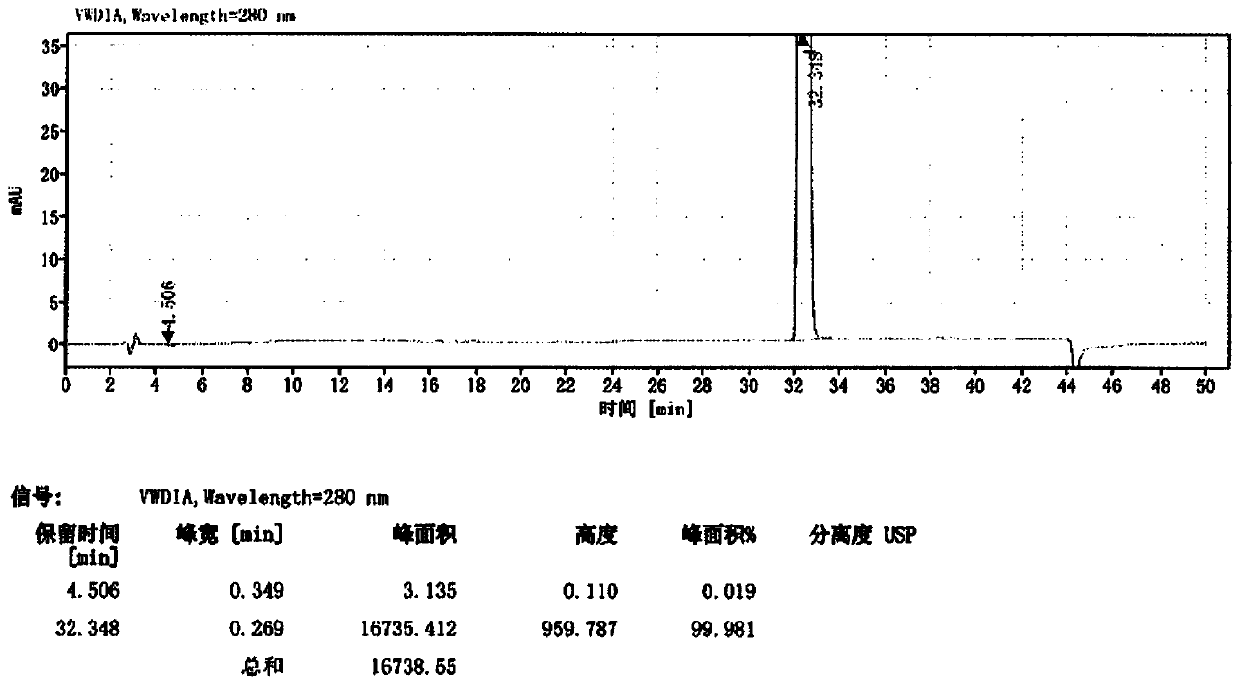
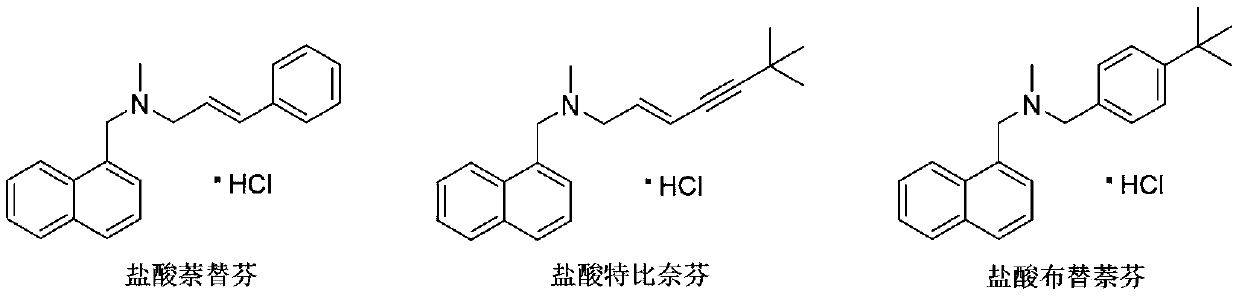
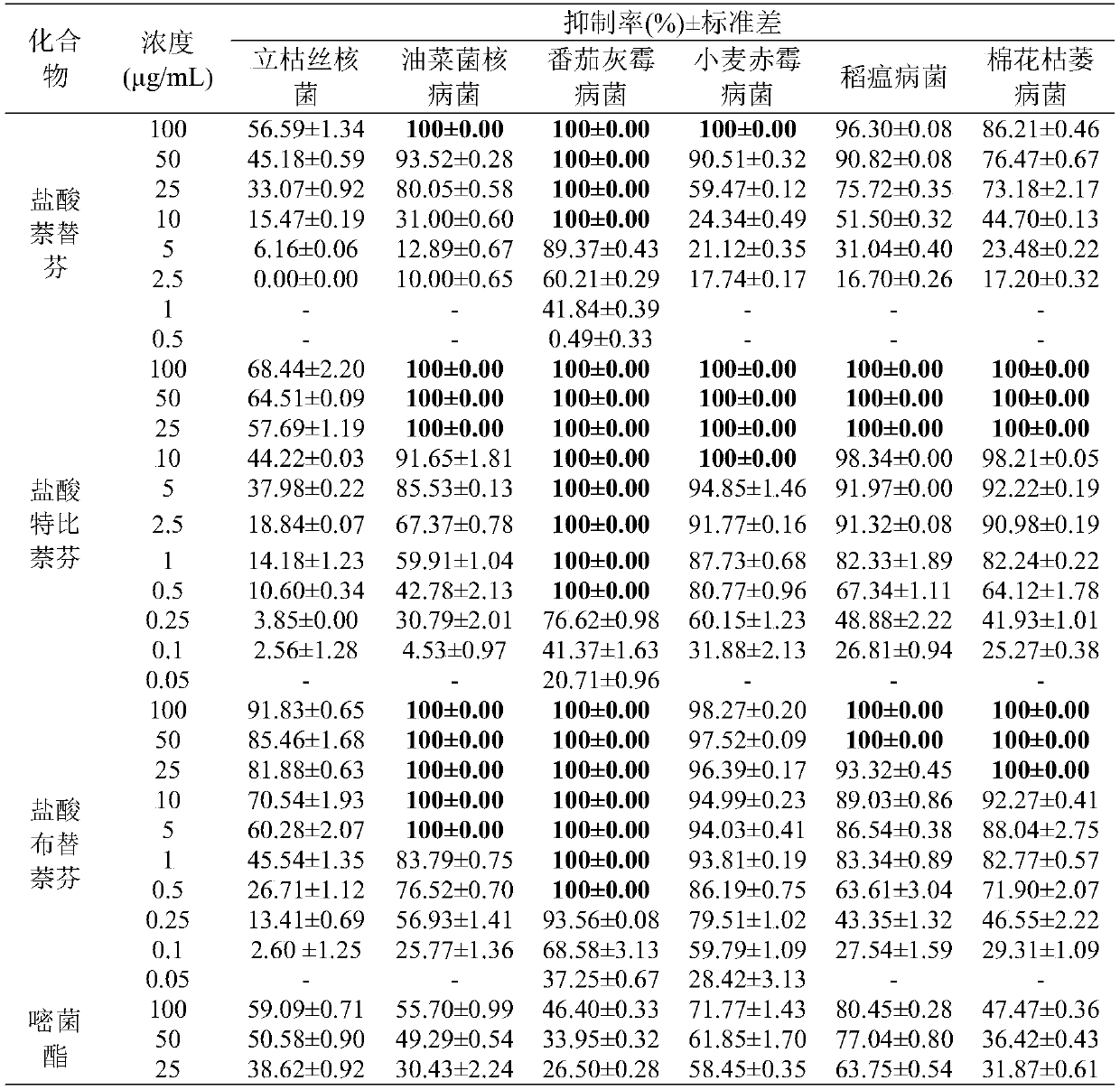
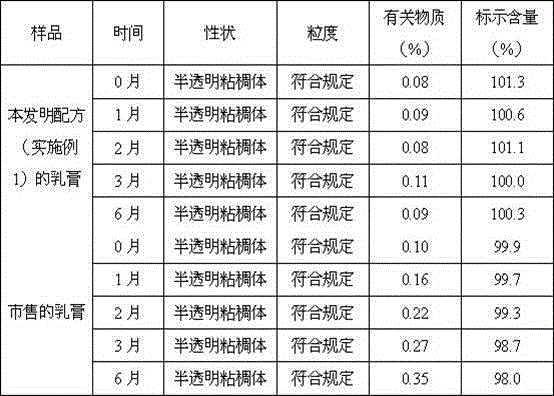
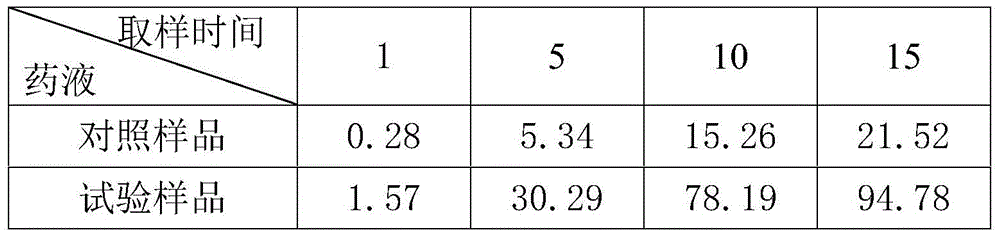
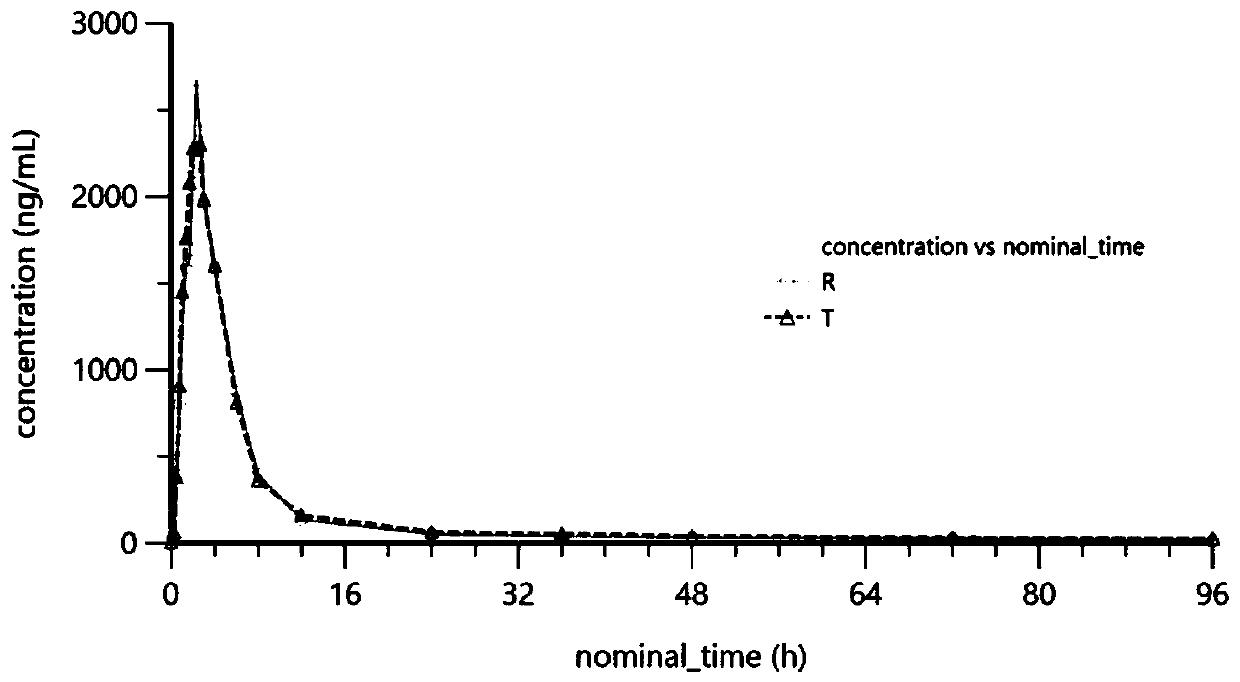
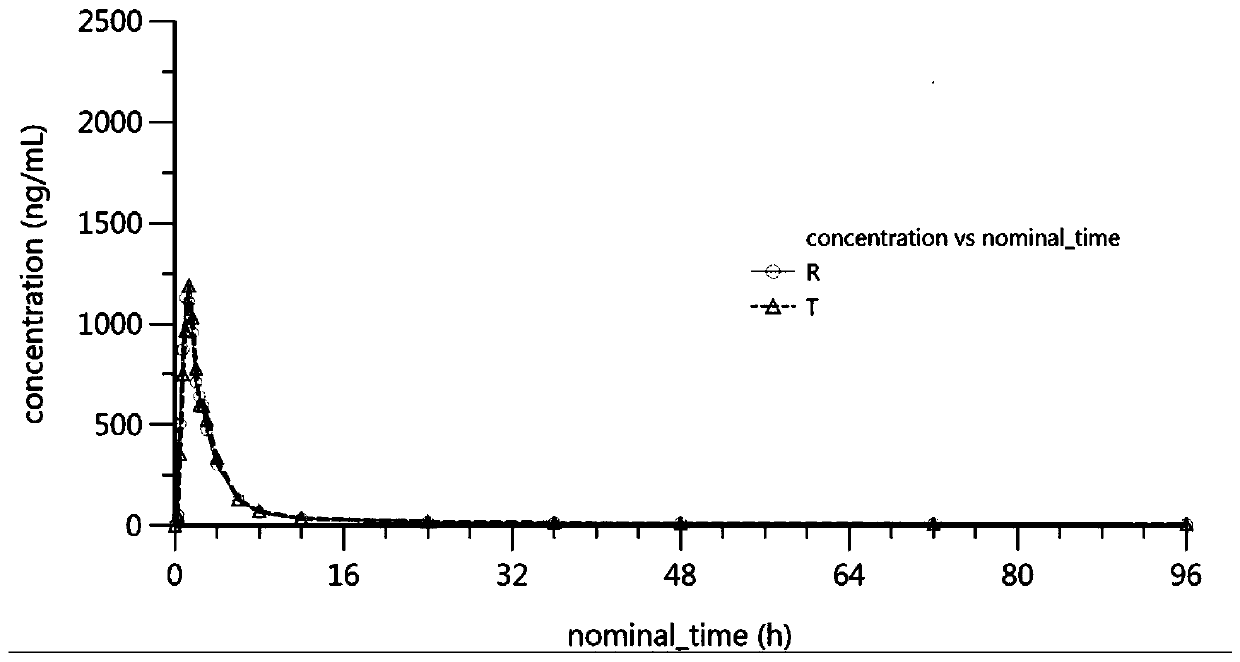
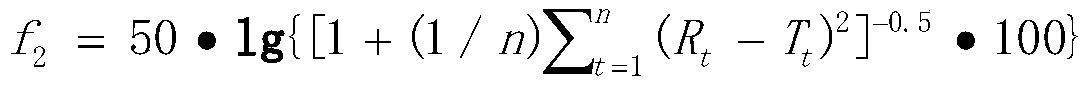Patents
Literature
93 results about "Terbinafine Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A synthetic allylamine derivative structurally related to naftifine, antifungal Terbinafine Hydrochloride blocks ergosterol biosynthesis by inhibition of squalene epoxidase, part of the sterol synthesis pathway for the fungal cell membrane. Highly lipophilic, it tends to accumulate in skin, nails, and fatty tissues. Terbinafine is active against dermatophytes. (NCI04)
Synthesis of terbinafine hydrochloride
InactiveCN1362400ALow costFix security issuesAmino preparation from aminesAmino compound preparation by condensation/addition reactionsGrignard reagentTert butyl
The method for synthesizing terbinafine hydrochloride includes the following steps: using tert-butyl acetylene as raw material, converting it into 3,3-dimethyl-1-butyne litihium or 3,3-dimethyl-1-butyne Grignard reagent, then synthesizing intermediate 1-halo-6,6-diemthyl-2-hepten-4-acetylene, heating said intermediate and N-methyl-1-naphthoamine and making them produce condensation reaction in alkaline system, using HCl to form salt to obtain the invented product.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI +2
Antimycotic externally used drug
InactiveCN101129409APromote regenerationGood curative effectOrganic active ingredientsAntimycoticsDiseaseBeriberi
The invention discloses an externally-used medicament for treating skin mycosis diseases, which is prepared from the raw materials of (by weight percent) terbinafine hydrochloride or miconazole or ketoconazole 0. 5-2%, Clobetasol Propionate 0. 01-0. 2%, neomycin sulphate 0. 3-1%, bee glue 5-25%, and balancing distilled water.
Owner:程贵昌
Transdermal delivery system of terbinafine hydrochloride long-acting spraying agent
ActiveCN107638423AWaterproofFriction-resistantOrganic active ingredientsAntimycoticsPharmaceutical drugMedicinal chemistry
The present invention belongs to the technical field of pharmaceutical preparations, discloses a transdermal delivery system for long-acting treatment of fungal skin infections, ie., a terbinafine hydrochloride long-acting spraying agent, and particularly provides a terbinafine hydrochloride water-soluble stable system preparation method, wherein the terbinafine hydrochloride long-acting sprayingagent can form a true film layer after the terbinafine hydrochloride long-acting spraying agent is sprayed onto the skin surface, such that the maintenance time of the preparation on the skin can be prolonged, the drug can be slowly released on the skin, and can act and be stored at lesions, and the long-acting treatment of fungal skin infections can be achieved.
Owner:CHINA PHARM UNIV
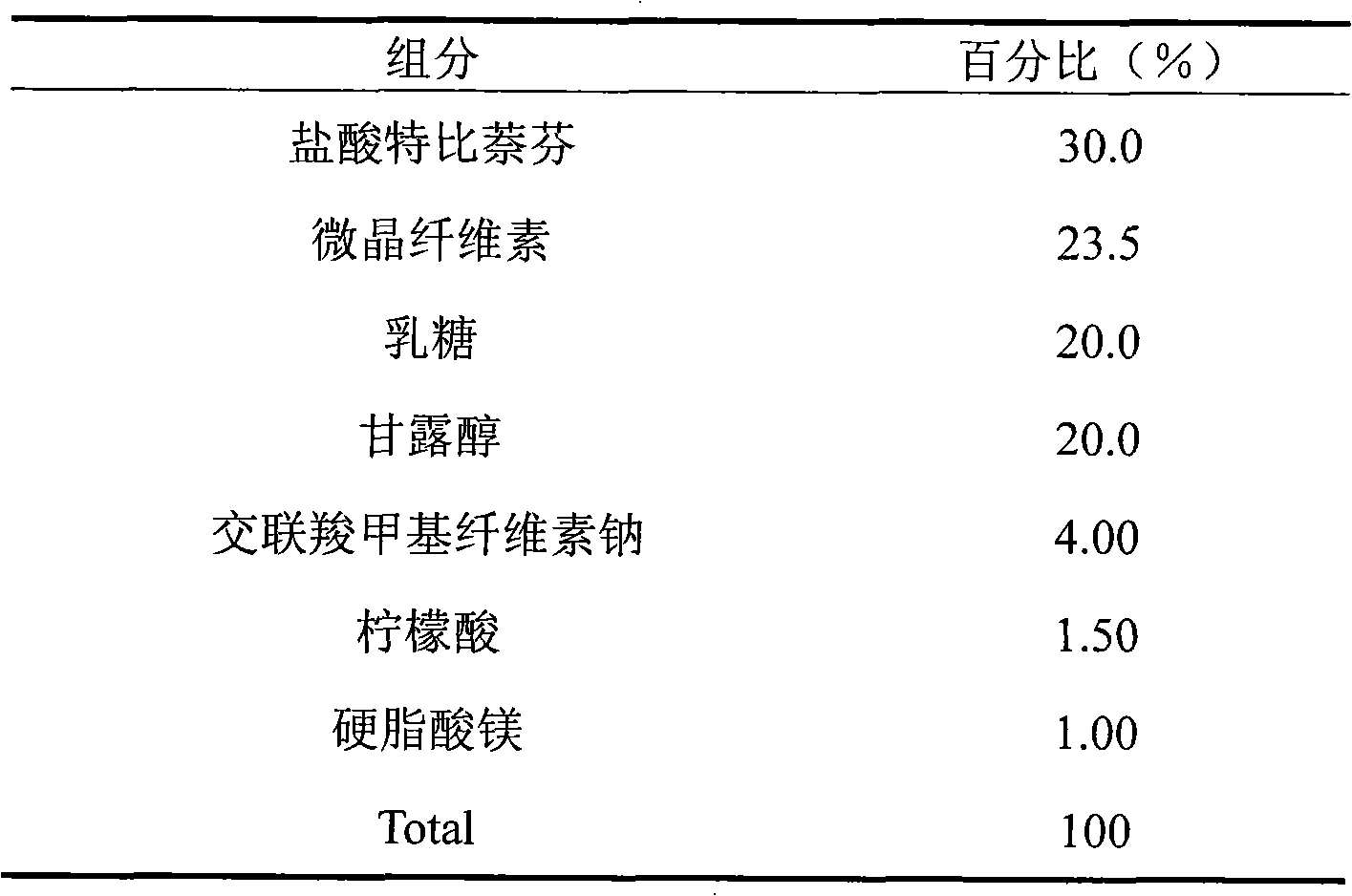
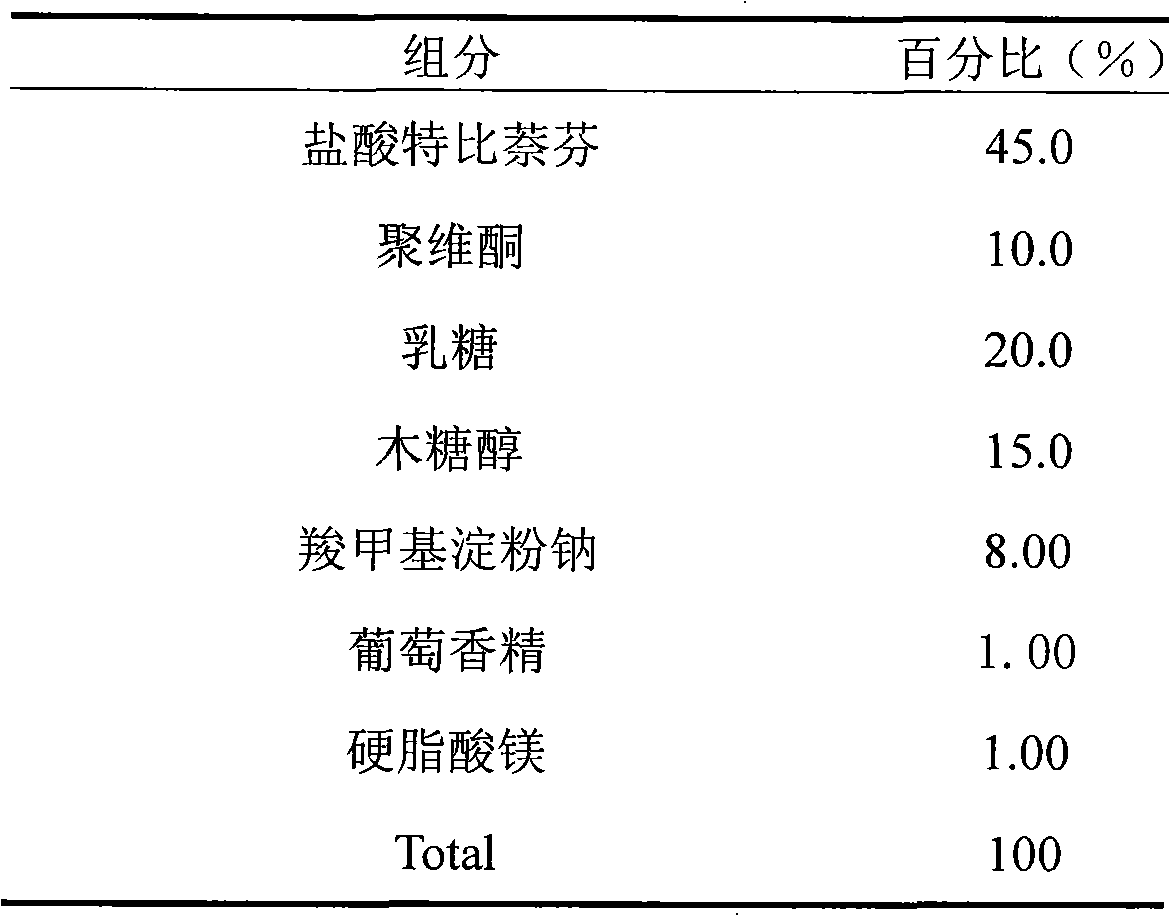
Dispersing tablet containing terbinafine hydrochloride
ActiveCN101548958AEasy to takePromote dissolutionOrganic active ingredientsAntimycoticsAdditive ingredientActive component
The invention discloses a dispersing tablet containing terbinafine hydrochloride. The dispersing tablet comprises active components of the terbinafine hydrochloride and medical auxiliary materials and is clinically used for killing or inhibiting fungus.
Owner:AVENTIS PHARMA HAINAN
Eye antifungal nano-micelle solution containing terbinafine hydrochloride
ActiveCN105012235ALess irritatingConvenient eye dropsOrganic active ingredientsSenses disorderAntifungalIrritation
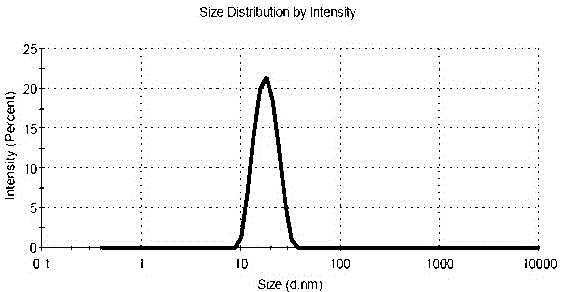
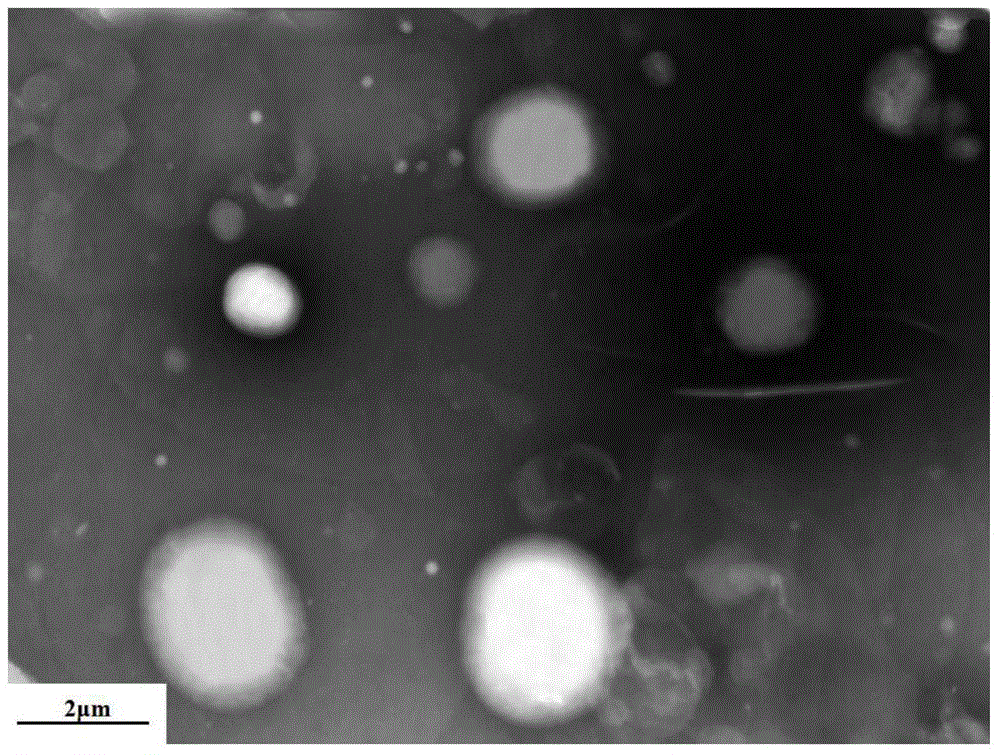
The invention discloses an eye antifungal nano-micelle solution containing terbinafine hydrochloride. The eye antifungal nano-micelle solution consists of the following raw materials in parts by weight: 0.05-1 part of terbinafine hydrochloride, 0.5-10 parts of a solubilizer, 0.5-10 parts of glycerinum, 0.003-0.05 part of a bacteriostatic agent, 0.05-0.2 part of a thickening agent and 90-99.8 parts of water. According to the eye terbinafine hydrochloride antifungal nano-micelle solution disclosed by the invention, terbinafine hydrochloride nano-micelle is prepared from the solubilizer by self aggregation, the average particle size of the terbinafine hydrochloride nano-micelle is smaller than 30nm, the characters are not greatly changed after the terbinafine hydrochloride nano-micelle is subjected to freezing redissolution and boiling sterilization for 30 minutes and then cooled to room temperature, and the physicochemical properties are also stable. Water is taken as a solvent of the eye antifungal nano-micelle solution, and no organic solvent is used in the preparation process, so that the eye antifungal nano-micelle solution is small in irritation, relatively convenient to drop into eyes, free of phenomenon of adhesion or fuzziness, relatively long in eye acting time, relatively good in absorption by eyes, and relatively good in antifungal effect, and use comfort degree of patients can be greatly increased.
Owner:河南省眼科研究所
Spray for treating fungus infection on body surface of pet and preparation method of spray
ActiveCN104288290AHigh cure rateInhibits squalene epoxidaseAntimycoticsAerosol deliveryAlcoholEugenol
The invention discloses a spray for treating fungus infection on the body surface of a pet and a preparation method of the spray and aims to provide a spray which is used for treating fungus infection on the body surface of the pet and is simple in preparation method, convenient to use, good in curative effect and high in safety. The technical key points are as follows: on one hand, the invention provides the spray for treating fungus infection on the body surface of the pet, and the spray is composed of the following components in parts by weight: 65-95 parts of alcohol, 0.1-5 parts of terbinafine hydrochloride, 0.1-5 parts of azone, 5-20 parts of extracts of a traditional Chinese medicine composition and 0.1-5 parts of eugenol, wherein the traditional Chinese medicine composition is prepared from the following raw materials in parts by weight: 5-20 parts of fructus cnidii, 3-10 parts of rhubarb, 2-8 parts of litsea cubeba, 2-5 parts of cortex dictamni and 3-7 parts of fructus kochiae; and on the other hand, the invention provides a preparation method of the spray. The spray and the preparation method thereof belong to the technical field of medicines for animals.
Owner:WUHAN CHOPPER LVYA BIOLOGICAL SCI & TECH CO LTD
Preparation method of terbinafine hydrochloride microcapsule antibacterial agent for shoes
A preparation method of a terbinafine hydrochloride microcapsule antibacterial agent for shoes comprises the steps: with chitosan and Arabic gum as a wall material and a terbinafine hydrochloride emulsifiable paste as a core material, dispersing the terbinafine hydrochloride emulsifiable paste in a capsule wall material aqueous solution, regulating a pH value, allowing the positively charged chitosan and the negatively charged Arabic gum to generate an electrostatic effect, attracting each other, then making the solubility reduced, generating phase separation, forming a gel layer, precipitating the gel layer around the core material, and finally adding glutaraldehyde to make the obtained microcapsule cured. The prepared terbinafine hydrochloride microcapsule has uniform distribution of particle size, is regularly spherical in shape, can better penetrate into the interior of fiber, and achieves the purpose of lasting bacteria resistance whenever and wherever possible; and the prepared microcapsule also has antifungal and antibacterial performance, is more suitable for shoe material bacteria resistance, and has the characteristics of being simple and easy to implement.
Owner:SHAANXI UNIV OF SCI & TECH
Application of composition of thymol, fipronil and terbinafine hydrochloride, animal skin disease prevention and curing drug and preparation method thereof
The invention discloses application of composition of thymol, fipronil and terbinafine hydrochloride, an animal skin disease prevention and curing drug and a preparation method thereof. The animal skin disease prevention and curing drug comprises the thymol, the fipronil and the terbinafine hydrochloride; the preparation method of the animal skin disease prevention and curing drug comprises the steps that the thymol, the fipronil and the terbinafine hydrochloride are added into acceptable ingredients, stirred and dissolved to obtain the animal skin disease prevention and curing drug. The animal skin disease prevention and curing composition can cure the animal skin disease in a broad spectrum, the curative effect is good, and the action speed is fast.
Owner:FOSHAN NANHAI EASTERN ALONG PHARMA CO LTD
Terbinafine hydrochloride emulsifiable paste and preparation method thereof
ActiveCN101467960AHigh glossGood spreadabilityOrganic active ingredientsAntimycoticsPolyethylene glycolDissolution
The invention relates to a self-emulsified terbinafine hydrochloride cream and preparation method thereof, comprising, main medicaments, terbinafine hydrochloride of 0.5-3%, TEFOSE63 (polyethylene glycol-7 stearate) of 6-18%, span 80 of 1-6%, glycerin monostearate of 1-5%, and light liquid petrolatum of 1-4%; the remainder being purified water. Self-emulsifier, surfactant and solvent are heated to 75-80 DEG C, rapidly stirred for 30-45 minutes after complete dissolution, and cooled to around 40 DEG C; maintaining the temperature, the terbinafine hydrochloride is added and stirred continuously; triethanolamine of proper amount is added to regulate the pH value between 4-6, vacuumized, and continuously stirred to the room temperature to obtain the cream with good brightness, good coating ability, less consistency change with the environmental temperature change, good stability and transdermal absorption. Compared with the traditional preparation technology, the preparation method thereof is simple in operation, low in the energy consumption and low in device investment.
Owner:ZHEJIANG CONBA PHARMA
Pet all-in-one insect expelling spray and preparation method thereof
InactiveCN105688168AReduce infectionProtect the skinAntibacterial agentsHydroxy compound active ingredientsFipronilIrritation
The invention relates to a pet all-in-one insect expelling spray and a preparation method thereof, and belongs to the field of pet articles. The pet all-in-one insect expelling spray is prepared from the following raw materials in percentage by weight: 30% to 60% of distilled water, 3% to 7% of olive oil, 8% to 12% of propylene glycol, 5% to 8% of glycerinum, 8% to 12% of high-quality castor oil, 3% to 5% of aloe extract, 3% to 5% of ginger extract, 0.5% to 1% of borneol, 0.3% to 0.7% of eucalyptus oil, 0.12% to 0.15% of pyrethroid, 0.2% to 0.5% of abamectin, 0.1% to 0.3% of Fipronil, 0.5% to 0.7% of N-(2-ethylhexyl)-5-norbornene-2,3-dicarboximide, 1% to 2% of terbinafine hydrochloride, 10% to 14% of ethyl alcohol, and 1% to 2% of camphor extract. The pet all-in-one insect expelling spray has the advantages that by adding multiple plant extracts, the bacteria infection of the pet can be decreased, the damaged hair follicles are restored, the growth of hair follicles is promoted, and the pet skin is effectively protected; various types of parasites at the surface of the pet can be effectively removed, and the attack by the parasites can be prevented; the plant extraction is safe and is free from irritation, so that the pet is cared in all directions.
Owner:SHANGHAI SHENYA ANIMAL HEALTH PROD FUYANG CO LTD
Acne cream and preparing method thereof
ActiveCN104688808AThorough curative effectSignificant effectHydroxy compound active ingredientsAerosol deliveryDiseaseWestern medicine
The invention relates to the technical field of drugs for skin diseases, in particular to acne cream and a preparing method thereof. The acne cream is made from terbinafine hydrochloride, rifampicin, clindamycin, borneol, puerarin powder, poria cocos powder and dandelion. By means of compatibility of medicines contained in the acne cream, the acne cream can comprehensively treat pathomycete, bacteria and viruses which cause acne in the view of Western medicine and heat, poison and dampness which cause acne in the view of traditional Chinese medicine, treatment effect is thorough and remarkable, and patients who have acne can be cured comprehensively at low cost within a short period of time so as to return to normal life. Furthermore, the preparing method of the acne cream enables the treatment effect of the acne cream to be guaranteed, operation is easy, production cost is low, and the acne cream is convenient to carry and take.
Owner:太原市福星生物科技有限公司
Compound medicament for treating proventriculitis and gizzard erosion of poultry and preparation method thereof
ActiveCN103394021AGuaranteed normal needsQuick cureHydroxy compound active ingredientsDigestive systemSnow moldTherapeutic effect
A disclosed compound medicament for treating proventriculitis and gizzard erosion of poultry comprises the following components in parts by weight: 5-20 parts of terbinafine hydrochloride, 10-40 parts of ampicillin sodium, 50-150 parts of amomum villosum, 50-100 parts of poria cocos, 5-15 parts of vitamin A and 10-20 parts of methionine. By aiming at the symptoms of proventriculitis and gizzard erosion of poultry and inducing factors, the medicines used for inhibiting fungus and mould, the traditional Chinese medicines capable of controlling gastric acid secretion and other medicines are selected for the medicament of the invention, and the formula is reasonable; and the medicament has the characteristics of production simplicity, low price, convenient production and the like.
Owner:CHENGDU CENTURY INVESTMENT
Terbinafine hydrochloride cream and preparation method thereof
ActiveCN103126976AGood application effectMedication convenienceOrganic active ingredientsAntimycoticsIrritationTherapeutic effect
The invention relates to a terbinafine hydrochloride cream. According to the invention, terbinafine hydrochloride with an effective amount is adopted as a main raw material, and the cream is prepared with the main raw material and pharmaceutically acceptable auxiliary materials of sodium dodecyl sulfate, propylene glycol, glycerol monostearate, octadecanol, white vaseline, light liquid paraffin, ethyl paraben, and water. The cream provided by the invention has the advantages of moisturizing cream body, good spreadability, convenient medication, low irritation to skins, good treatment effect, and stable quality.
Owner:TIANSHENG PHARMA GROUP
Preparation method of Terbinafine hydrochloride
InactiveCN101870655ARaw materials are cheap and easy to getThorough responseAntimycoticsPhysical/chemical process catalystsSodium iodideAlkyne
The invention relates to a preparation method of Terbinafine hydrochloride, which comprises the following steps: dissolving a certain amount of catalyst, i.e. sodium iodide (or potassium iodide) in an organic solvent, adding 1-chloro-6,6-dimethyl-2-heptylene-4-alkyne, sequentially adding alkali and N-methyl-1-naphthalene methylamine hydrochloride, carrying out condensation at 10-40 DEG C, salifying the obtained product in an alcohol system with hydrogen chloride to obtain the Terbinafine hydrochloride, and carrying out recrystallization in an alcohol / water mixed solution to obtain the qualified product. In the method, the raw materials are cheap and easily-acquired, the reaction is complete, and the reaction time is greatly shortened. Therefore, the method is completely suitable for industrialized mass production.
Owner:YANGTZE RIVER PHARMA GRP BEIJING HAIYAN PHARMA +1
Terbinafine hydrochloride vaginal effervescent tablet and preparation method thereof
InactiveCN101543479AImprove matchExpand the scope of the filterOrganic active ingredientsPill deliveryOrganic acidEffervescent tablet
The invention discloses a terbinafine hydrochloride vaginal effervescent tablet and a preparation method thereof. The terbinafine hydrochloride vaginal effervescent tablet mainly comprises a basic remedy of terbinafine hydrochloride, an effervescent auxiliary material and other auxiliary materials, wherein the effervescent auxiliary material consists of organic acid and carbonate; and other auxiliary materials consist of one or more of a disintegrant, a binding agent, a diluting agent and a lubricating agent and a surfactant. The invention is suitable for women to administrate in vagina; and the medicament can be disintegrated in a shortest time, generates fine and even foam and is favorable for fully absorbing pharmacodynamical ingredients, so patients are easy to accept the medicament, and the medicament is suitable to be clinically popularized and applied. The invention also discloses the preparation method which ensures that the quality of products is stable, the preparation process is simple, the quality is controllable and the cost is lower, so the preparation method is suitable for industrial large-scale production. The terbinafine hydrochloride vaginal effervescent tablet prepared by the invention is mainly applied to monilial vaginitis and has unique effect.
Owner:杜波 +1
Compound terbinafine preparation and applications thereof
InactiveCN104771405AQuick effectSignificant relief of symptomsOrganic active ingredientsAntimycoticsSide effectCure rate
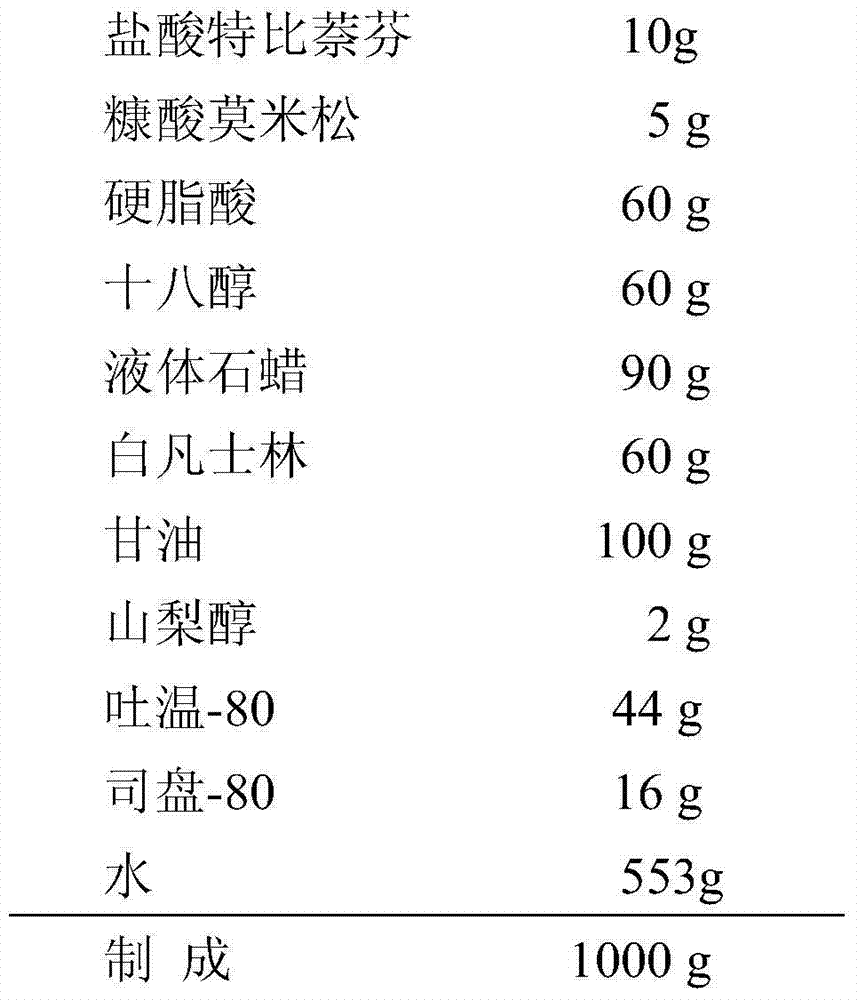
The invention belongs to the technical field of medicines, and provides a compound terbinafine preparation. The active component of the preparation is mainly composed of terbinafine hydrochloride and mometasone furoate. The provided compound terbinafine preparation is suitable for treating superficial fungal infection, and has the advantages of high curative effect, quick effect, safety, and little side effect of hormone. In the treatments of tinea of feet and hands, the clinical cure rate is 71.7%, the total effective rate is 96.7%, and the fungi eliminating rate is 100%. In the treatments of tinea corporis and cruris, the clinical cure rate is 72.4%, the total effective rate is 97.1%, and the fungus eliminating rate is 100%. The preparation does not cause prominent allergic reactions and hormone side effects.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Preparation method for improving purity of terbinafine hydrochloride
ActiveCN110423200AMeet production requirementsAvoid potential hazardsAmino compound purification/separationAmino compound preparation by condensation/addition reactionsAlkyneEthyl acetate
The invention discloses a preparation method for improving the purity of terbinafine hydrochloride, and the method comprises the following steps: 1) adding an acid binding agent into water, stirring and dissolving, adding N-methylnaphthylamine and 1-chlorine-6,6-dimethyl-2-heptene-4-alkyne, and cooling to room temperature; adding ethyl acetate for extraction, filtering, adding a hydrochloric acidaqueous solution, stirring, and filtering to obtain crude terbinafine hydrochloride; and 2) purifying the terbinafine hydrochloride, namely adding the crude terbinafine hydrochloride in the step 1) and purified water into a reaction kettle, heating and refluxing to dissolve, cooling to room temperature, and filtering to obtain a finished product of the terbinafine hydrochloride. According to the preparation method of the terbinafine hydrochloride, only water is used as a crystallization solvent and no toxic solvent is used in the final step reaction of the bulk drug, so that potential harm oftoxic organic solvents to human bodies is avoided; moreover, the solvent amount is extremely small, the cost is low, the generated waste liquid is less, the pollution to the environment is reduced, the product purity and yield are high, the reaction operation is simple, the control is easy, the production period is short, and commercial production is facilitated.
Owner:SICHUAN OPEN MEDICINE CO LTD
Terbinafine hydrochloride capsule
InactiveCN1440746AGood disintegrationFast absorptionOrganic active ingredientsAntimycoticsAdditive ingredientTerbinafine Hydrochloride
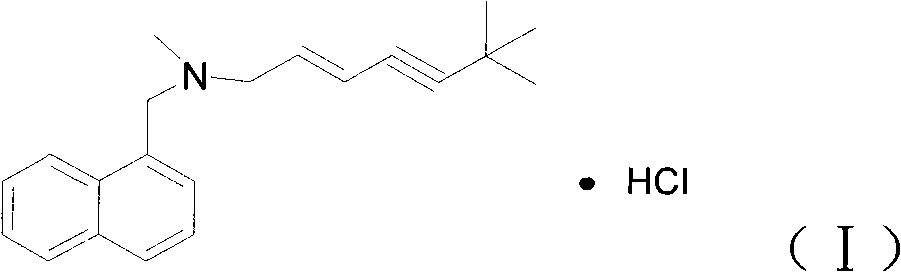
The Terbinafine hydrochloride capsule preparation contains Terbinafine hydrochloride as main medicine component and auxiliary components. Terbinafine hydrochloride has the chemical name of (E)-N-(6,6-dimethyl-2-heptene-4-yne)-N-methyl-1-menaphthyl amine hydrochloride, and the chemical general expression of C21H25N.HCl. The auxiliary components include common additive for preparing capsule. The Terbinafine hydrochloride capsule preparation is superior to Terbinafine hydrochloride tablet, powder and other preparation forms, is lightproof and moistureproof, and can mask the bad taste of medicine component, delay medicine decomposition and promote the absorption of medicine component.
Owner:成都锦绣生物医药科技有限责任公司
External-use ointment for treatment of dermatopathy
InactiveCN106668864AWide range of treatmentHigh cure rateHydroxy compound active ingredientsAerosol deliveryDiseaseTherapeutic effect
The invention relates to an external-use ointment for treatment of dermatopathy. The ointment is prepared by mixing and blending the following medicines (by weight): 6-9 parts of sulfamethoxazole, 5-8 parts of chloramphenicol, 5-8 parts of prednisone, 3-5 parts of borneol, 0-8 parts of sulfur flour, 5-8 parts of vitamin E, 4-8 parts of vitamin complex B, 6-10 parts of VC, 5-7 parts of B1, 0.01-0.04 part of triamcinolone acetonide, 10 parts of urea ointment, 30 parts of a Chinese herbal antibacterial paste, 8-10 parts of chlorpheniramine, 4-8 parts of chloramphenicol raw powder, 6-10 parts of metronidazole, 10 parts of B6 ointment, 0-15 parts of terbinafine hydrochloride emulsifiable paste and 0-10 parts of ketoconazole emulsifiable paste. The ointment of the invention is used for treatment of skin diseases such as dermatitis, eczema, acne, yellow fluid ulcers, manus and tinea pedis, psoriasis, etc., can be used for realizing antibiosis, anti-inflammation, itching-relieving and antiallergic functions and the function of boosting body immunity, reduces exudation and inflammatory response and promotes inflammatory resorption of wounds and growth of granulation tissues, and has a good comprehensive treatment effect.
Owner:毕明华
Application of naphthylamine antifungal drug in prevention and treatment of agricultural diseases
The invention discloses a new application of a naphthylamine antifungal drug as a pesticide bactericide in crop disease control. The napthalene benzylamine antifungal drugs are naftifine hydrochloride, terbinafine hydrochloride and butenafine hydrochloride. Crop diseases caused by rhizoctonia solani, sclerotinia sclerotiorum, botrytis cinerea, fusarium graminearum, magnaporthe oryzae and fusariumoxysporum of cotton can be prevented and treated; especially, terbinafine hydrochloride and butenafine hydrochloride have excellent antibacterial effects on sclerotinia sclerotiorum, botrytis cinerea,fusarium graminearum, magnaporthe grisea and fusarium oxysporum of cotton, and the antibacterial effects are significantly higher than those of commercial bactericides azoxystrobin, so that the terbinafine hydrochloride and butenafine hydrochloride can be applied to prevention and treatment of crop diseases.
Owner:LANZHOU UNIVERSITY
Terbinafine hydrochloride gel and preparation method thereof
InactiveCN105342986AImprove solubilityEvenly dispersedAntibacterial agentsOrganic active ingredientsRaw materialChemistry
The invention provides terbinafine hydrochloride gel and a preparation method thereof. The terbinafine hydrochloride gel is prepared from the following raw materials in parts by weight: 1 part of terbinafine hydrochloride, 1-3 part of carbomer, 10-30 parts of propylene glycol, 5-16 parts of polyethylene glycol 400, 4-6 parts of hexadecanol, 0.001-0.01 part of butylated hydroxyanisole, 4-12 parts of polysorbate 80, 0.3-3 parts of triethanolamine, 0.05-0.15 part of edetate disodium, 20-40 parts of ethanol and 8-50 parts of distilled water. The preparation method comprises the following steps: (1) swelling carbomer by adopting purified water; (2) emulsifying the swelled carbomer, and then emulsifying propylene glycol, polyethylene glycol 400, hexadecanol, and butylated hydroxyanisole to form a gel substrate; (3) dissolving terbinafine hydrochloride with ethanol, adding polysorbate 80, uniformly mixing the dissolved terbinafine hydrochloride and polysorbate 80, adding the mixed solution into the gel substrate obtained in step (2), and stirring for emulsifying; and (4) adding triethanolamine and the rest of purified water to a product in step (3), stirring for emulsifying, and uniformly mixing the obtained mixture with a gel to obtain the terbinafine hydrochloride gel. The terbinafine hydrochloride gel has the positive effects that the stability of the medicine is improved, the curative effect is high, the coating and removing by washing are easy, the irritation to the skin is eliminated, and no odor exists.
Owner:吉林修正药业新药开发有限公司
Terbinafine hydrochloride emulsifiable paste and preparation method thereof
ActiveCN101467960BHigh glossGood spreadabilityOrganic active ingredientsAntimycoticsPolyethylene glycolDissolution
The invention relates to a self-emulsified terbinafine hydrochloride cream and preparation method thereof, comprising, main medicaments, terbinafine hydrochloride of 0.5-3%, TEFOSE63 (polyethylene glycol-7 stearate) of 6-18%, span 80 of 1-6%, glycerin monostearate of 1-5%, and light liquid petrolatum of 1-4%; the remainder being purified water. Self-emulsifier, surfactant and solvent are heated to 75-80 DEG C, rapidly stirred for 30-45 minutes after complete dissolution, and cooled to around 40 DEG C; maintaining the temperature, the terbinafine hydrochloride is added and stirred continuously; triethanolamine of proper amount is added to regulate the pH value between 4-6, vacuumized, and continuously stirred to the room temperature to obtain the cream with good brightness, good coating ability, less consistency change with the environmental temperature change, good stability and transdermal absorption. Compared with the traditional preparation technology, the preparation method thereof is simple in operation, low in the energy consumption and low in device investment.
Owner:ZHEJIANG CONBA PHARMA
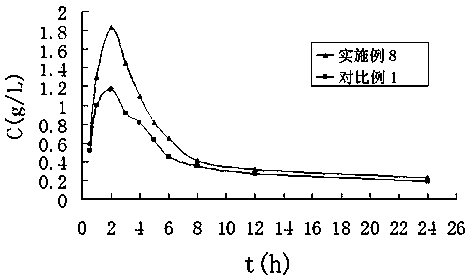
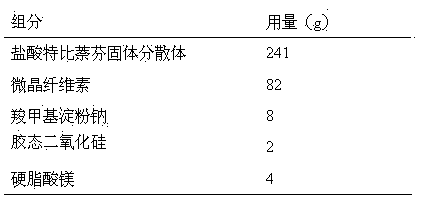
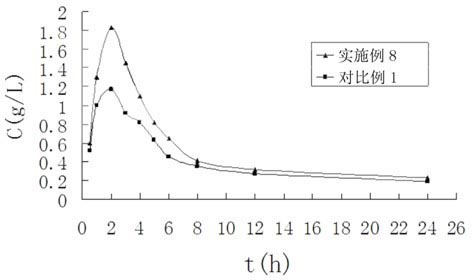
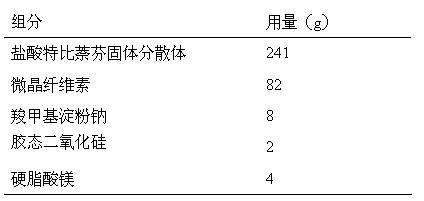
Terbinafine hydrochloride solid dispersoid and tablet thereof
ActiveCN102641252BImprove in vitro dissolutionReduce dosageOrganic active ingredientsAntimycoticsBULK ACTIVE INGREDIENTPharmaceutical Substances
The invention relates to a terbinafine hydrochloride solid dispersoid, which is composed of terbinafine hydrochloride and carrier materials, wherein the proportion by weight between the terbinafine hydrochloride and the carrier materials is 1 / 2-1 / 15. The invention further relates to a tablet prepared by the terbinafine hydrochloride solid dispersoid. Compared with the prior art, the terbinafine hydrochloride tablet has the advantages of (1) improving dissolution in vitro of terbinafine hydrochloride preparation; (2) solving the problem that the terbinafine hydrochloride is poor in oral administration absorption and low in biological availability; (3) covering harmful odor and irritation of the terbinafine hydrochloride; (4) keeping pesticide effects of large dose while reducing using quantity of active ingredients in the preparation per unit, and reducing drug side-effects; and (5) being simple in preparation process and suitable for industrial production.
Owner:NANJING CHENGONG PHARM CO LTD
Preparation method of terbinafine hydrochloride liniment
InactiveCN111067883AHigh glossGood spreadabilityOrganic active ingredientsAntimycoticsDrug releasePharmaceutical Substances
The invention discloses a preparation method of a terbinafine hydrochloride liniment. The preparation method comprises steps as follows: materials are prepared in parts by weight according to a formula in the following; after all materials in the formula are uniformly crushed, the crushed materials are put in a stirrer, 30-40 parts of distilled water are added, and the mixture is mixed and stirredat the stirring speed of 2,500 r / min; after the mixture is uniformly stirred, the mixture is heated to 90-100 DEG C for 30-50 min and then sieved by a sieve of 150 meshes and a filtrate is retained;after the filtrate is concentrated in a vacuum manner to be sticky, and a mixed sticky drug with the relative density being 1.1-1.3 is obtained; the pH value is adjusted to be 3-6, and the drug is stirred uniformly and cooled to the room temperature; the drug is emulsified for 30-50 min until uniformly mixed colloid is obtained, that is, the terbinafine hydrochloride liniment is obtained. The liniment has good glossiness and coating property, paste has small consistence changes with changes of environment temperature and is stable, the drug release speed is high, the transdermal absorption property is good, and compared with a conventional preparation technology, the preparation method has the advantages that the preparation method is simple and easy to operate, quality control is easy, medication is convenient, and the drug has small irritation to skin, good in treatment effect and stable in quality.
Owner:安徽汇喻科技服务有限公司
Compound teribinafine hydrochloride solution for animal
InactiveCN1883475ASignificantly heals pets (dogsSignificant feline) fungal skin disease effectOrganic active ingredientsAntimycoticsDiseaseCypermethrin
The invention discloses a compound terbinafine hydrochloride solution for animals, which is prepared mainly from terbinafine hydrochloride, Metronidazole, thiabendazole and cypermethrin. The solution has substantial effect in treating pet granuloma skin diseases.
Owner:NANJING JINDUN ANIMAL PHARMA
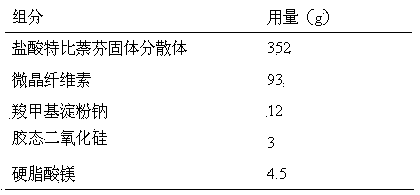
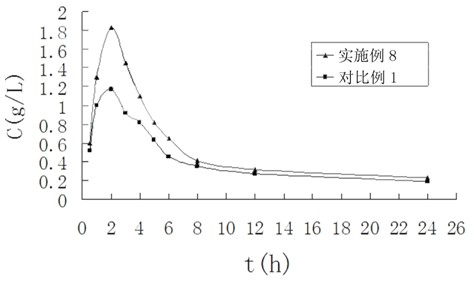
Terbinafine hydrochloride solid dispersoid and tablet thereof
ActiveCN102641252AImprove in vitro dissolutionReduce dosageOrganic active ingredientsAntimycoticsBULK ACTIVE INGREDIENTPharmaceutical Substances
The invention relates to a terbinafine hydrochloride solid dispersoid, which is composed of terbinafine hydrochloride and carrier materials, wherein the proportion by weight between the terbinafine hydrochloride and the carrier materials is 1 / 2-1 / 15. The invention further relates to a tablet prepared by the terbinafine hydrochloride solid dispersoid. Compared with the prior art, the terbinafine hydrochloride tablet has the advantages of (1) improving dissolution in vitro of terbinafine hydrochloride preparation; (2) solving the problem that the terbinafine hydrochloride is poor in oral administration absorption and low in biological availability; (3) covering harmful odor and irritation of the terbinafine hydrochloride; (4) keeping pesticide effects of large dose while reducing using quantity of active ingredients in the preparation per unit, and reducing drug side-effects; and (5) being simple in preparation process and suitable for industrial production.
Owner:NANJING CHENGONG PHARM CO LTD
Eye ointment for treating fungal keratitis
InactiveCN102526051AExtended retention timeAntifungal effect is obviousSenses disorderAntimycoticsTreatment effectSide effect
The invention discloses eye ointment for treating fungal keratitis. The eye ointment is a mixture containing terbinafine hydrochloride and atropine. The eye ointment solves the problems that the anti-fungal eye medicine lacks and the conventional anti-fungal eye drop is expensive and short in persistence time in eyes, has a poor anti-fungal curative effect and cannot relieve secondary iridocyclitis of the fungal keratitis and the like. The invention has the advantages that: the eye ointment has long persistence time in the eyes and an obvious anti-fungal curative effect, the effective rate reaches 98 percent, and the eye ointment has a treatment effect on the secondary iridocyclitis, obviously relieves the eye pain symptom of a patient and does not have obvious toxic or side effect; the preparation method is simple, and has the feasibility of industrialized production; and the eye ointment is expected to play an important role in treating the clinical fungal keratitis.
Owner:HARBIN MEDICAL UNIVERSITY
Cactus mask for treating acne and preparation method
InactiveCN105496934ATo promote metabolismImprove smoothnessCosmetic preparationsOrganic active ingredientsPrawnPolyvinyl alcohol
The invention discloses a cactus mask for treating acne and a preparation method. The cactus mask is prepared from, by weight, terbinafine hydrochloride, cacti, blueberries, radix glycyrrhizae preparata, carmine radish, nagens groundsel, hemigraphis procumbens (Lour.) Merr., holboellia latifolia wall, all-grass of Taiwan lettuce, roots of angled bittersweet, prawn shells, chamomile essential oil, sandalwood essential oil, pearls, gypsum, para aminobenzoic acid, carboxymethylcellulose, sorbitol and polyvinyl alcohol. The preparation method comprises the steps of material preparation, juicing, extraction of effective ingredients of Chinese herbal materials and preparation of the mask. According to the mask, all the herbal materials are matched properly, and the mask is mild and effective and can effectively treat acne, remove blackheads, whiten and nourish skin, lighten color spots and scars, resist oxidation, promote skin metabolism, enhance blood vessels and skin elasticity, boost blood circulation, improve skin smoothness, inhibit inflammations and allergies and increase skin resistance.
Owner:LIAOCHENG UNIV
Terbinafine hydrochloride tablets and preparation method thereof
The invention discloses terbinafine hydrochloride tablets and a preparation method thereof. The terbinafine hydrochloride tablets consist of terbinafine hydrochloride and pharmaceutic adjuvants, wherein the pharmaceutic adjuvants comprise a filling agent, a disintegrating agent, an adhesive and a lubricating agent. By adopting a wet granulation process, the terbinafine hydrochloride tablet is simple in preparation process, easy to operate, controllable in quality, good in reproducibility and suitable for industrial large-scale production, and the obtained terbinafine hydrochloride tablet is smooth in surface, high in dissolution rate, small in dissolution difference among batches, high in in in-vivo bioavailability, bioequivalent to a reference preparation in human body, and consistent with the reference preparation in quality.
Owner:NANJING CHENGONG PHARM CO LTD
Method for improving silkworm cocoon yield stability
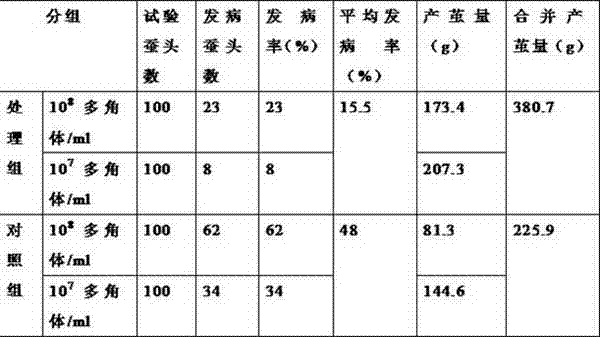
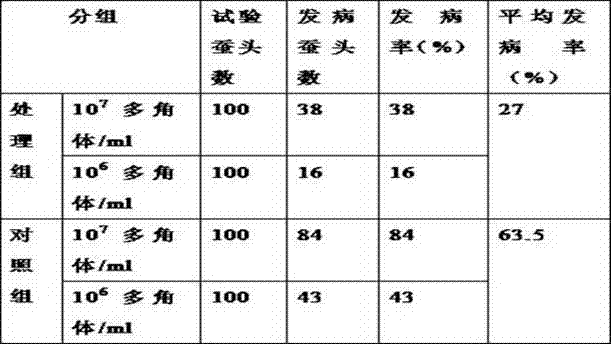
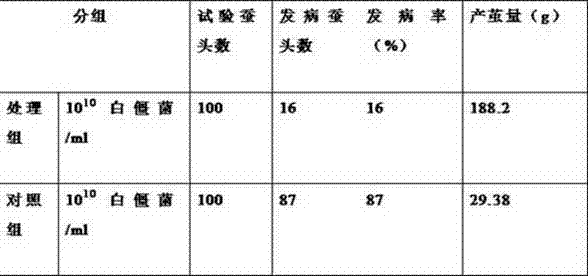
InactiveCN103565829ASolve the problem of reduced productionGuaranteed stabilityOrganic active ingredientsAntimycoticsDiseaseNuclear Polyhedrosis Virus
The invention discloses a method for improving silkworm cocoon yield stability, which comprises the following steps: (1), a mixture composed of virazole, terbinafine hydrochloride and ammonium chloride is used to prepare an aqueous solution according to the mass fraction of 10-15 to 3-7 to 75-85; (2), after the prepared aqueous solution is sprayed to mulberry leaves, mulberry leaves are dried in the air; (3), the mulberry leaves obtained in the step (2) are used for feeding silkworms. According to the invention, by adding the aqueous solution of the mixture composed of virazole, terbinafine hydrochloride and ammonium chloride, prevention and treatment for diseases of the silkworms, such as nuclear polyhedrosis virus, cytoplasmic polyhedrosis and white muscardine, can be achieved, the problem of silkworm cocoon yield reduction caused by the diseases can be solved, and the silkworm cocoon yield stability is ensured.
Owner:王羽骋 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com