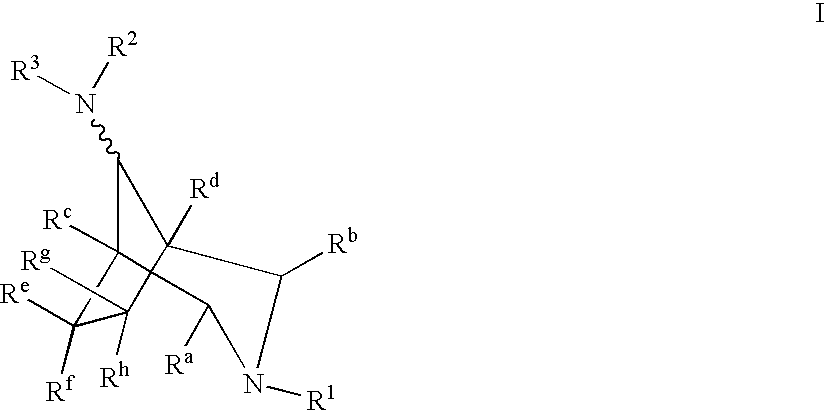
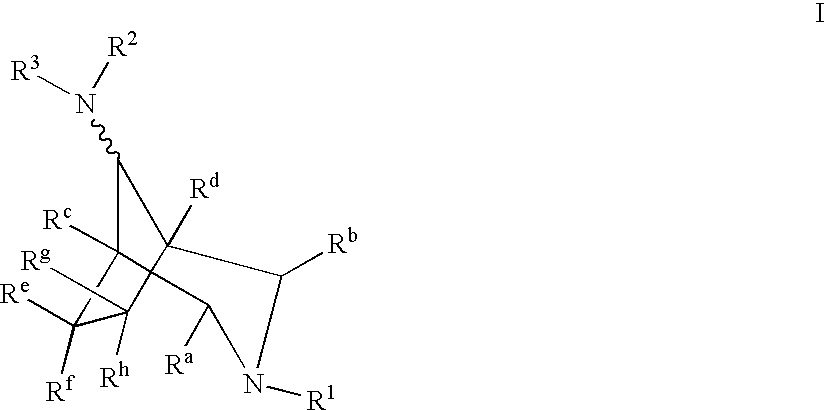
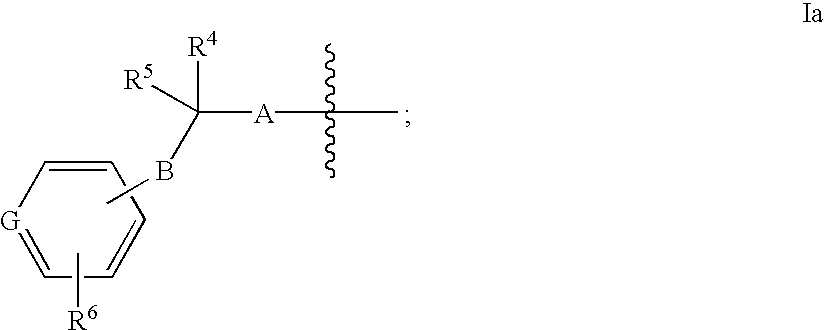Azabicyclooctane derivatives useful in the treatment of cardiac arrhythmias
a technology of azabicyclooctane and derivatives, which is applied in the direction of biocide, drug composition, cardiovascular disorder, etc., can solve problems such as (turning points
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation b
3-Benzyl-N-methyl-3-azabicyclo[3.2.1]octan-8-amine
A mixture of sodium cyanoborohydride (35.0 g, 0.557 mol) and zinc chloride (37.9 g, 0.278 mol) in methanol (500 mL) was added dropwise to a mechanically stirred mixture of 3-benzyl-3-azabicyclo[3.2.1]octan-8-one (Preparation A; 120 g, 0.557 mol) and methylamine hydrochloride (151 g, 2.23 mol) in methanol (1.0 L) at 25.degree. C. under nitrogen. After 2.5 h of stirring, 6 N NaOH (90 mL) was added dropwise. The mixture was filtered through a pad of Celite.RTM., washing with methanol (500 mL). The filtrate was concentrated in vacuo. Water (500 mL) was added, and the aqueous mixture was extracted with ethyl acetate (3.times.500 mL). The combined organic extracts were dried (Na.sub.2 SO.sub.4), and concentrated in vacuo. The residue was chromatographed, eluting with methanol dichloromethane (1:10), to afford 63.0 g (49%) of the sub-title compound as a solid.
.sup.1 H NMR (300 MHz, CCDl.sub.3): .delta. 7.40-7.10 (m, 5H), 3.88 (s, 2H), 3.60 ...
preparation d
tert-Butyl 3-Azabicyclo[3.2.1]oct-8-yl(methyl)carbamate
A solution of 1 M HCl in diethyl ether (730 mL, 730 mmol) was added dropwise to a solution tert-butyl 3-benzyl-3-azabicyclo[3.2.1]oct-8-yl(methyl)carbamate (Preparation C; 241 g, 0.730 mol) in diethyl ether (1.5 L). The resulting precipitate was collected via filtration through a sintered glass funnel and then dried in vacuo. The solid was dissolved in methanol (4.0 L), and 10% palladium on carbon (24 g) was added. The mixture was stirred under one atmosphere of hydrogen at 40.degree. C. overnight. The catalyst was filtered through a pad of Celite.RTM., washing with methanol (1.0 L). The filtrate was concentrated in vacuo, and chromatographed, eluting with dichloromethane:methanol:concentrated ammonium hydroxide (88:10:2) to afford 70 g (40%) of an oil. A solution of this oil (70 g, 296 mmol) in diethyl ether (1.0 L) was treated with 1 M HCl in diethyl ether (300 mL), added dropwise. The resulting precipitate was removed via fil...
example 1
tert-Butyl 8-{[3-(4-Cyanoanilino)propyl](methyl)amino}-3-azabicyclo[3.2. 1]octane-3-carboxylate
A mixture of 4-({3-[3-azabicyclo[3.2.1]oct-8-yl(methyl)amino]propyl}amino)benzonitrile (Preparation H; 170 mg, 0.569 mmol) and di-tert-butyldicarbonate (124 mg, 0.569 mmol) in dichloromethane (3.0 mL) was stirred for 2 h at 25.degree. C. under nitrogen. The mixture was concentrated in vacuo and the residue chromatographed on silica gel, eluting with ethyl acetate:dichloromethane (1:2), to yield 90 mg (40%) of the title compound as a colorless oil.
.sup.1 H NMR (300 MHz, CDCl.sub.3): .delta. 7.40 (d, J=8.2 Hz, 2H), 6.50 (d, J=8.2 Hz, 2H), 5.45 (s, 1H), 3.68-3.60 (m, 1H), 3.52-3.40 (m, 1H), 3.32-3.10 (m, 4H), 2.60 (s, 2H), 2.30 (s, 3H), 2.22-2.15 (m, 3H), 1.92-1.60 (m, 6H), 1.45 (s, 9H). .sup.13 C NMR (75 MHz, CDCl.sub.3): .delta. 156.4, 151.6, 133.6, 120.6, 112.0, 108.1, 79.4, 67.8, 54.2, 45.0, 44.0, 42.7, 39.8, 35.6, 28.4, 26.0, 25.4. CI-MS: (M+1)=399 m / z.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com