A kind of raw material of cephalosporins and preparation method thereof
A technology of cephalosporins and pharmaceutical preparations, applied in the field of medicine, can solve the problems of corrosiveness, difficulty in preservation, crystallization and inability to stabilize heptahydrate compounds, etc., and achieve the effects of improving quality, saving costs, and reducing waste of reagents and energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
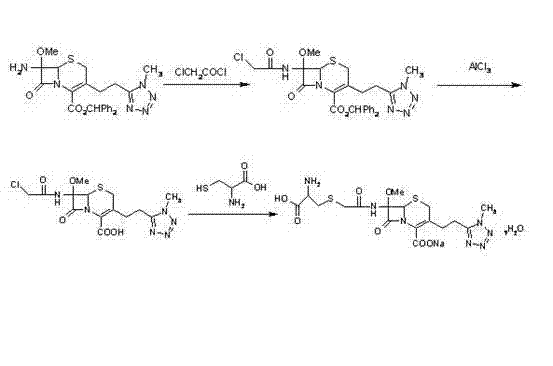
[0024] Take 77g of 7-MAC, add 550ml of ethyl acetate, dissolve completely, at 0°C, add 5ml of p-methylaniline, keep the temperature at 0°C, add 24ml of chloroacetyl chloride, add 60ml of ethyl acetate, keep warm for 1 hour, add water, Static layering, followed by saturated KHSO 4 150ml of water, 150ml of water, 150ml of saturated sodium bicarbonate to wash the organic solvent layer, add 20g of anhydrous magnesium sulfate, keep the organic solvent layer to obtain solution B; mix 117g of anhydrous aluminum trichloride, 128ml of anisole, and 385ml of ethyl acetate Mix and dissolve, add solution B at minus 20°C, react at temperature for 1 hour, and filter; after filtration, dissolve the solid in a mixed solution of 385ml of water, 50ml of concentrated hydrochloric acid, 385ml of ethyl acetate, and 38.5g of sodium chloride, and control the temperature 5°C, stir to dissolve completely, stand to separate layers, extract the water layer with 150ml of ethyl acetate to obtain an organi...
Embodiment 2
[0028] Take 400g of 7-MAC, add 2700ml of dichloromethane, dissolve completely, add 25ml of N,N-dimethylaniline at minus 20°C, keep the temperature at minus 20°C, add 120ml of chloroacetyl chloride, add 310ml of methylene chloride, Insulate for 5 hours, add water, let stand to separate layers, and then use saturated KHSO 4 750ml, water 750ml, saturated sodium bicarbonate 750ml, wash the organic solvent layer, add anhydrous magnesium sulfate 100g, keep the organic solvent layer, and obtain solution B; anhydrous aluminum chloride 575g, isopropyl ether 635ml, and dichloromethane 1900ml Mix and dissolve, add solution B at 0°C, control the temperature and react for 1 hour, then filter; after filtration, dissolve the solid in a mixed solution of 1900ml of water, 270ml of concentrated hydrochloric acid, 1925ml of ethyl acetate, and 190g of sodium chloride, and control the temperature at 20°C , stir to dissolve completely, stand to separate layers, extract the water layer with 800ml o...
Embodiment 3
[0032] Take 150 g of 7-MAC, add 1100ml of tetrahydrofuran, dissolve completely, add 80ml of p-nitroaniline at minus 5°C, keep the temperature at minus 5°C, add 50ml of chloroacetyl chloride, add 121ml of tetrahydrofuran, keep warm for 2 hours, add water, and keep the temperature at minus 5°C. Set the layers, followed by saturated KHSO 4 Wash the organic solvent layer with 310ml of water, 300ml of water and 310ml of saturated sodium bicarbonate, add 100g of anhydrous magnesium sulfate, keep the organic solvent layer, and obtain solution B; mix and dissolve 240g of anhydrous aluminum trichloride, 260ml of diethyl ether, and 790ml of tetrahydrofuran, and dissolve in Add solution B at minus 5°C, react at temperature for 4 hours, and filter; after filtration, dissolve the solid in a mixed solution of 790ml of water, 110ml of concentrated hydrochloric acid, 770ml of ethyl acetate, and 77g of sodium chloride, control the temperature at 10°C, and stir completely The water layer was e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com