Improved tadalafil preparation method
A tadalafil and reaction technology, applied in the field of chemical raw material preparation, can solve the problems of low total yield, flammable and explosive, high price, etc., and achieve the effects of improving total yield, stable process and reducing cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
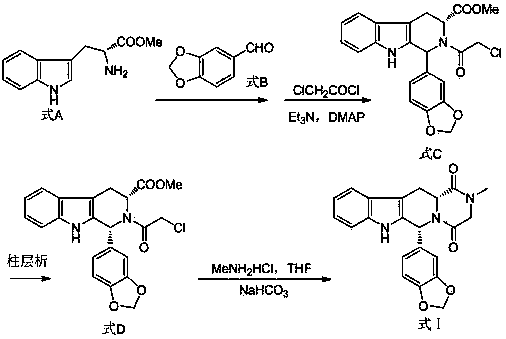
[0023] Example 1: 25.5 g (0.1 mol) of D-tryptophan methyl ester hydrochloride and 250 mL of dichloromethane were sequentially added into a 500 mL reaction flask, and stirred at room temperature for 1 h. 15.0 g (0.1 mol) of piperonal was added, the temperature was raised to reflux for 12 hours, and the end point of the reaction was controlled by TLC. Cool down to room temperature, add 20.2g (0.2mol) of triethylamine, 0.12g (about 0.001moL) of 4-dimethylaminopyridine, stir for 1h, cool down to 0°C, and keep the temperature at 0~5°C and add ethyl chloride dropwise Acyl chloride in dichloromethane solution 50mL (containing 11.3g of chloroacetyl chloride, about 0.1mol), stirred at room temperature for 1h after the drop was completed. The reaction solution was washed 3 times with purified water (100 mL×3), dichloromethane solution, and dried by adding anhydrous sodium sulfate. Filtration, dichloromethane was distilled off to obtain the intermediate 1-(1,3-benzodioxol-5-yl)-2-(chl...
Embodiment 2
[0024] Example 2: 28.0 g (0.11 mol) of D-tryptophan methyl ester hydrochloride and 250 mL of dichloromethane were sequentially added into a 500 mL reaction flask, and stirred at room temperature for 1 h. 16.5 g (0.11 mol) of piperonal was added, the temperature was raised to reflux for 12 hours, and the end point of the reaction was controlled by TLC. Cool down to room temperature, add 22.0g (0.22mol) of triethylamine, 0.12g (about 0.001moL) of 4-dimethylaminopyridine, stir for 1h, cool down to 0°C, and keep the temperature at 0~5°C and add ethyl chloride dropwise Acyl chloride in dichloromethane solution 50mL (containing 12.4g of chloroacetyl chloride, about 0.11mol), stirred at room temperature for 1h after the drop was completed. The reaction solution was washed 3 times with purified water (100 mL×3), dichloromethane solution, and dried by adding anhydrous sodium sulfate. Filtration, dichloromethane was distilled off to obtain the intermediate 1-(1,3-benzodioxol-5-yl)-2-(c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com