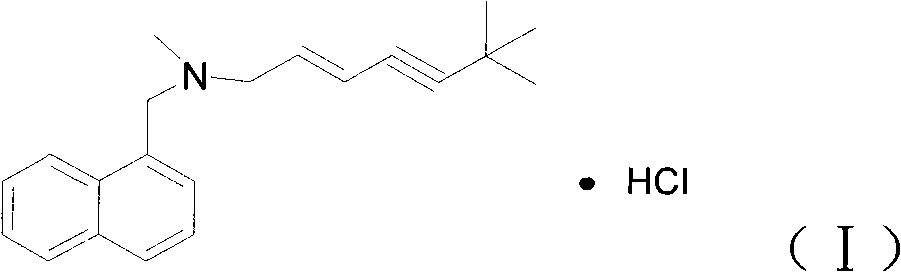
Preparation method of Terbinafine hydrochloride
A technology of terbinafine hydrochloride and naphthylmethylamine hydrochloride is applied in the field of preparation of terbinafine hydrochloride, can solve the problems of high reaction temperature, high price, long reaction time and the like, and achieves complete reaction , the effect of shortened reaction time, cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0020] At room temperature, add 650ml of N,N-dimethylformamide into a 1000ml three-necked flask, add 7.5 grams (0.05 moles) of sodium iodide, stir to dissolve, add dropwise 78.5 grams (0.5 moles) of 1-chloro-6,6 -Dimethyl-2-hepten-4-yne, reacted for 1 hour. Add 106 grams (1 mole) Na in turn to the reaction solution 2 CO 3 , 104 grams (0.5 moles) of N-methyl-1-naphthylmethylamine hydrochloride, reacted at 20° C.-30° C. for 4 hours.
[0021] The solid in the reaction liquid was removed by filtration, and the concentrated brown liquid was added to 600 ml of ethyl acetate, mixed well, washed with an appropriate amount of water, the water layer was separated, the organic layer was dried, and concentrated to obtain 140 g of brown liquid. With 500 ml of absolute ethanol, under stirring, an appropriate amount of dry hydrogen chloride was introduced into the reaction solution, stirred at room temperature for half an hour, and concentrated to obtain 200 g of a yellow solid.
[0022] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com