Simple and convenient preparation method of relebactam
A technology of relebactam and compounds, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of low atom economy, cumbersome operation, and unfavorable industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
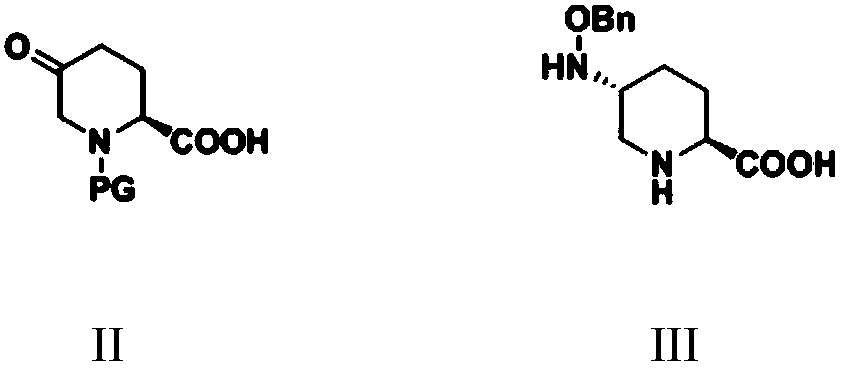
[0089] Embodiment 1: the preparation of L-diethyl glutamate hydrochloride (preparation of formula II compound intermediate)
[0090] Add 2.4 L of absolute ethanol to a 5 L reaction flask equipped with a spherical condenser, drying tube, tail gas absorption device and constant pressure dropping funnel, and add 294.0 g (2.0 moles) of L-glutamic acid under stirring. 593.5 grams (2.0 moles) of triphosgene were dissolved in 1.5 L of dichloromethane. At 0°C, add triphosgene in dichloromethane dropwise into the reaction system containing L-glutamic acid, keeping the temperature below 25°C. After the dropwise addition, the temperature was raised to 50° C. and the reaction was refluxed until the solid was completely dissolved. The reaction was stopped, the temperature of the reaction system was cooled to room temperature, and the solvent was evaporated under reduced pressure to obtain a transparent light yellow oil. Add 800ml methyl tert-butyl ether to make a slurry. Suction filtrat...
Embodiment 2
[0091] Embodiment 2: the preparation of N-methoxycarbonylmethyl-N-benzyl-L-glutamic acid diethyl ester (preparation of formula II compound intermediate)
[0092] Add 4.0 L of acetonitrile to a 5 L reaction flask equipped with a spherical condenser, a drying tube, a thermometer and a constant pressure dropping funnel. Add 480.0 g (2.0 moles) of L-glutamic acid diethyl ester hydrochloride prepared according to the method in Example 1 to the system, stir and dissolve at room temperature. Add 829.2 g (6.0 moles) of anhydrous potassium carbonate to the system and stir at room temperature. 219.85 g (2.02 mol) of methyl chloroacetate and 33.85 g (0.2 mol) of potassium iodide were successively added to the system. The system was heated to 80°C for reaction. After the reaction was detected by GC, 258.56 grams (2.04 moles) of benzyl chloride were added to the system, and the temperature was kept at 80°C. After the reaction was detected by GC, the reaction was stopped and cooled to room...
Embodiment 3
[0093] Embodiment 3: Preparation of (S)-N-benzyl-5-oxo-2-piperidinecarboxylic acid (II)
[0094] Under the protection of nitrogen, add 500 mL of pretreated toluene and 32.16 g (0.47 moles) of sodium ethoxide solid to a 1 L reaction flask equipped with a mechanical stirrer, a condenser tube, a drying tube, a thermometer and a constant pressure dropping funnel, stir, and heat for 110 °C to reflux. 132.85 g (0.36 mol) of N-methoxycarbonylmethyl-N-benzyl-L-glutamic acid diethyl ester prepared in Example 2 was dissolved in 100 mL of toluene and transferred to a constant pressure dropping funnel. Under the condition of reflux, start to drop the toluene solution of the raw material, and continue the reflux reaction after the dropwise addition is completed. After the completion of the reaction detected by GC, the reaction was stopped, and the temperature was lowered to room temperature. The reaction system was slowly added dropwise to 400 mL of saturated ammonium chloride solution, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com