1 aryl-3-azabicyclo[3.1.0]hexanes](https://images-eureka.patsnap.com/patent_img/002eb78a-c362-4ec3-9a97-cfe15b8f7b2a/US20080058535A1-20080306-C00001.png)
![Methods and compositions for production, formulation and use of 1 aryl-3-azabicyclo[3.1.0]hexanes Methods and compositions for production, formulation and use of 1 aryl-3-azabicyclo[3.1.0]hexanes](https://images-eureka.patsnap.com/patent_img/002eb78a-c362-4ec3-9a97-cfe15b8f7b2a/US20080058535A1-20080306-C00002.png)
![Methods and compositions for production, formulation and use of 1 aryl-3-azabicyclo[3.1.0]hexanes Methods and compositions for production, formulation and use of 1 aryl-3-azabicyclo[3.1.0]hexanes](https://images-eureka.patsnap.com/patent_img/002eb78a-c362-4ec3-9a97-cfe15b8f7b2a/US20080058535A1-20080306-C00003.png)
![8-azabicyclo[3.2.1]octyl-2-hydroxybenzamide compounds as mu opioid receptor antagonists 8-azabicyclo[3.2.1]octyl-2-hydroxybenzamide compounds as mu opioid receptor antagonists](https://images-eureka.patsnap.com/patent_img/a69c13e6-21b8-4c16-ac64-9f6969e1a2a6/US20090062333A1-20090305-C00001.png)
![8-azabicyclo[3.2.1]octyl-2-hydroxybenzamide compounds as mu opioid receptor antagonists 8-azabicyclo[3.2.1]octyl-2-hydroxybenzamide compounds as mu opioid receptor antagonists](https://images-eureka.patsnap.com/patent_img/a69c13e6-21b8-4c16-ac64-9f6969e1a2a6/US20090062333A1-20090305-C00002.png)
![8-azabicyclo[3.2.1]octyl-2-hydroxybenzamide compounds as mu opioid receptor antagonists 8-azabicyclo[3.2.1]octyl-2-hydroxybenzamide compounds as mu opioid receptor antagonists](https://images-eureka.patsnap.com/patent_img/a69c13e6-21b8-4c16-ac64-9f6969e1a2a6/US20090062333A1-20090305-C00003.png)
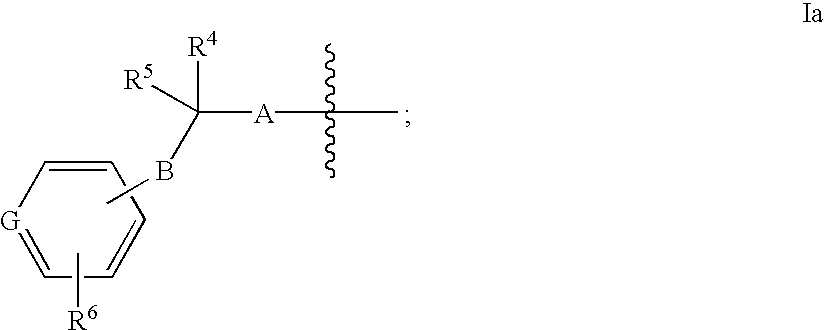
![3-trifluoromethyl-5-tert-butoxycarbonyl-2,5-diheterobicyclo[2.2.1]heptane and preparation method thereof 3-trifluoromethyl-5-tert-butoxycarbonyl-2,5-diheterobicyclo[2.2.1]heptane and preparation method thereof](https://images-eureka.patsnap.com/patent_img/be7bfea3-56f2-4e84-bcde-c66c03d5c00c/FSA00000037749500011.png)
![3-trifluoromethyl-5-tert-butoxycarbonyl-2,5-diheterobicyclo[2.2.1]heptane and preparation method thereof 3-trifluoromethyl-5-tert-butoxycarbonyl-2,5-diheterobicyclo[2.2.1]heptane and preparation method thereof](https://images-eureka.patsnap.com/patent_img/be7bfea3-56f2-4e84-bcde-c66c03d5c00c/FSA00000037749500012.png)
![3-trifluoromethyl-5-tert-butoxycarbonyl-2,5-diheterobicyclo[2.2.1]heptane and preparation method thereof 3-trifluoromethyl-5-tert-butoxycarbonyl-2,5-diheterobicyclo[2.2.1]heptane and preparation method thereof](https://images-eureka.patsnap.com/patent_img/be7bfea3-56f2-4e84-bcde-c66c03d5c00c/FSA00000037749500041.png)
![8-azabicyclo[3.2.1]octane-8-carboxamide derivative 8-azabicyclo[3.2.1]octane-8-carboxamide derivative](https://images-eureka.patsnap.com/patent_img/080609b7-ce93-49cf-857b-38ec891a590e/206868DEST_PATH_IMAGE020.png)
![8-azabicyclo[3.2.1]octane-8-carboxamide derivative 8-azabicyclo[3.2.1]octane-8-carboxamide derivative](https://images-eureka.patsnap.com/patent_img/080609b7-ce93-49cf-857b-38ec891a590e/293084DEST_PATH_IMAGE027.png)
![8-azabicyclo[3.2.1]octane-8-carboxamide derivative 8-azabicyclo[3.2.1]octane-8-carboxamide derivative](https://images-eureka.patsnap.com/patent_img/080609b7-ce93-49cf-857b-38ec891a590e/527757DEST_PATH_IMAGE028.png)
![Method for preparing 2-hydroxyl-2,2-diphenyl acetic acid-3alpha-(8-azabicyclo[3,2,1]-3-octyl ester Method for preparing 2-hydroxyl-2,2-diphenyl acetic acid-3alpha-(8-azabicyclo[3,2,1]-3-octyl ester](https://images-eureka.patsnap.com/patent_img/294f2403-8724-4bb6-a08b-c9b9c3645f57/A20081014732600031.PNG)
![Method for preparing 2-hydroxyl-2,2-diphenyl acetic acid-3alpha-(8-azabicyclo[3,2,1]-3-octyl ester Method for preparing 2-hydroxyl-2,2-diphenyl acetic acid-3alpha-(8-azabicyclo[3,2,1]-3-octyl ester](https://images-eureka.patsnap.com/patent_img/294f2403-8724-4bb6-a08b-c9b9c3645f57/A20081014732600051.PNG)
![Synthesis method of 6, 6-dimethyl-3-azabicyclo [3.1. 0] hexane Synthesis method of 6, 6-dimethyl-3-azabicyclo [3.1. 0] hexane](https://images-eureka.patsnap.com/patent_img/cf2764f6-2c9b-4a8b-9792-3ced56e5b675/220120131032.png)
![Synthesis method of 6, 6-dimethyl-3-azabicyclo [3.1. 0] hexane Synthesis method of 6, 6-dimethyl-3-azabicyclo [3.1. 0] hexane](https://images-eureka.patsnap.com/patent_img/cf2764f6-2c9b-4a8b-9792-3ced56e5b675/220120131036.png)
![Synthesis method of 6, 6-dimethyl-3-azabicyclo [3.1. 0] hexane Synthesis method of 6, 6-dimethyl-3-azabicyclo [3.1. 0] hexane](https://images-eureka.patsnap.com/patent_img/cf2764f6-2c9b-4a8b-9792-3ced56e5b675/220120131039.png)
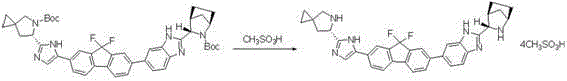

![Process for preparing (1r,2s,5s)-n-[(1S)-3-amino-1-(cyclobutylmethyl)-2,3-dioxopropyl]-3-[(2S)-2-[[[(1,1-dimethylethyl)amino]-carbonyl]amino]-3,3-dimethyl-1-oxobutyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide Process for preparing (1r,2s,5s)-n-[(1S)-3-amino-1-(cyclobutylmethyl)-2,3-dioxopropyl]-3-[(2S)-2-[[[(1,1-dimethylethyl)amino]-carbonyl]amino]-3,3-dimethyl-1-oxobutyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide](https://images-eureka.patsnap.com/patent_img/614e0028-f413-4b25-b55c-13aea3b0735a/US20100145013A1-20100610-C00001.png)
![Process for preparing (1r,2s,5s)-n-[(1S)-3-amino-1-(cyclobutylmethyl)-2,3-dioxopropyl]-3-[(2S)-2-[[[(1,1-dimethylethyl)amino]-carbonyl]amino]-3,3-dimethyl-1-oxobutyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide Process for preparing (1r,2s,5s)-n-[(1S)-3-amino-1-(cyclobutylmethyl)-2,3-dioxopropyl]-3-[(2S)-2-[[[(1,1-dimethylethyl)amino]-carbonyl]amino]-3,3-dimethyl-1-oxobutyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide](https://images-eureka.patsnap.com/patent_img/614e0028-f413-4b25-b55c-13aea3b0735a/US20100145013A1-20100610-C00002.png)
![Process for preparing (1r,2s,5s)-n-[(1S)-3-amino-1-(cyclobutylmethyl)-2,3-dioxopropyl]-3-[(2S)-2-[[[(1,1-dimethylethyl)amino]-carbonyl]amino]-3,3-dimethyl-1-oxobutyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide Process for preparing (1r,2s,5s)-n-[(1S)-3-amino-1-(cyclobutylmethyl)-2,3-dioxopropyl]-3-[(2S)-2-[[[(1,1-dimethylethyl)amino]-carbonyl]amino]-3,3-dimethyl-1-oxobutyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide](https://images-eureka.patsnap.com/patent_img/614e0028-f413-4b25-b55c-13aea3b0735a/US20100145013A1-20100610-C00003.png)
![Preparation and therapeutic applications of (2S,3R)-N-2-((3-pyridinyl)methyl)-1-azabicyclo[2.2.2]OCT-3-yl)-3,5-difluorobenzamide Preparation and therapeutic applications of (2S,3R)-N-2-((3-pyridinyl)methyl)-1-azabicyclo[2.2.2]OCT-3-yl)-3,5-difluorobenzamide](https://images-eureka.patsnap.com/patent_img/84b482ca-4376-4767-9e08-baf32651a33c/US08476296-20130702-D00001.png)
![Preparation and therapeutic applications of (2S,3R)-N-2-((3-pyridinyl)methyl)-1-azabicyclo[2.2.2]OCT-3-yl)-3,5-difluorobenzamide Preparation and therapeutic applications of (2S,3R)-N-2-((3-pyridinyl)methyl)-1-azabicyclo[2.2.2]OCT-3-yl)-3,5-difluorobenzamide](https://images-eureka.patsnap.com/patent_img/84b482ca-4376-4767-9e08-baf32651a33c/US08476296-20130702-D00002.png)
![Preparation and therapeutic applications of (2S,3R)-N-2-((3-pyridinyl)methyl)-1-azabicyclo[2.2.2]OCT-3-yl)-3,5-difluorobenzamide Preparation and therapeutic applications of (2S,3R)-N-2-((3-pyridinyl)methyl)-1-azabicyclo[2.2.2]OCT-3-yl)-3,5-difluorobenzamide](https://images-eureka.patsnap.com/patent_img/84b482ca-4376-4767-9e08-baf32651a33c/US08476296-20130702-D00003.png)
![Derivatives of [2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)alkyl]phosphonic acid and methods of making them Derivatives of [2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)alkyl]phosphonic acid and methods of making them](https://images-eureka.patsnap.com/patent_img/b9e051b4-a37d-4522-b172-3060820d2c95/US20060079679A1-20060413-C00001.png)
![Derivatives of [2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)alkyl]phosphonic acid and methods of making them Derivatives of [2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)alkyl]phosphonic acid and methods of making them](https://images-eureka.patsnap.com/patent_img/b9e051b4-a37d-4522-b172-3060820d2c95/US20060079679A1-20060413-C00002.png)
![Derivatives of [2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)alkyl]phosphonic acid and methods of making them Derivatives of [2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)alkyl]phosphonic acid and methods of making them](https://images-eureka.patsnap.com/patent_img/b9e051b4-a37d-4522-b172-3060820d2c95/US20060079679A1-20060413-C00003.png)
![5-amino-2-azabicyclo [2.2.1] heptane-3-carboxyl acid derivatives, and preparation thereof 5-amino-2-azabicyclo [2.2.1] heptane-3-carboxyl acid derivatives, and preparation thereof](https://images-eureka.patsnap.com/patent_img/4579d657-5d2e-4117-b463-fb7d47b8f6a7/A200710094605C00021.PNG)
![5-amino-2-azabicyclo [2.2.1] heptane-3-carboxyl acid derivatives, and preparation thereof 5-amino-2-azabicyclo [2.2.1] heptane-3-carboxyl acid derivatives, and preparation thereof](https://images-eureka.patsnap.com/patent_img/4579d657-5d2e-4117-b463-fb7d47b8f6a7/A200710094605C00031.PNG)
![5-amino-2-azabicyclo [2.2.1] heptane-3-carboxyl acid derivatives, and preparation thereof 5-amino-2-azabicyclo [2.2.1] heptane-3-carboxyl acid derivatives, and preparation thereof](https://images-eureka.patsnap.com/patent_img/4579d657-5d2e-4117-b463-fb7d47b8f6a7/A200710094605C00041.PNG)