Cephalosporin compound, crystal thereof, and preparation method and application thereof
A compound and crystal technology, applied in the field of chemical pharmaceuticals, can solve problems such as bacterial resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
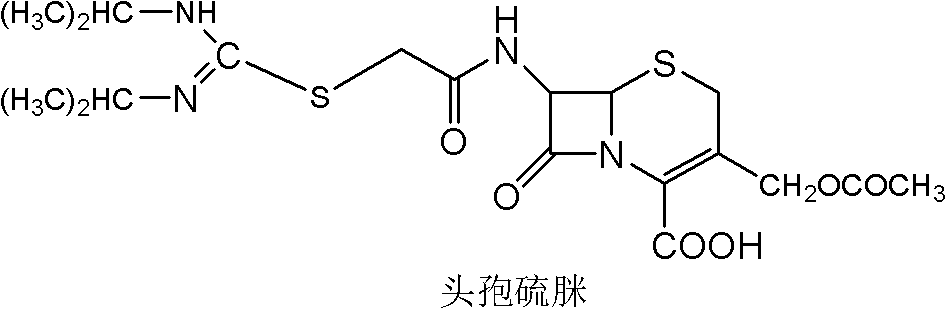
[0034] Example 1: Preparation of 7-bromoacetamido-3-[(acetoxy)methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2 - Formic acid (Intermediate A)
[0035] At room temperature, 30 g (0.11 mol) of 7-ACA was added to 300 ml of water, and triethylamine was slowly added dropwise under stirring at 120 rpm until 7-ACA was completely dissolved to obtain a clear solution. Add 50ml of dichloromethane to the above clear solution, adjust the temperature to 0-5°C, add dropwise sodium bicarbonate solution (24g dissolved in 250ml water) and 13ml bromoacetyl bromide (0.15mol). Keep warm at 0-5°C, react for 30-60 minutes, let stand to separate layers, separate the water phase, slowly add 3mol / L sulfuric acid solution to the water phase, keep the temperature at 0-5°C, and start to precipitate solids when the pH is about 2.5 , until the pH is about 1.0 to 1.5 as the titration end point, and continue to grow crystals for 1h. After suction filtration, the filter residue was washed three times wit...
Embodiment 2
[0036] Embodiment 2: Preparation of Intermediate A
[0037] At room temperature, 30 g (0.11 mol) of 7-ACA was added to 500 ml of water, and sodium bicarbonate solution (27.6 g dissolved in 250 ml of water) was added dropwise with stirring until 7-ACA was completely dissolved to obtain a clear solution. Add 100ml of toluene to the above clear solution, adjust the temperature to 30-35°C, continue to add dropwise the remaining sodium bicarbonate solution and 12ml of bromoacetyl bromide (0.138mol). Keep warm at 30-35°C, react for 30-60 minutes, let stand to separate layers, separate the water phase, adjust the temperature of the water phase to room temperature, slowly add 3mol / L hydrochloric acid to the water phase, and start to precipitate when the pH is about 2.5 Solid, until the pH is about 1.0-1.5 is the titration end point, and continue to grow crystals for 1h. After suction filtration, the filter residue was washed three times with water and dried in vacuum to obtain about ...
Embodiment 3
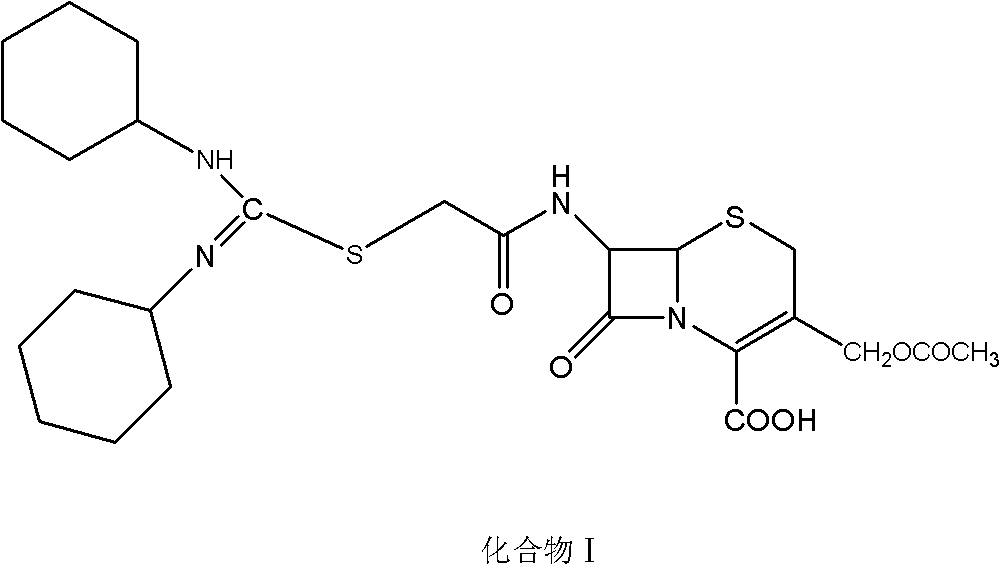
[0038] Embodiment 3: preparation compound I
[0039] Adjust the temperature of 500ml of dichloromethane to 15-20°C, add 39.3g (0.1mol) of intermediate A, and slowly add triethylamine dropwise until intermediate A is completely dissolved to obtain a clear solution, then add N,N'-di Cyclohexylthiourea 36g (0.15mol), slowly adjust the temperature to 25-30°C. After reacting for 2h, suction filtered, and the filter residue was washed 3 times with dichloromethane. After vacuum drying at room temperature, about 39 g of compound I was finally obtained. The purity was determined to be 93.2% by HPLC method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com