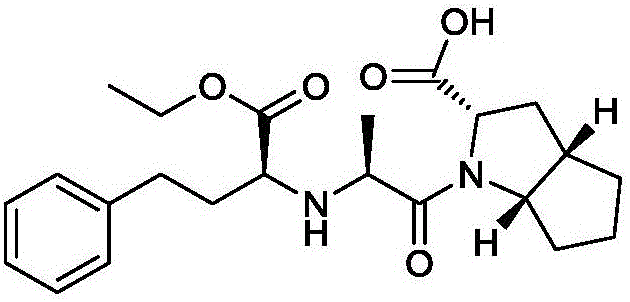
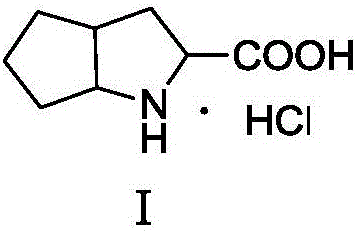
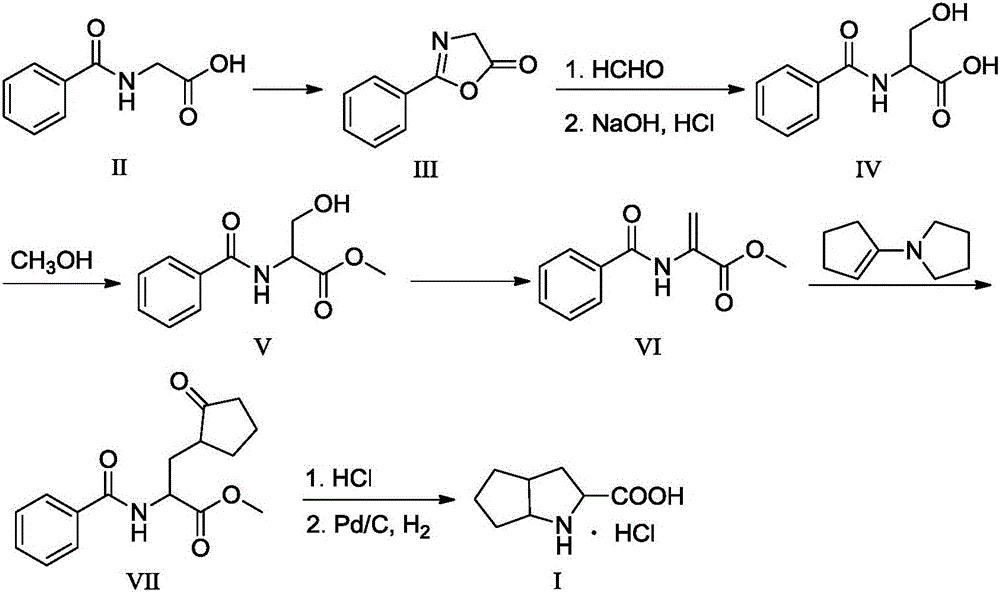
Synthesis method of ramipril key intermediate
A synthesis method and technology for intermediates, which are applied in the field of synthesis of pharmaceutical and chemical intermediates, can solve problems such as unfavorable environmental protection, high production cost, and many reaction steps, and achieve the effects of being suitable for industrial production, avoiding pollution problems, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Preparation of 2-phenyloxazol-5-(4H)-one (III)
[0043] Add N-benzoylglycine (35.8g, 0.2mol), solvent ethyl acetate 100mL, and acetic anhydride 10mL into a 250mL three-necked flask equipped with mechanical stirring and a thermometer, heat to 65°C, react for 4 hours, and use anhydrous sulfuric acid Sodium drying, after filtration, the crude product was purified by methanol recrystallization to obtain 25.60 g of light yellow solid, the yield was 79.5%, and the product purity was 97.5%
[0044] (2) Preparation of N-benzoylserine (IV)
[0045] Add 2-phenyloxazol-5-(4H)-one (18.7g, 0.096mol), 56.0mL of 35% aqueous formaldehyde solution, and 3.8mL of pyridine into a 250mL three-necked flask equipped with mechanical stirring and a thermometer, and react at room temperature After 17h, it was concentrated to remove water and pyridine. Add sodium hydroxide solution to adjust the pH to 8-11, continue stirring at room temperature for 1 h, add 50 mL of ethyl acetate for extrac...
Embodiment 2
[0058] (1) Preparation of 2-phenyloxazol-5-(4H)-one (III)
[0059] Add N-benzoylglycine (35.8g, 0.2mol), solvent ethyl acetate 100mL, and acetic anhydride 12mL into a 250mL three-necked flask equipped with mechanical stirring and a thermometer, heat to 65°C, react for 4 hours, and use anhydrous sulfuric acid Sodium drying, after filtration, the crude product was purified by methanol recrystallization to obtain 28.8 g of light yellow solid, the yield was 86.5%, and the product purity was 98.2%. (2) Preparation of N-benzoylserine (IV)
[0060] Add 2-phenyloxazol-5-(4H)-one (18.7g, 0.096mol), 40.0mL of 35% aqueous formaldehyde solution, and 11.6mL of pyridine into a 250mL three-necked flask equipped with mechanical stirring and a thermometer, and react at room temperature After 15h, concentrate to remove water and pyridine. Add sodium hydroxide solution to adjust the pH to 8-11, continue stirring at room temperature for 4 h, add 50 mL of ethyl acetate for extraction, and adjust ...
Embodiment 3
[0070] (1) Preparation of 2-phenyloxazol-5-(4H)-one (III)
[0071] Add N-benzoylglycine (35.8g, 0.2mol), solvent ethyl acetate 100mL, and acetic anhydride 28mL into a 250mL three-neck flask equipped with mechanical stirring and a thermometer, heat to 65°C, react for 4 hours, and use anhydrous sulfuric acid After drying with sodium, the crude product was filtered and refined by methanol recrystallization to obtain 27.1 g of a light yellow solid with a yield of 85.2% and a product purity of 98.7%.
[0072] (2) Preparation of N-benzoylserine (IV)
[0073] Add 2-phenyloxazol-5-(4H)-one (18.7g, 0.096mol), 18.7mL of 35% aqueous formaldehyde solution, and 15.5mL of pyridine in a 250mL three-necked flask equipped with mechanical stirring and a thermometer, and react at room temperature After 10 h, it was concentrated to remove water and pyridine. Add sodium hydroxide solution to adjust the pH to 8-11, continue stirring at room temperature for 3 h, add 50 mL of ethyl acetate for extr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com