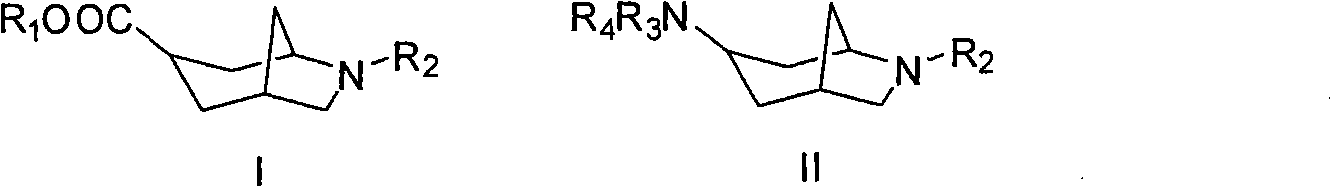
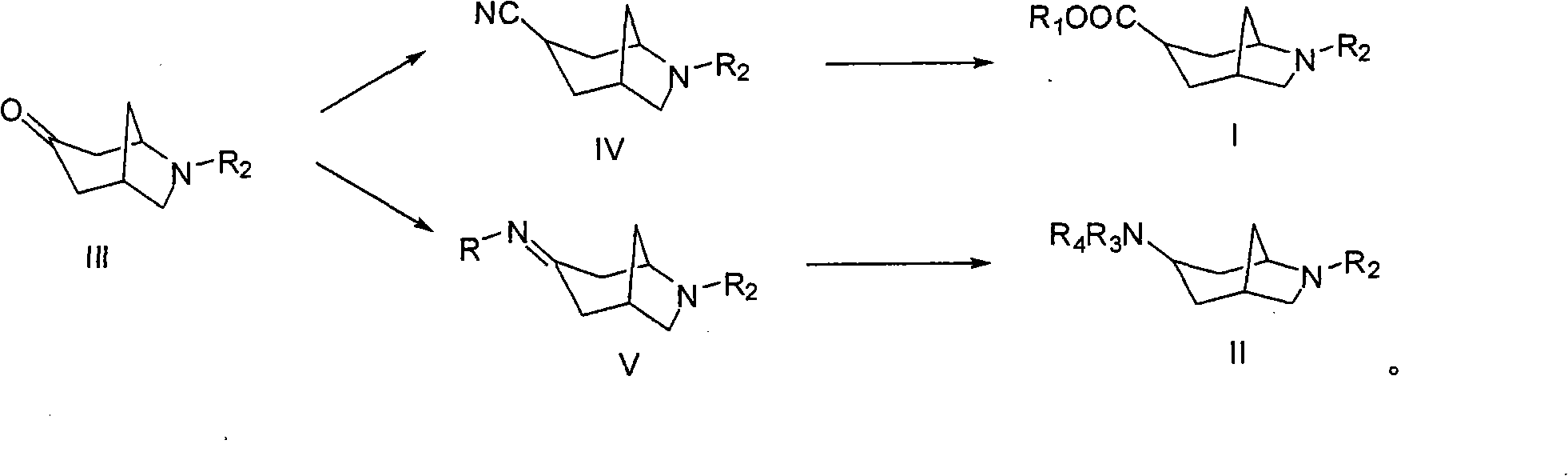
6-azabicyclo (3.2.1) nonane-3-substituted derivative and preparation method thereof
An azabicyclo and derivative technology, which is applied in the field of 6-azabicyclo[3.2.1]octane-3-substituted derivatives and preparation, can solve the problems of poor water solubility of compounds, limitation of spatial structure extension, etc. Physiological activity, the effect of improving diversity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of 3-hydroxyl-5-(methylbenzylamine) cyclohexene
[0042]
[0043] Steps:
[0044] Under the protection of nitrogen, in a dry three-necked flask, add lithium aluminum hydride (1.75g, 43.75mmol) and tetrahydrofuran (170mL), add 3-hydroxyl-5-formylbenzylamide cyclohexene (5g , 0.0216mol). The reaction solution was stirred at 0°C for 0.5 hours, slowly raised to 65°C and stirred overnight, quenched with saturated ammonium chloride aqueous solution, adjusted to pH 7 with 3N aqueous sodium hydroxide solution, filtered, concentrated filtrate, added water, extracted with ethyl acetate , the organic phase was dried and concentrated to obtain 8.5 g of the product, with a yield of 90%.
[0045] HNMR (DMSO) δ: 7.277-7.338 (m, 5H), 5.597 (s, 2H), 4.982 (s, 2H), 2.900 (s, 2H), 1.734-2.148 (m, 3H), 1.497-1.734 (m , 3H), 1.113-1.142(m, 2H).
Embodiment 2
[0046] Embodiment 2: Preparation of 3-carbonyl-6-benzyl-6-azabicyclo[3.2.1]octane
[0047]
[0048] Steps:
[0049] 3-Hydroxy-5-(methylbenzylamine)cyclohexene (20g, 92mmol) was dissolved in 800mL of anhydrous dichloromethane, and activated manganese dioxide (16.0g, 183.9mmol) was added in one go under nitrogen protection. The reaction system was vigorously stirred for 2 hours. A yellow solid was filtered and washed with dichloromethane (2*50 mL). The filtrates were combined and concentrated to obtain an orange oily crude product, which was solidified and recrystallized in a hot n-hexane / ether system to obtain 13.4 g of the product, with a yield of 68%.
[0050] HNMR (MeOD) δ: 7.216-7.335 (m, 5H), 3.716-3.797 (m, 2H), 3.352 (t, J 1 = 4Hz,J 2 =4.8Hz, 1H), 2.832-2.869(m, 1H), 2.742(d, J=10Hz, 1H), 2.626(s, 1H), 2.566(t, J 1 = 1.6Hz,J 2 =2.4Hz, 2H), 2.403(d, J=2Hz, 1H), 2.102-2.122(m, 1H), 1.912(d, J=12Hz, 1H).
Embodiment 3
[0051] Example 3: Preparation of 3-carbonyl-6-tert-butoxycarbonyl-6-azabicyclo[3.2.1]octane
[0052]
[0053] Steps:
[0054] In a dry three-necked flask, add 3-carbonyl-6-benzyl-6-azabicyclo[3.2.1]octane (4g, 18.6mmol), 100mL of anhydrous dichloromethane, then cool to 0°C, Chloroethyl α-chloroformate (2 mL, 22.5 mmol) was added dropwise under nitrogen protection. The reaction solution was stirred at 0°C for 10 minutes, then warmed to room temperature and stirred for 1.5 hours. The reaction solution was concentrated, anhydrous methanol (37.5 mL) was added, and the reaction was refluxed for 2 hours. The reaction solution was concentrated, anhydrous dichloromethane (100 mL) was added to form a suspension, triethylamine (20 mL, 150 mmol) and di-tert-butyl carbonate (5 g, 24 mmol) were added sequentially under ice cooling, warmed to room temperature, and reacted for 64 hours. The reaction solution was diluted with dichloromethane, washed successively with water (100 mL), 1N ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com