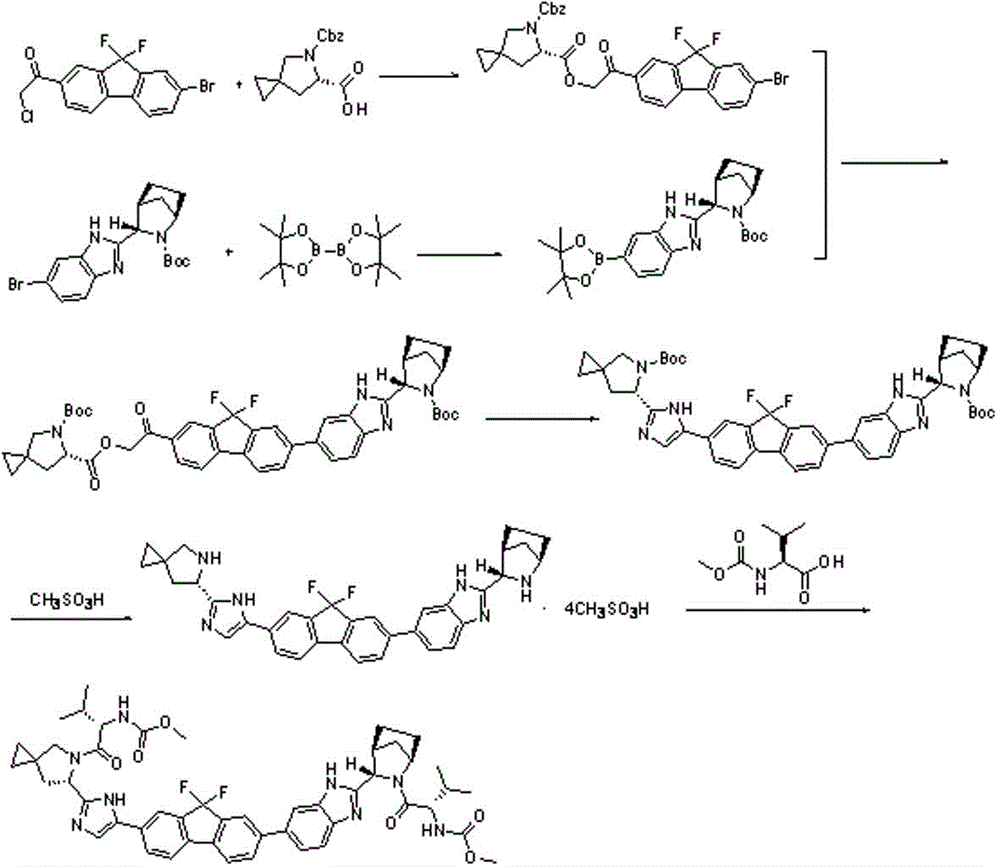
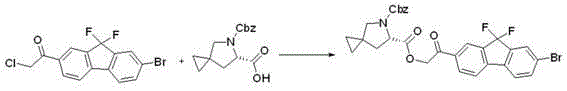
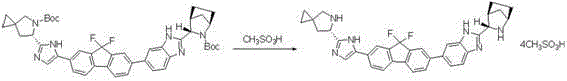Preparation method for novel NS5A inhibitor medicine
A technology for inhibitors and drugs, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of difficult solidification and purification of intermediates, long synthesis process routes, and complicated preparation processes, and achieves short process routes, simple preparation processes, The effect of high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1). Preparation of intermediate L1:
[0038] In the reaction flask, add 40g of (1R,3S,4S)-3-(6-bromo-1H-benzimidazol-2-yl)-2-azabicyclo[2.2.1]heptane-2-carboxylate Acetate tert-butyl ester, 38.2g diboronic acid pinacol ester, 4g potassium propionate and 500mL isopropyl acetate, stir, cool down to 10-15°C, stir, add 2g tetrakistriphenylphosphopalladium, and then under nitrogen protection The temperature was raised to 60-65° C. for the reaction, and the reaction was monitored by TLC. After the reaction is complete, add 300mL of water, stir, let stand for liquid separation, extract the aqueous layer twice with 150mL isopropyl acetate, combine the organic phases, wash with 300mL saturated sodium chloride solution, dry over anhydrous sodium sulfate, filter with suction, and evaporate to dryness , to obtain 38.4g solid product, yield 85.6%.
[0039] Preparation of intermediate L2:
[0040] Add 35g of 1-(7-bromo-9,9-difluoro-9H-fluoren-2-yl)-2-chloroethanone, 49g o...
Embodiment 2
[0050] (1). Preparation of intermediate L1:
[0051] In the reaction flask, add 50g of (1R,3S,4S)-3-(6-bromo-1H-benzimidazol-2-yl)-2-azabicyclo[2.2.1]heptane-2-carboxylate Acetate tert-butyl ester, 48.7g biboronic acid pinacol ester, 5g potassium acetate and 800mL ethyl acetate, stir, cool down to 10-15°C, stir, add 3g palladium chloride, and then raise the temperature to 60-65°C under nitrogen protection The reaction was carried out at ℃, and the reaction was monitored by TLC. After completion of the reaction, add 500 mL of water, stir, let stand for liquid separation, extract the aqueous layer twice with 300 mL of ethyl acetate, combine the organic phases, wash with 300 mL of saturated sodium chloride solution, dry over anhydrous sodium sulfate, suction filter, and evaporate to dryness. Obtained 48.7g of solid product, yield 86.5%.
[0052] Preparation of intermediate L2:
[0053] Add 43.8g of 1-(7-bromo-9,9-difluoro-9H-fluoren-2-yl)-2-chloroethanone, 61.3g of (6S...
Embodiment 3
[0063] (1). Preparation of intermediate L1:
[0064] In the reaction flask, add 30g of (1R,3S,4S)-3-(6-bromo-1H-benzimidazol-2-yl)-2-azabicyclo[2.2.1]heptane-2-carboxylate Acetate tert-butyl ester, 30.5g diboronic acid pinacol ester, 3g sodium propionate and 400mL DMF, stir, cool down to 10-15°C, stir, add 2g bistriphenylphosphine palladium dichloride, and then under nitrogen protection The temperature was raised to 60-65° C. for the reaction, and the reaction was monitored by TLC. After completion of the reaction, add 500 mL of water, stir, let stand for liquid separation, extract the aqueous layer three times with 200 mL of ethyl acetate, combine the organic phases, wash with 200 mL of saturated sodium chloride solution, dry over anhydrous sodium sulfate, suction filter, and evaporate to dryness to obtain 29.7g of solid product, yield 87.9%.
[0065] Preparation of intermediate L2:
[0066] Add 26.3g of 1-(7-bromo-9,9-difluoro-9H-fluoren-2-yl)-2-chloroethanone, 36...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com