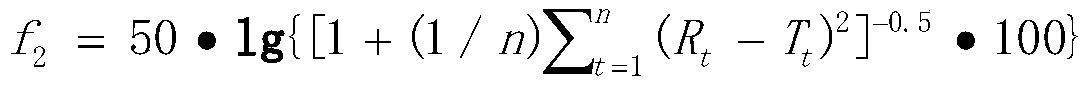Terbinafine hydrochloride tablets and preparation method thereof
A technology for terbinafine hydrochloride and naphthene tablets, which is applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. complex issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
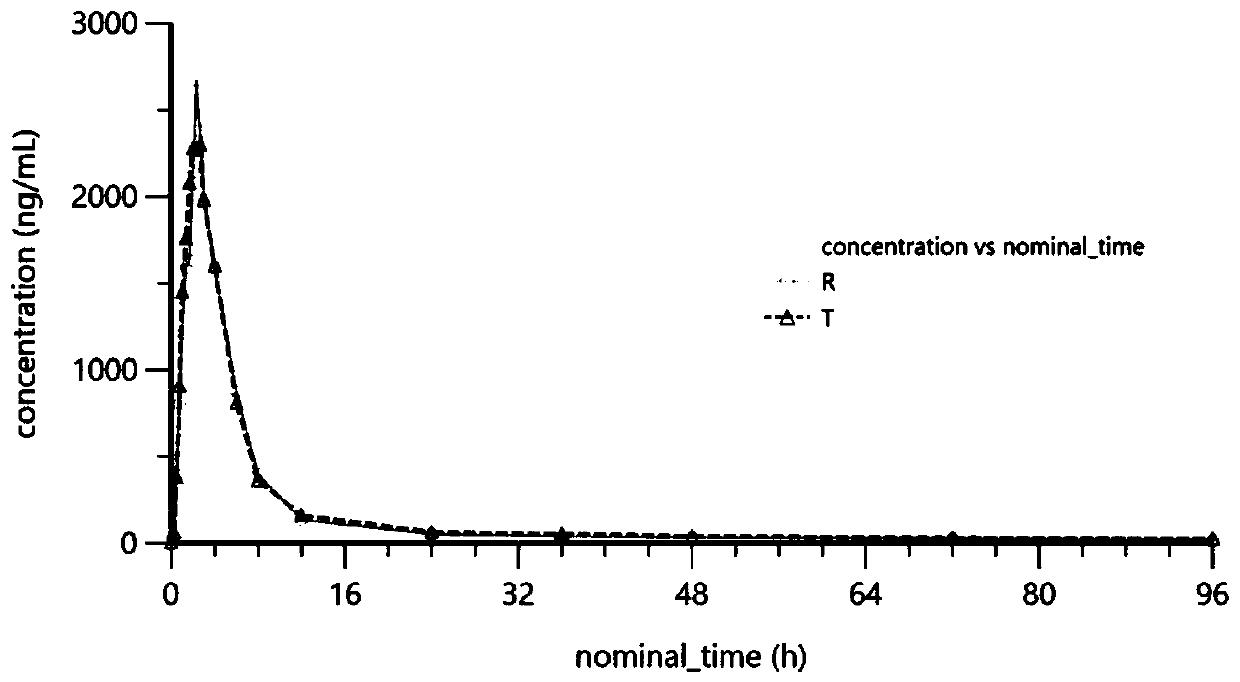
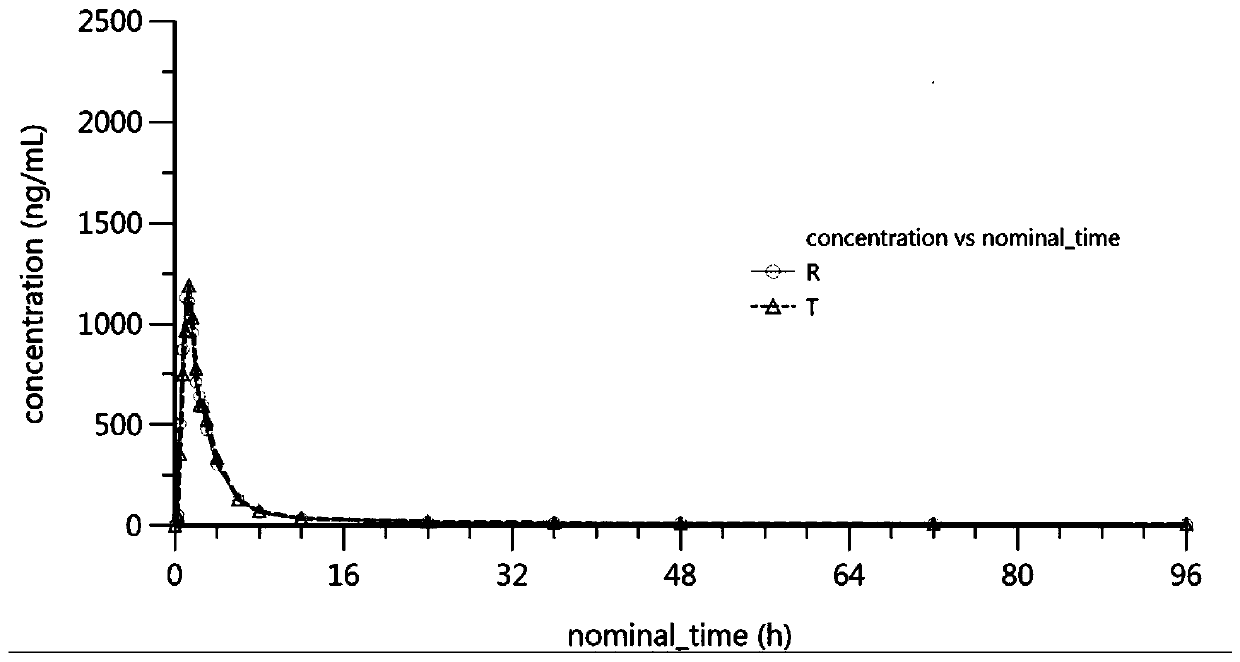
Image
Examples
Embodiment 1
[0034] Prepare Terbinafine Hydrochloride Tablets according to the following method: the Terbinafine Hydrochloride Tablets are made up of the following raw materials in parts by weight:
[0035] 250 parts of terbinafine hydrochloride, 67.5 parts of microcrystalline cellulose, 20 parts of sodium starch glycolate, 7 parts of hydroxypropyl methylcellulose, 4 parts of magnesium stearate, and 2 parts of silicon dioxide.
[0036] (1) micronizing the terbinafine hydrochloride for subsequent use;
[0037] (2) Adhesive preparation: weigh hydroxypropyl methylcellulose, add purified water under continuous stirring, stir evenly, and set aside;
[0038] (3) Granulation: the terbinafine hydrochloride, microcrystalline cellulose and half of the amount of sodium carboxymethyl starch were placed in a wet granulator and mixed for 10 to 20 minutes. Add the binder within seconds and continue granulation for a total of 2 to 4 minutes before discharging; tighten the screen, slowly add the soft mate...
Embodiment 2
[0044] Prepare Terbinafine Hydrochloride Tablets according to the following method: the Terbinafine Hydrochloride Tablets are made up of the following raw materials in parts by weight:
[0045] Terbinafine hydrochloride 300 parts, lactose 78 parts, croscarmellose sodium 40 parts, starch slurry 4 parts, talcum powder 4 parts.
[0046] (1) micronizing the terbinafine hydrochloride for subsequent use;
[0047] (2) Adhesive preparation: take starch slurry, add purified water under continuous stirring, stir evenly, and set aside;
[0048] (3) Granulation: place the croscarmellose sodium of described terbinafine hydrochloride, lactose and 2 / 3 amount in wet granulator and mix for 10~20 minutes, add binding agent, in 30 Finish adding the binder within ~60 seconds, continue to granulate for a total of 2 ~ 4 minutes, and discharge; tighten the screen, slowly add the soft material to the oscillating granulator, and pass through a 16-mesh to 24-mesh stainless steel sieve to granulate;
...
Embodiment 3
[0054] Prepare Terbinafine Hydrochloride Tablets according to the following method: the Terbinafine Hydrochloride Tablets are made up of the following raw materials in parts by weight:
[0055] 250 parts of terbinafine hydrochloride, 58 parts of mannitol and corn starch, 36 parts of crospovidone, 9 parts of ethyl cellulose and hydroxypropyl methyl cellulose, 16 parts of magnesium stearate.
[0056] (1) micronizing the terbinafine hydrochloride for subsequent use;
[0057] (2) Adhesive preparation: weigh ethyl cellulose and hydroxypropyl methyl cellulose, add purified water under constant stirring, stir evenly, and set aside;
[0058] (3) Granulation: the crospovidone of described terbinafine hydrochloride, mannitol and cornstarch and 20 parts of amounts is placed in wet granulator and mixes for 10~20 minutes, adds binding agent, in 30~60 Add the binder within seconds and continue granulation for a total of 2 to 4 minutes before discharging; tighten the screen, slowly add the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com