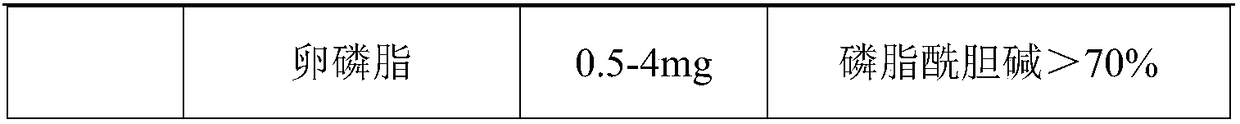
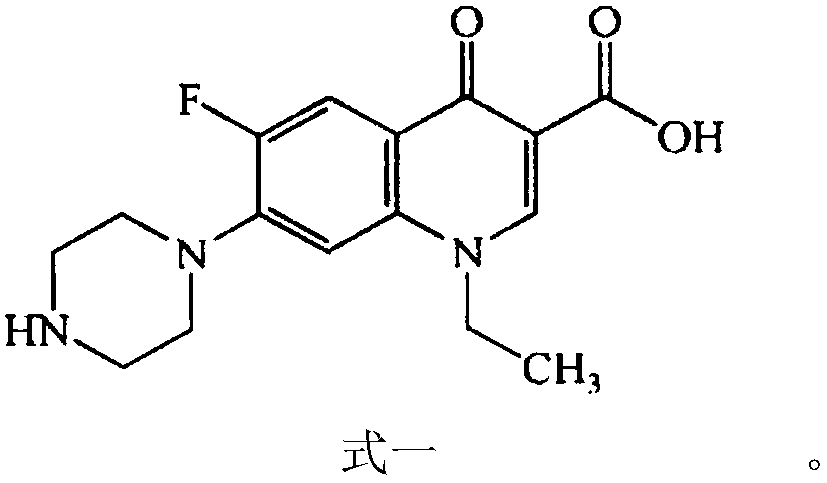
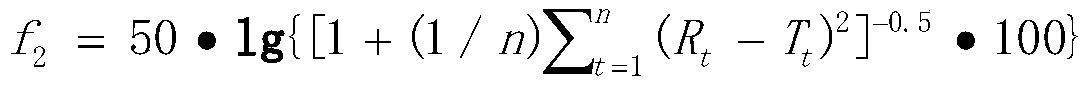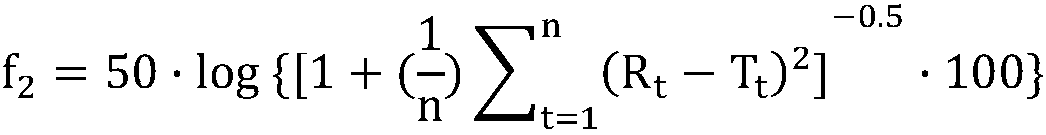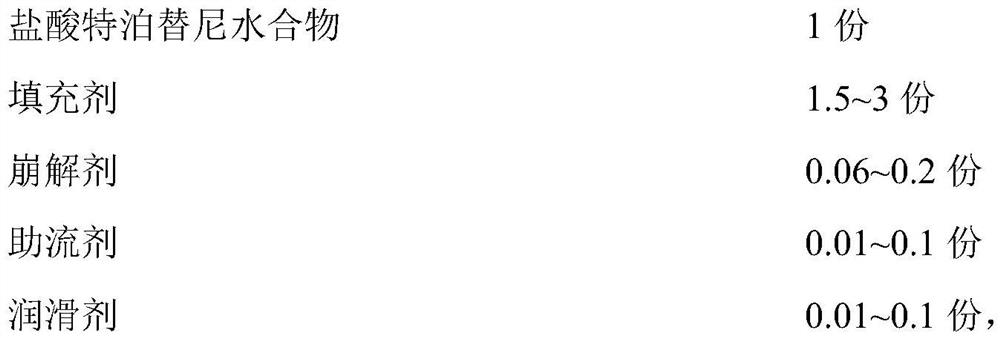Patents
Literature
64 results about "Reference preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
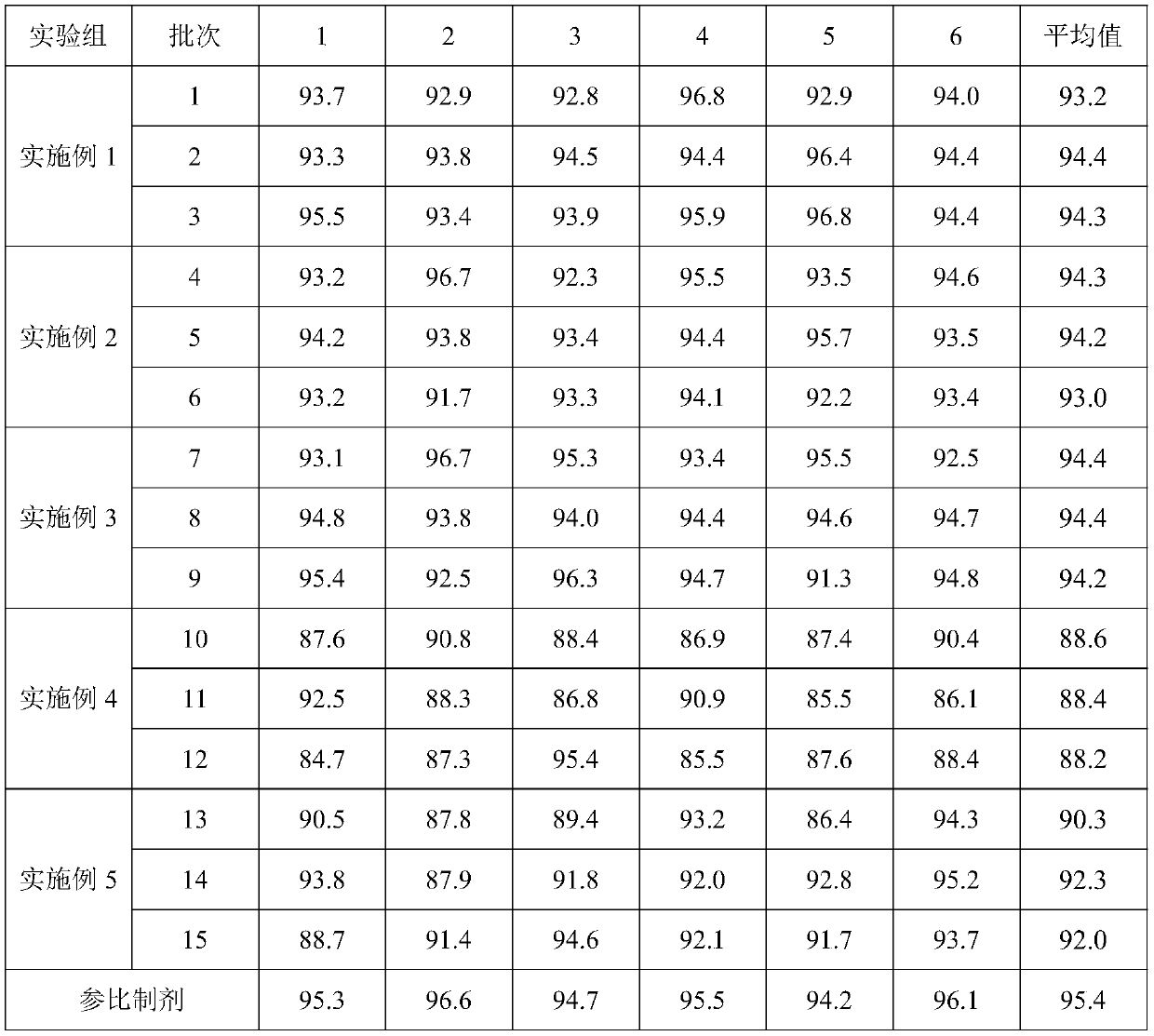
Preparation method of tumor sample sequencing reference
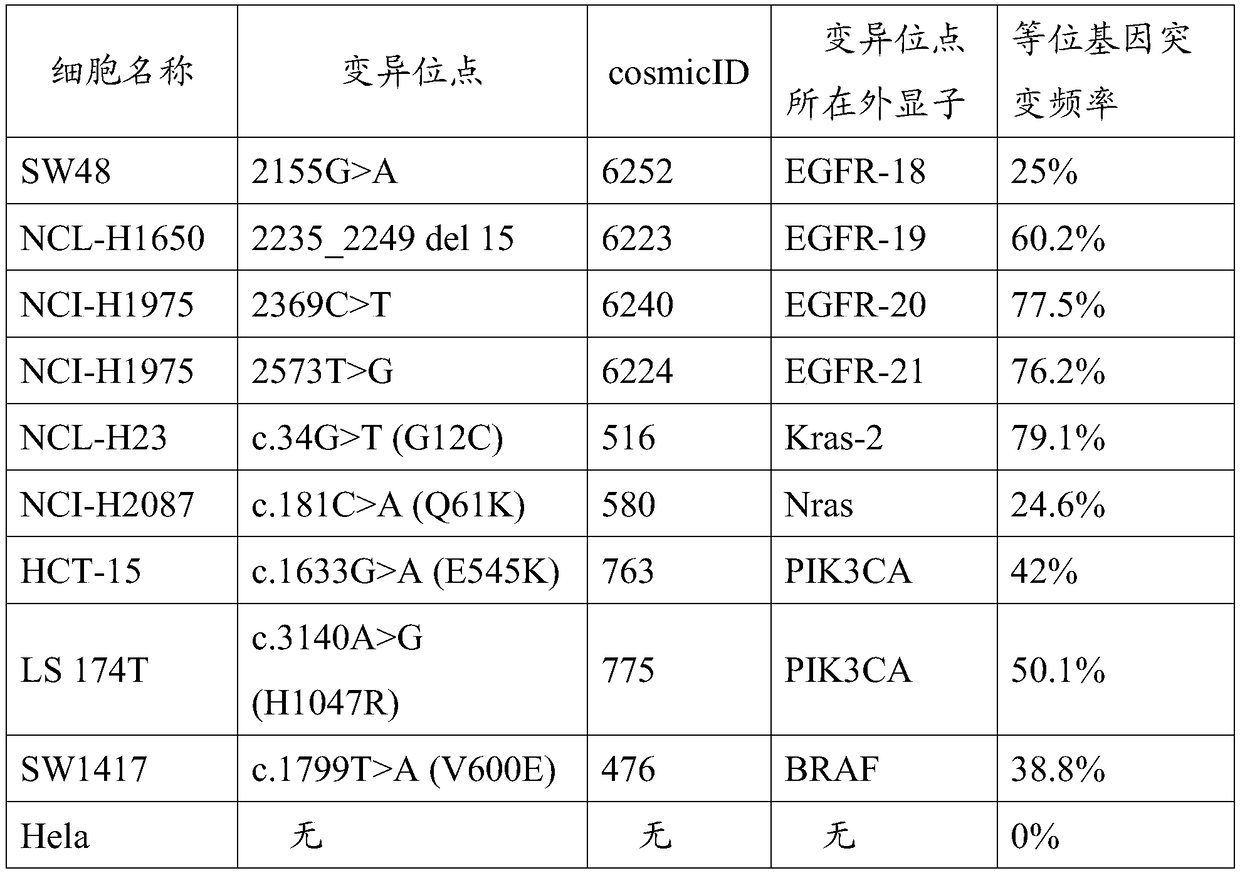
ActiveCN108728516AClear genetic backgroundAccurate countMicrobiological testing/measurementAllele frequencyMutation frequency
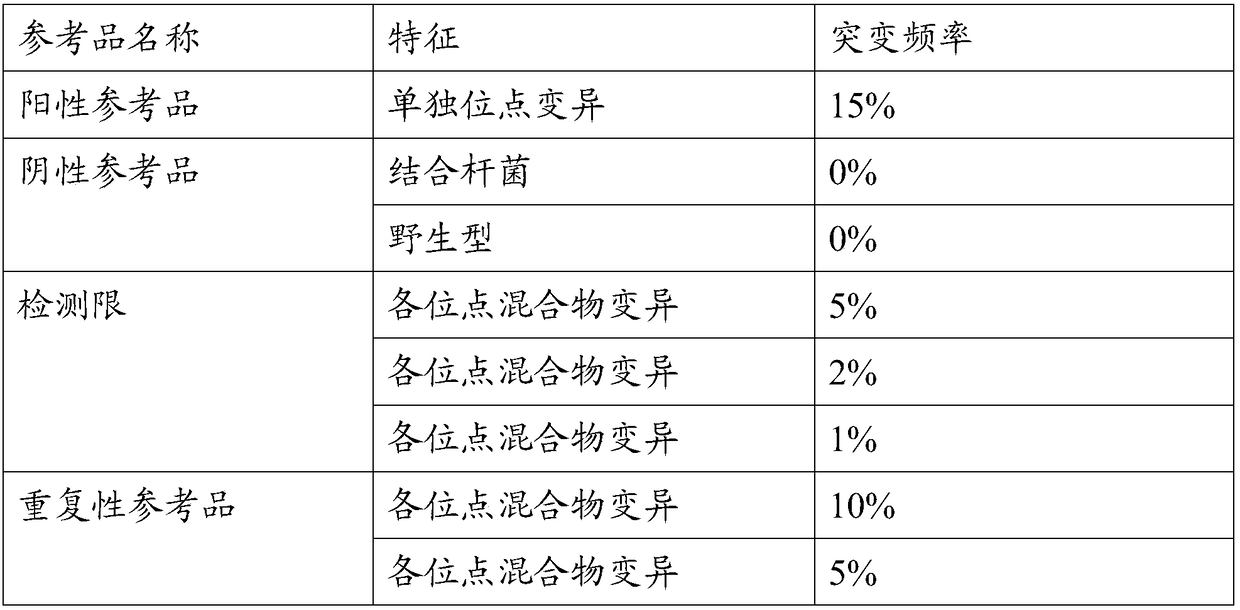
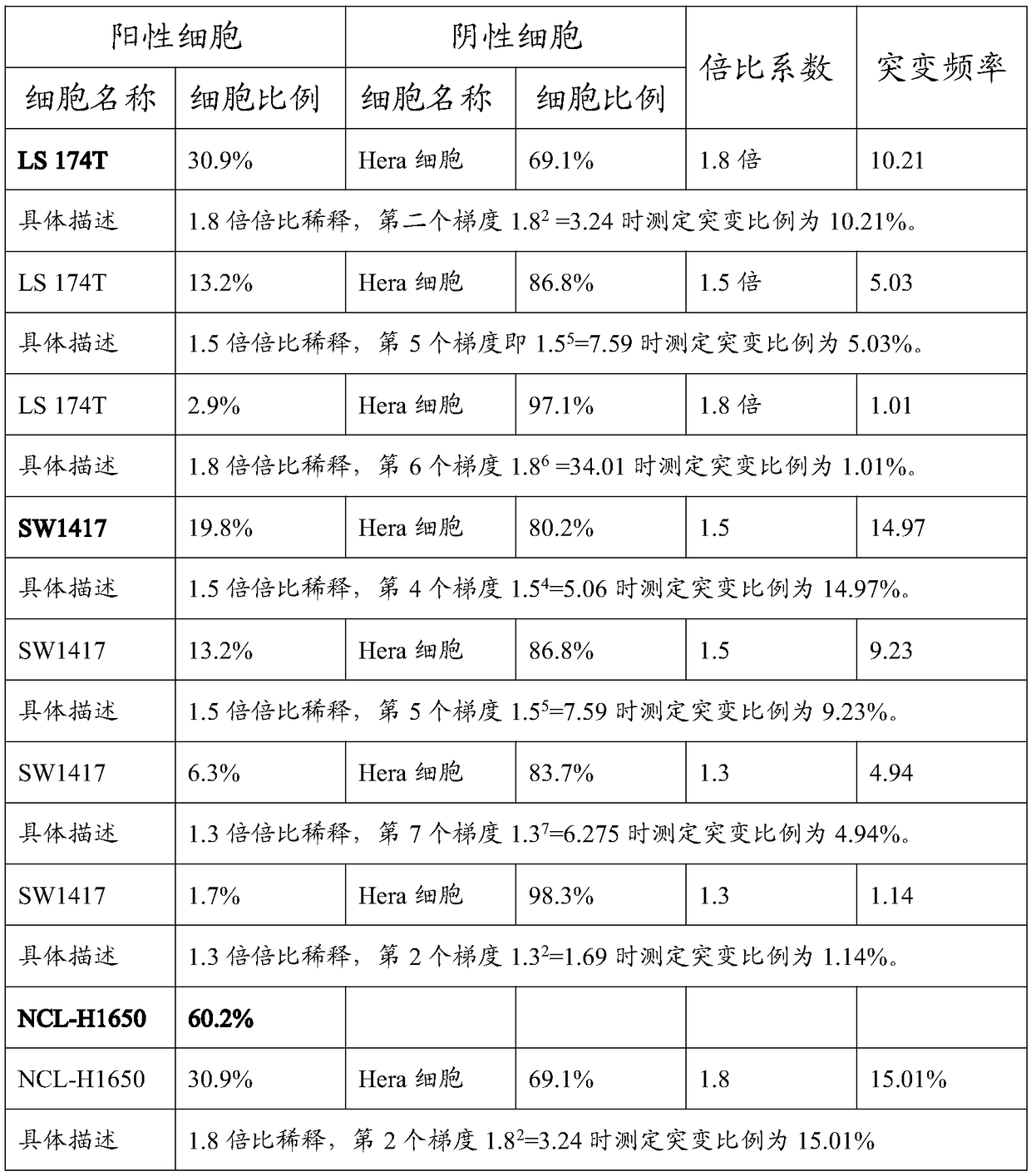
The invention provides a preparation method of a tumor sample sequencing reference, and belongs to the technical field of high-throughput sequencing. The method comprises the following steps: performing monoclonal culture on a tumor cell line to obtain a monoclonal cell line and extracting a whole genome; determining an allele frequency; determining a dilution multiple according to an allele mutation frequency and a target allele mutation frequency; diluting the monoclonal cell line with negative cells in multiple proportion to obtain mixed cells; extracting a whole genome of the mixed cells,and determining an allele mutation frequency; and according to the comparison between the allele mutation frequency and the target allele mutation frequency of the mixed cells, refining the multiple of multiple-proportion dilution, and diluting the monoclonal cell line with negative cells to obtain the reference. According to the method disclosed by the invention, the gene background of a cell line is determined by combination of cell strain monoclone and high-throughput sequencing; and a multiple-proportion dilution method with multi-step gradual refinement is combined with a flow cytometry to accurately count cells so as to ensure high repeatability of reference preparation.
Owner:安徽鼎晶生物科技有限公司 +1
Lurasidone hydrochloride oral preparation and preparing method of lurasidone hydrochloride oral preparation
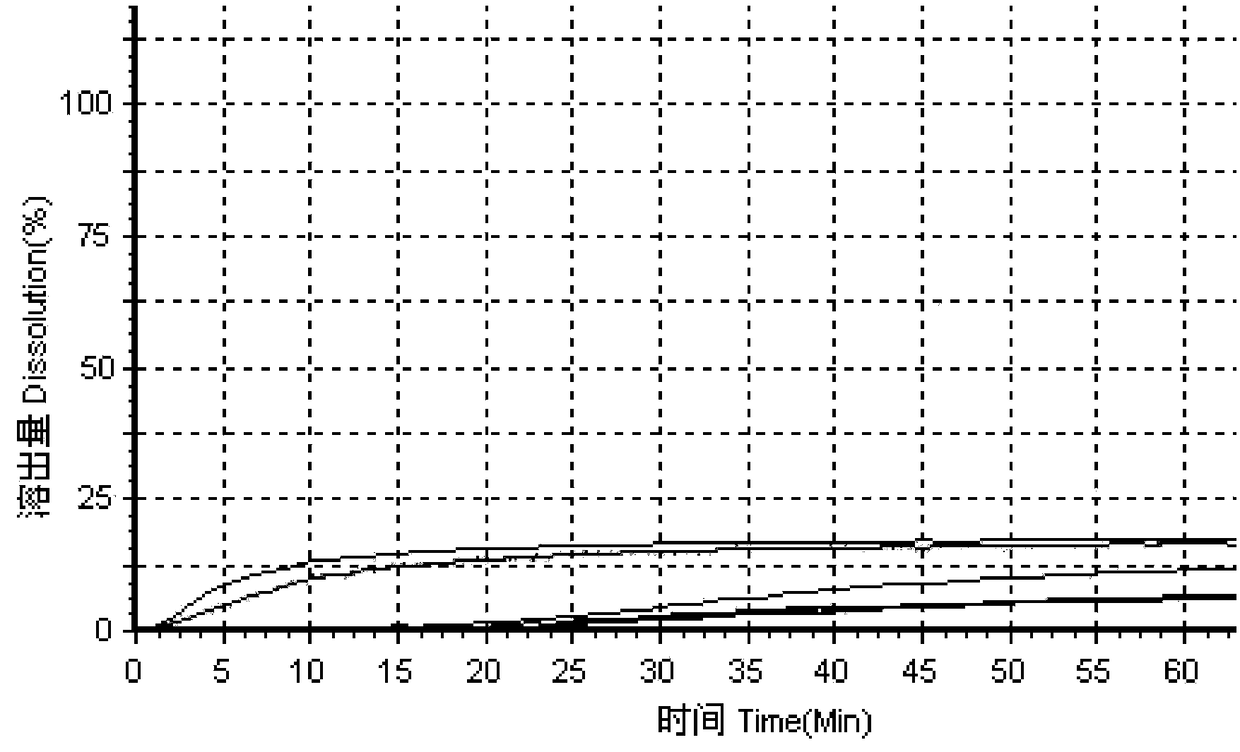
ActiveCN104337790ARapid dissolutionReduce lossesOrganic active ingredientsNervous disorderLurasidone HydrochlorideLow-substituted hydroxypropylcellulose
The invention provides a lurasidone hydrochloride oral preparation and a preparing method of the lurasidone hydrochloride oral preparation. The lurasidone hydrochloride oral preparation comprises lurasidone hydrochloride accounting for 20 to 45 weight percent of the oral preparation and cellulose derivatives accounting for 5 to 30 weight percent of the oral preparation, wherein the cellulose derivatives are a mixture of low-substituted hydroxypropyl cellulose and croscarmellose sodium. The lurasidone hydrochloride oral preparation has the advantages that the low-substituted hydroxypropyl cellulose and the croscarmellose sodium are simultaneously used as disintegrating agents, so that lurasidone hydrochloride tablets can be fast dissolved out, even when the content of active ingredients in the preparation is changed, the dissolution characteristics are also identical to the reference preparation, and the in-batch differences are small. In addition, according to the raw material processing mode, the raw material yield is higher, and the loss is less.
Owner:SHIJIAZHUANG NO 4 PHARMA
Soft nintedanib ethanesulfonate capsule and preparation method thereof
InactiveCN108078952AGreat physical stabilityThe bioavailability has a large impact onOrganic active ingredientsPharmaceutical non-active ingredientsMedicineBioavailability
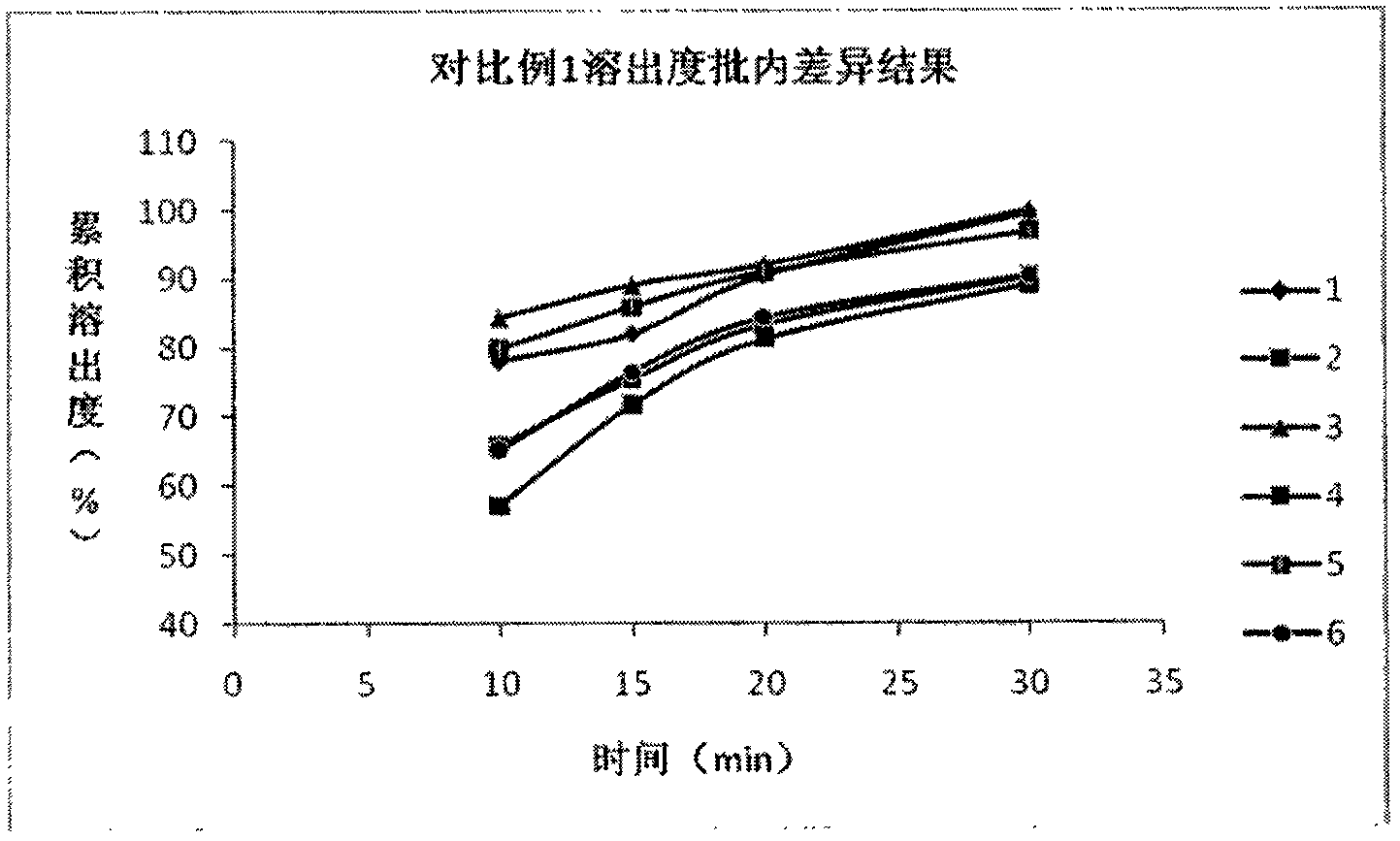
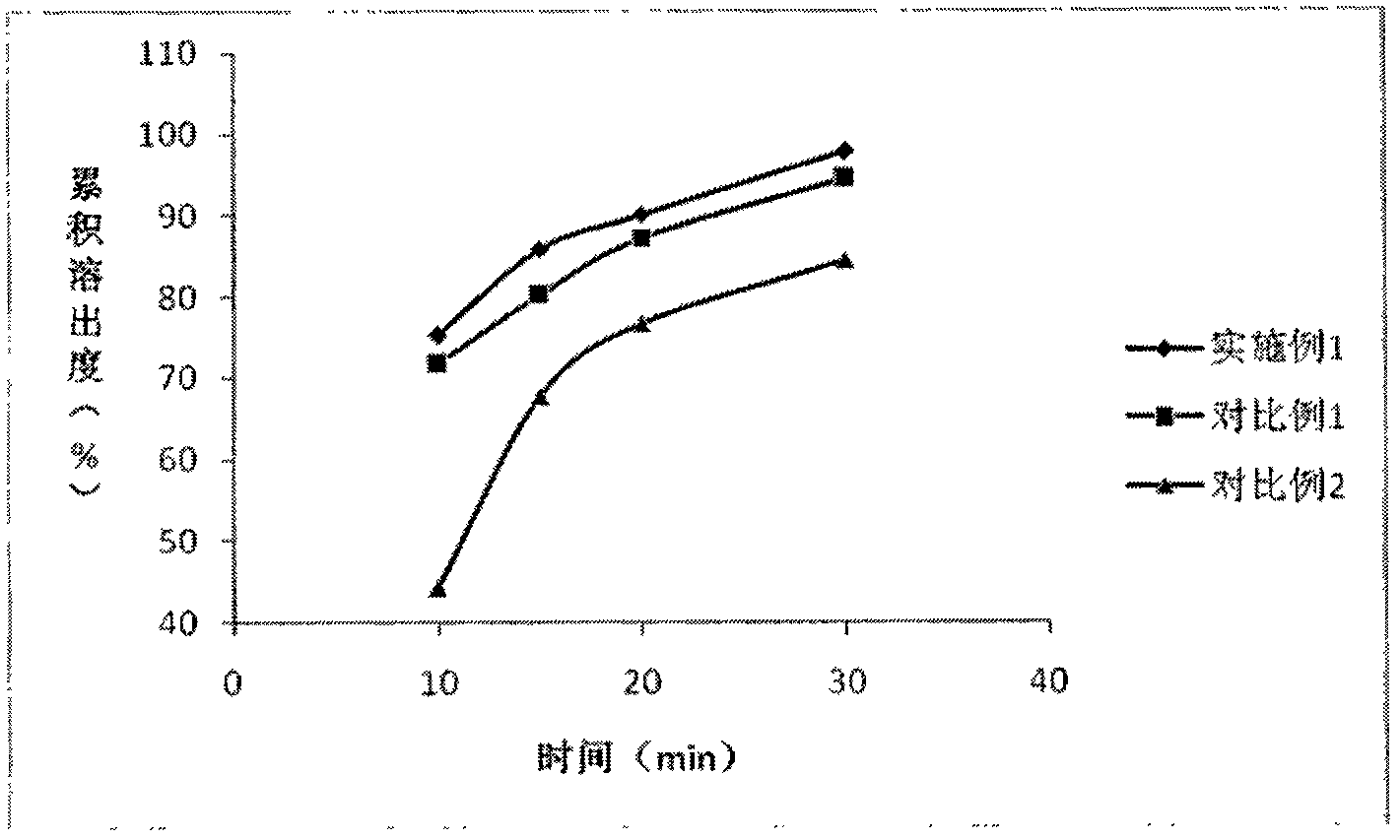
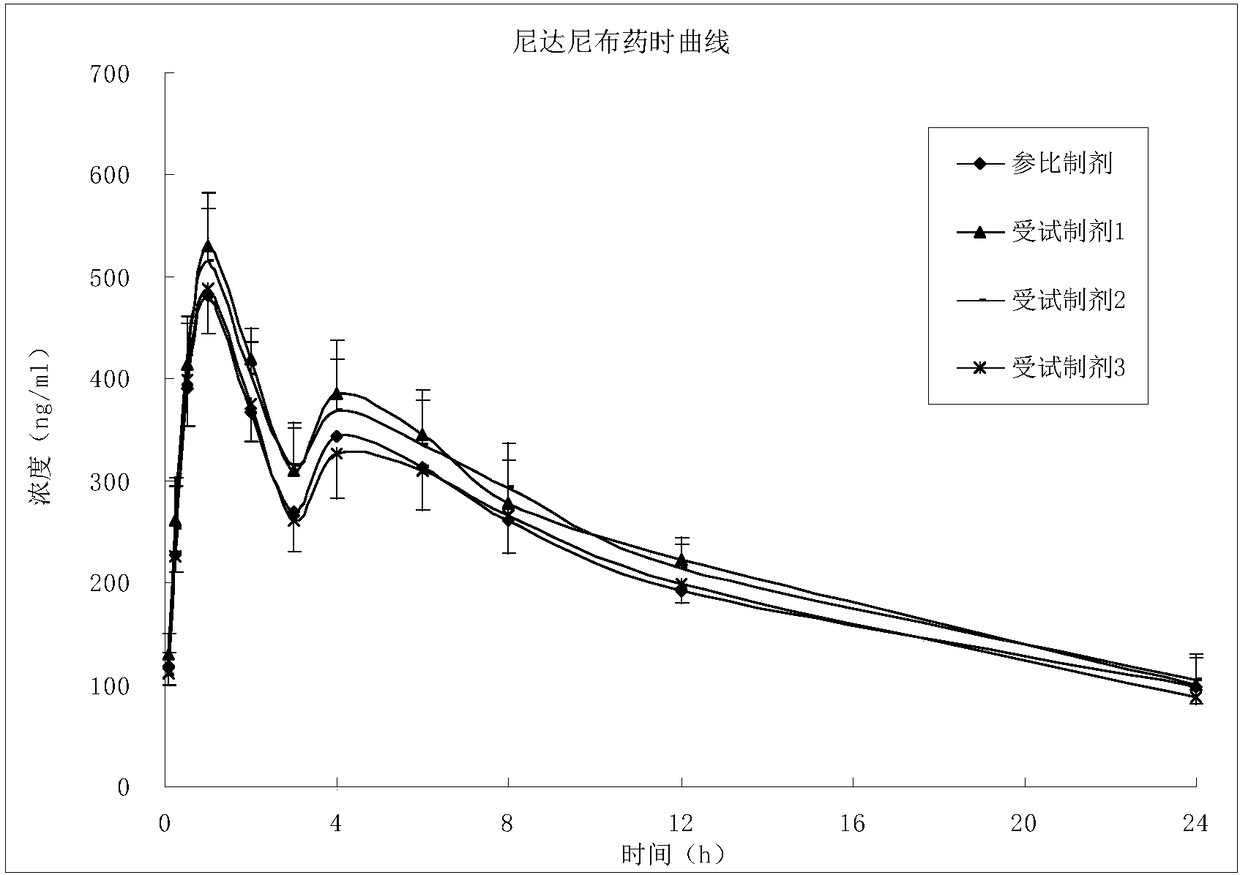
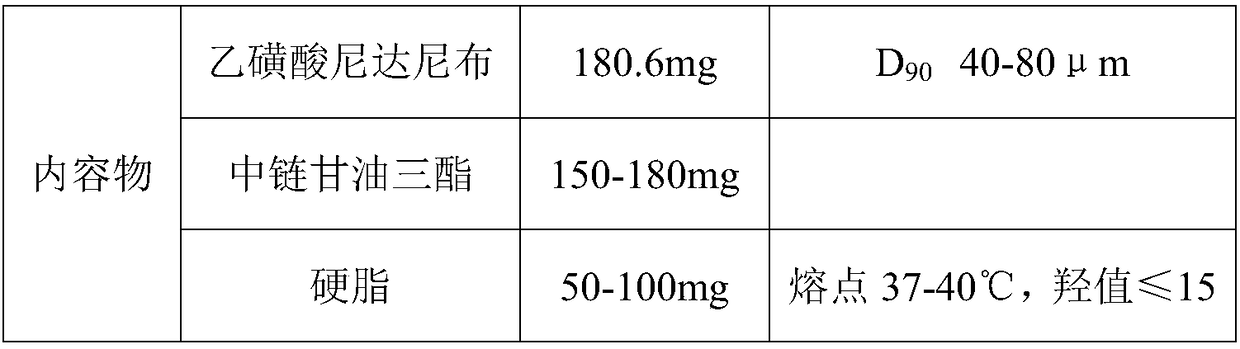
The invention belongs to the technical field of medicines, and particularly relates to a preparation method of a content of a soft nintedanib ethanesulfonate capsule. The content is prepared from nintedanib ethanesulfonate, wherein the grain size distribution range of the nintedanib ethanesulfonate is D90 from 40 mu m to 80 mu m. By studying the stability of a suspension system of the content of the soft nintedanib ethanesulfonate capsule and determining in-vivo pharmacokinetic parameters of wistar rats, the influence of the grain size of the nintedanib esylate as a raw material on the physical stability and bioavailability of the soft nintedanib ethanesulfonate capsule is discovered to be great, and the soft nintedanib ethanesulfonate capsule is ultimately discovered to have good physicalstability and be similar to pharmacokinetic behaviors of reference preparations in terms of absorption when the grain size of the nintedanib ethanesulfonate material is 40 mu m to 80 mu m, and moreover, since the grain size control range of the raw material is broadened, the production cost and the technological requirements are reduced. The invention further provides the preparation method of the content, the process of which is reasonable.
Owner:REYOUNG PHARMA
Telmisartan tablets and preparation method thereof
InactiveCN111249243AHigh similarityEasy to operateOrganic active ingredientsPharmaceutical non-active ingredientsDigestionPharmaceutical Aids
The invention relates to a preparation method of telmisartan tablets. The preparation method comprises steps as follows: telmisartan, sodium hydroxide and meglumine are subjected to salinization and recrystallization treatment, dry particles are obtained, the particles and auxiliary materials are granulated in a wet type granulator and dried in a fluidized bed, a lubricating agent is added after granulation for tabletting, and the telmisartan tablets with digestion performance which is highly similar to digestion performance of an original reference preparation are prepared. Telmisartan is adopted as a raw material, amorphous sodium salt is prepared from the telmisartan raw material by synthetic test equipment, the sodium salt has stable characters and can be stored for a long time, with the adoption of an optimized wet granulation technology, micronization equipment with harsh conditions is not needed, the technological operation is simple, meanwhile, similarity of multiple strippingcurves of an own product and a reference preparation is improved, the product has good stability and compressibility, and similarity of multiple stripping curves of a main drug and the reference preparation is improved.
Owner:CHONGQING CONQUER PHARML
Indapamide tablet and preparation method thereof
InactiveCN110538160ASolve the speed problemQuality improvementOrganic active ingredientsDrageesAdhesivePolyvinyl alcohol
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to an indapamide tablet and a preparation method thereof. The indapamide tablet is composed of a tablet core and a coating layer, and the tablet core is prepared from the following components in parts by weight: 0.5-2 parts of indapamide, 10-40 parts of a filler, 0.5-5 parts of an adhesive, and 0.5-10 parts of a lubricant, wherein the filler is any one or a combination of two or more of lactose anhydrous, lactose monohydrate, corn starch, microcrystalline cellulose lactose and starch lactose,the adhesive is any one or a composition of two or more of povidone K30, hydroxypropyl methylcellulose, polyvinyl alcohol and carboxymethylcellulose, and the lubricant is any one or a composition of two or more of talcum powder, magnesium stearate and silicon dioxide. The obtained indapamide tablet is a stable drug preparation which is lower in impurity content than a reference listed drug reference preparation, and has the bioequivalence.
Owner:TIANJIN PHARMA GROUP XINZHENG
Azithromycin dry suspension and preparation method thereof
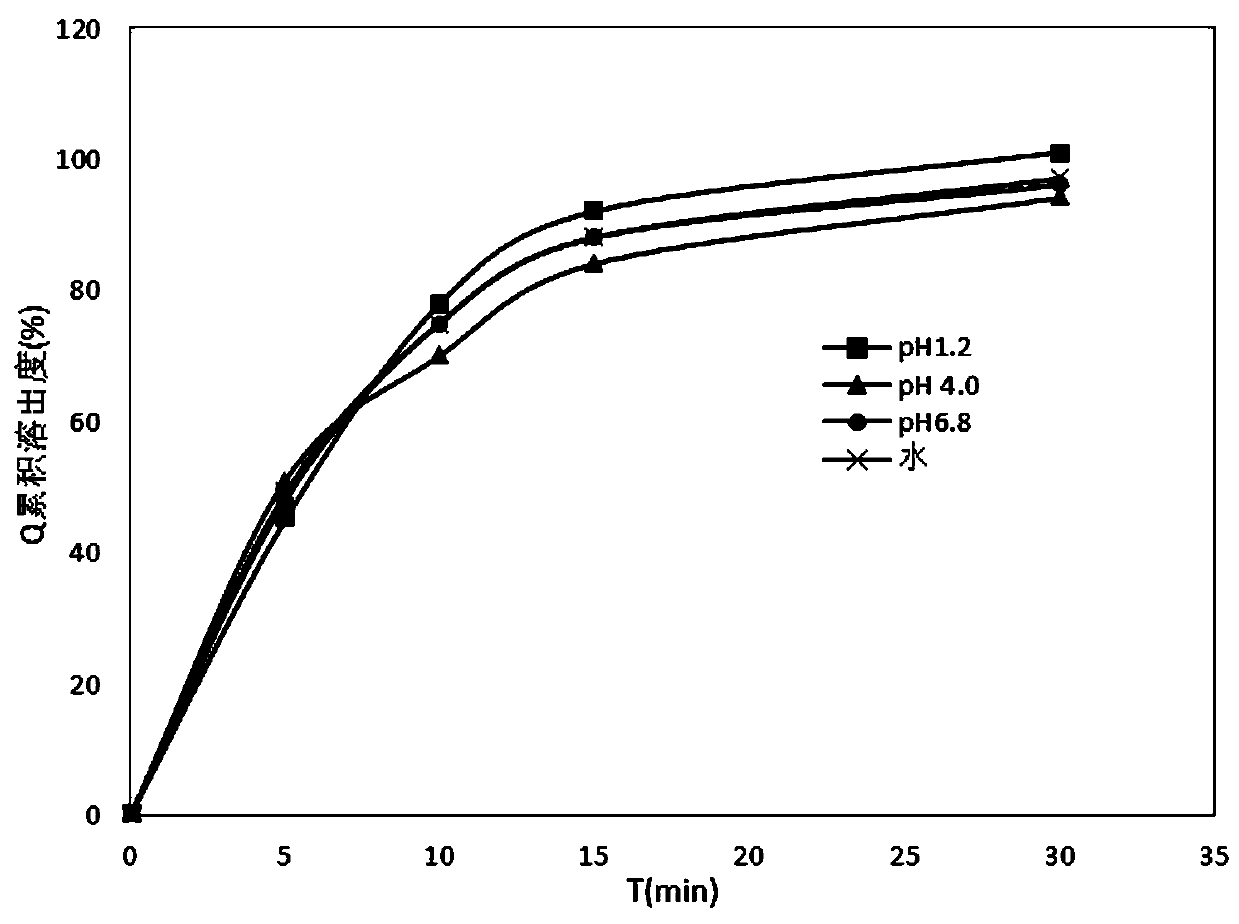
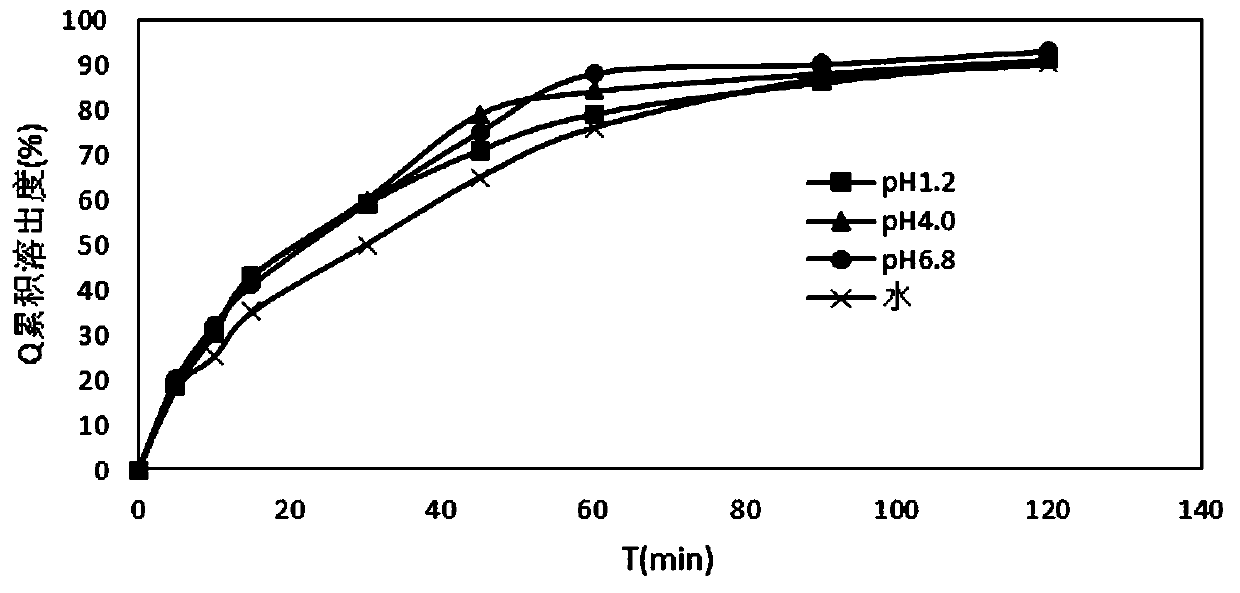
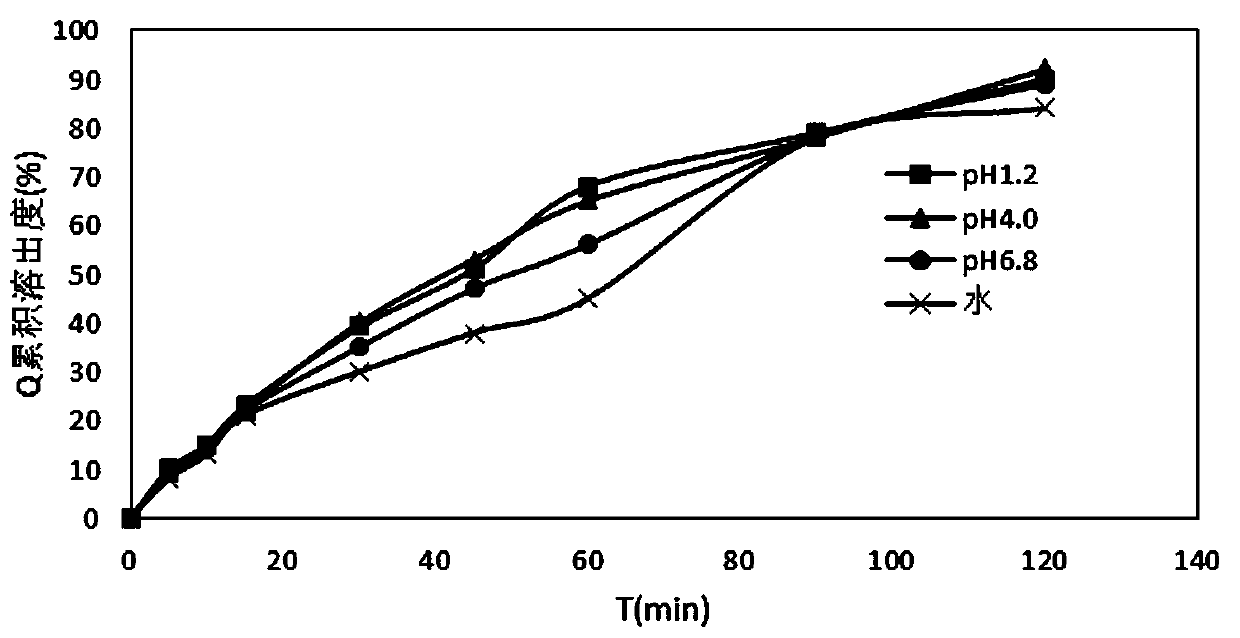
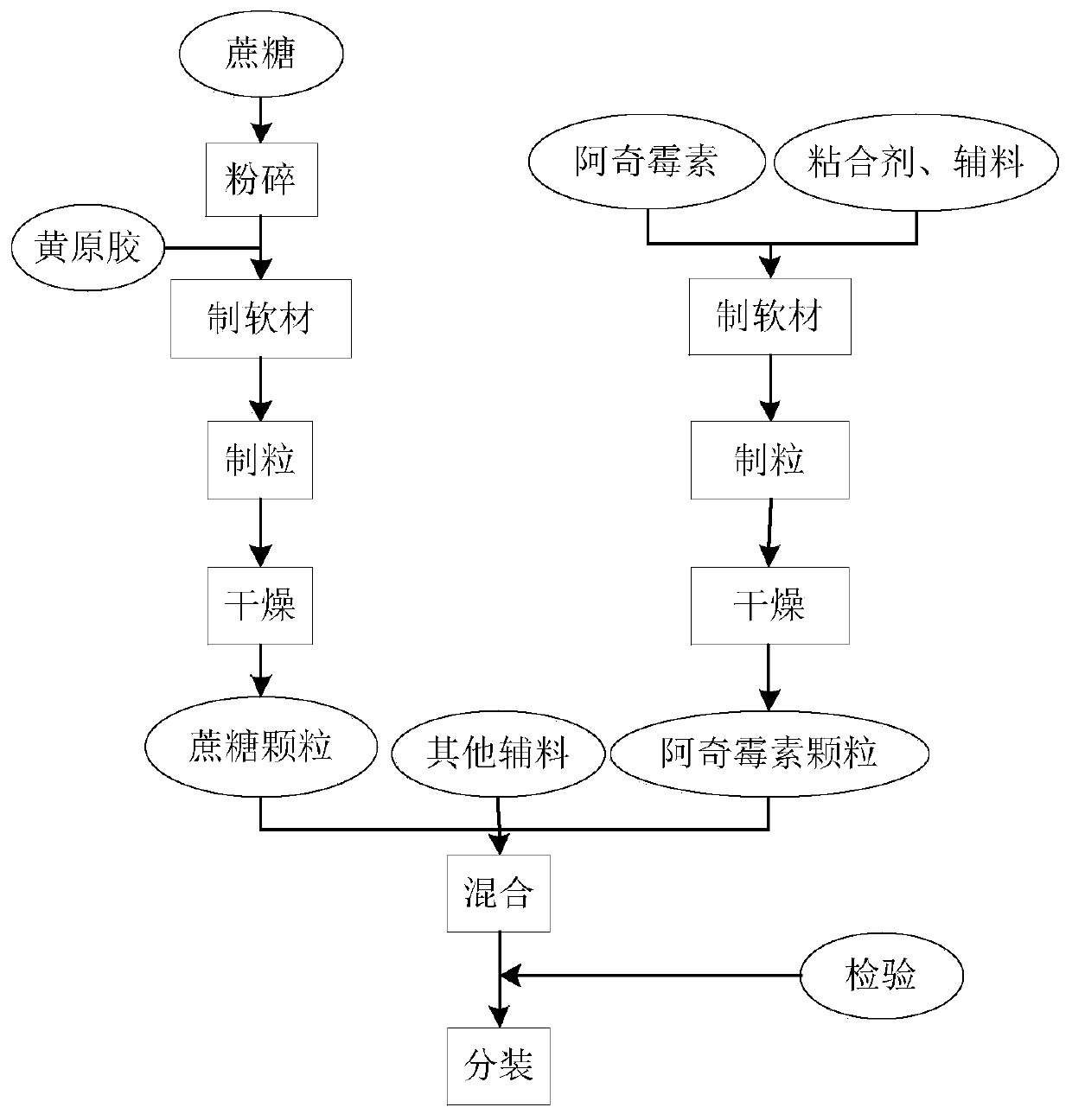
InactiveCN110478325AMask bitternessAddressing Adherence IssuesAntibacterial agentsOrganic active ingredientsAzithromycinMedicine
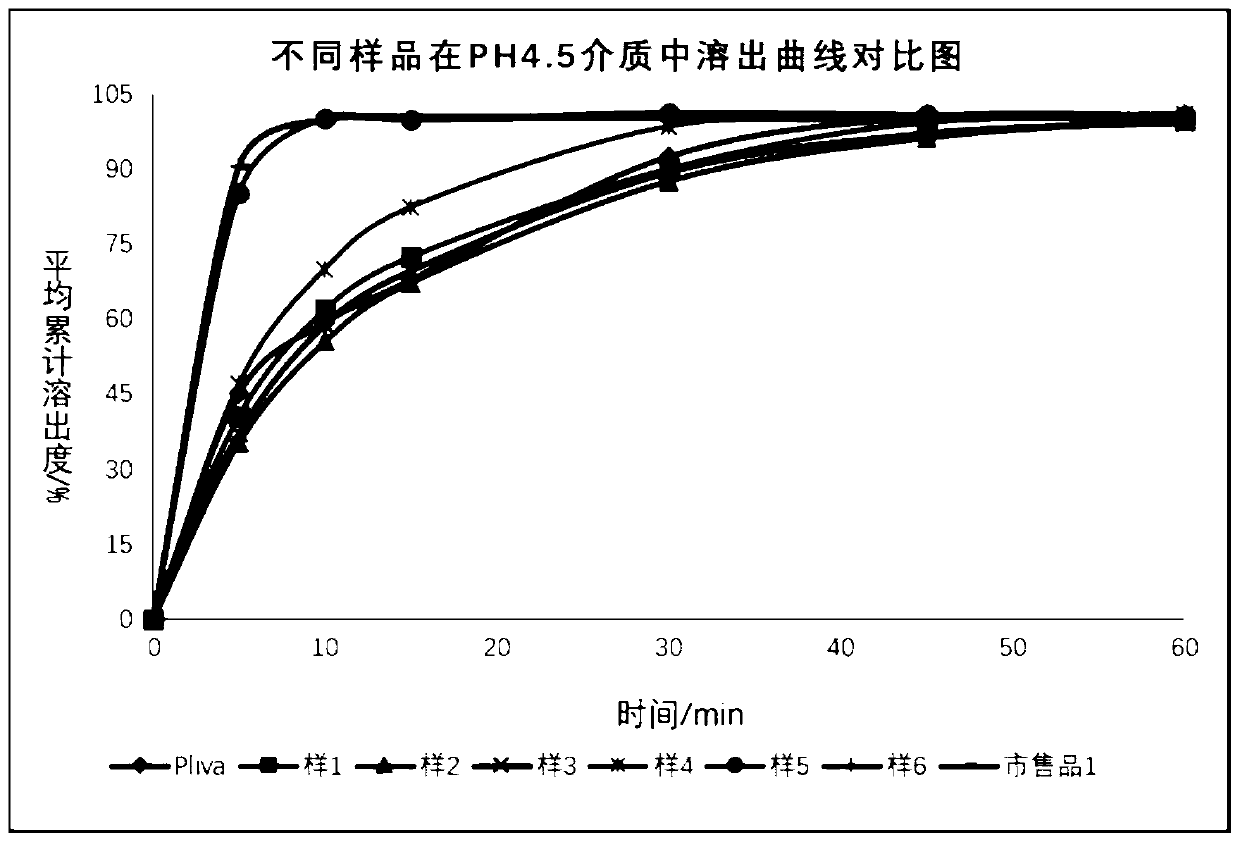
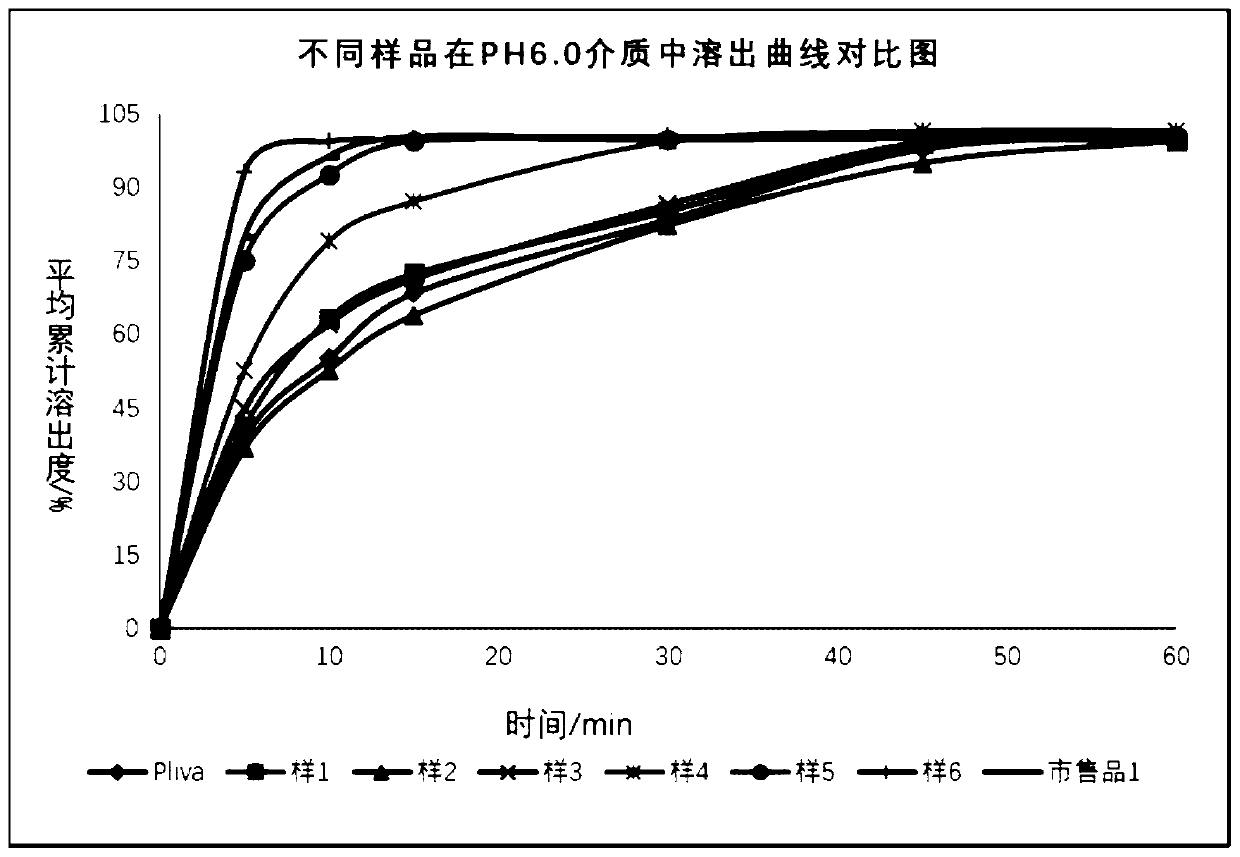
The invention relates to the field of pharmaceutical preparations, in particular to an azithromycin dry suspension and a preparation method thereof. In this method, azithromycin particles are firstlyprepared, and then the azithromycin particles are mixed with other excipients. The prepared azithromycin dry suspension has consistent in-vitro dissolution and in-vivo behavior with a reference preparation (azithromycin dry suspension Pliva) published by the NMP, that is, the azithromycin dry suspension of the invention has high consistency in quality and efficacy compared with the reference preparation. Meanwhile, the bitter taste of azithromycin can be well covered by the method, and the problem of children's drug compliance is solved.
Owner:BEIJING YUEKANGKECHUANG PHARM TECH CO LTD
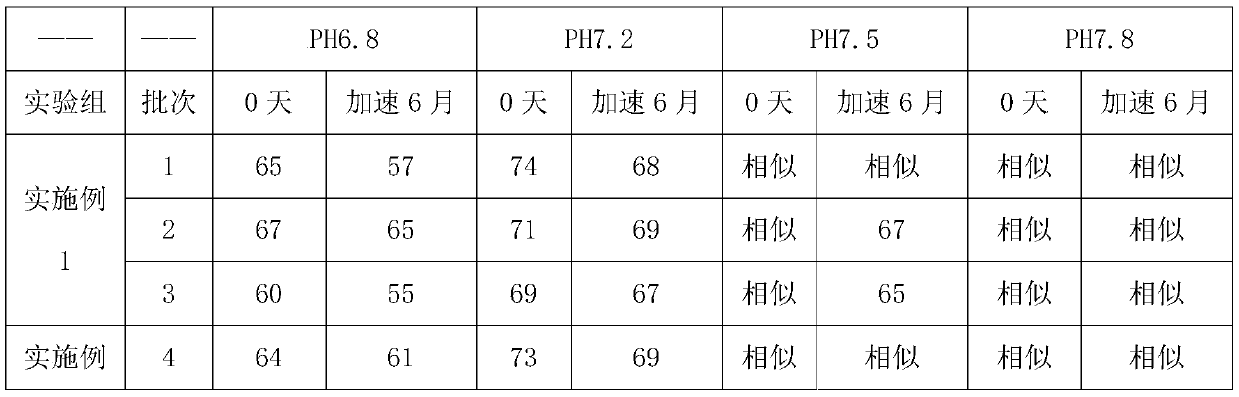
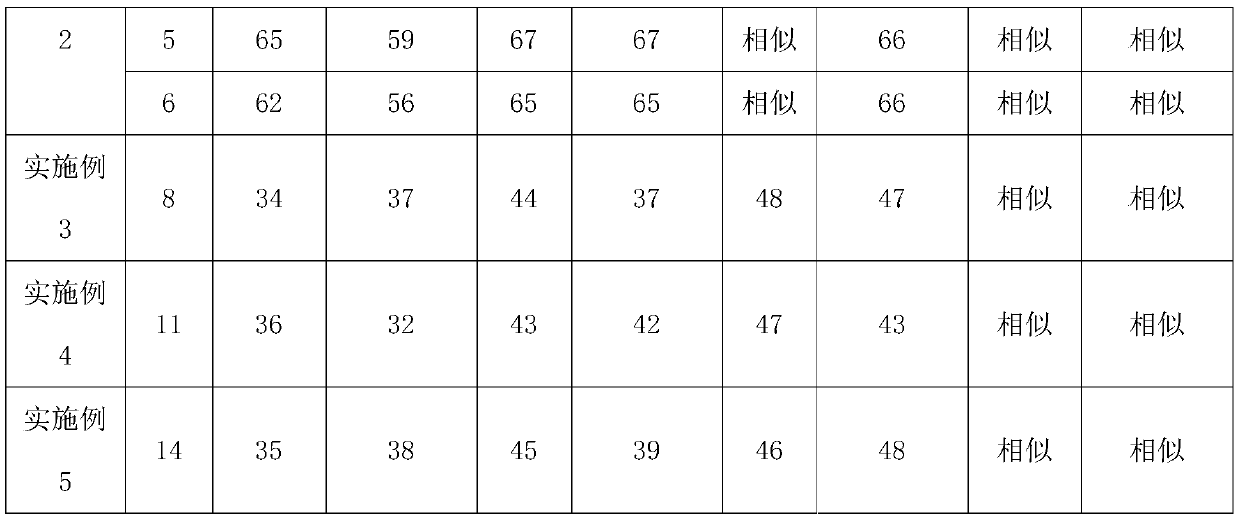
Method for detecting and evaluating in-vitro dissolution of enteric preparation
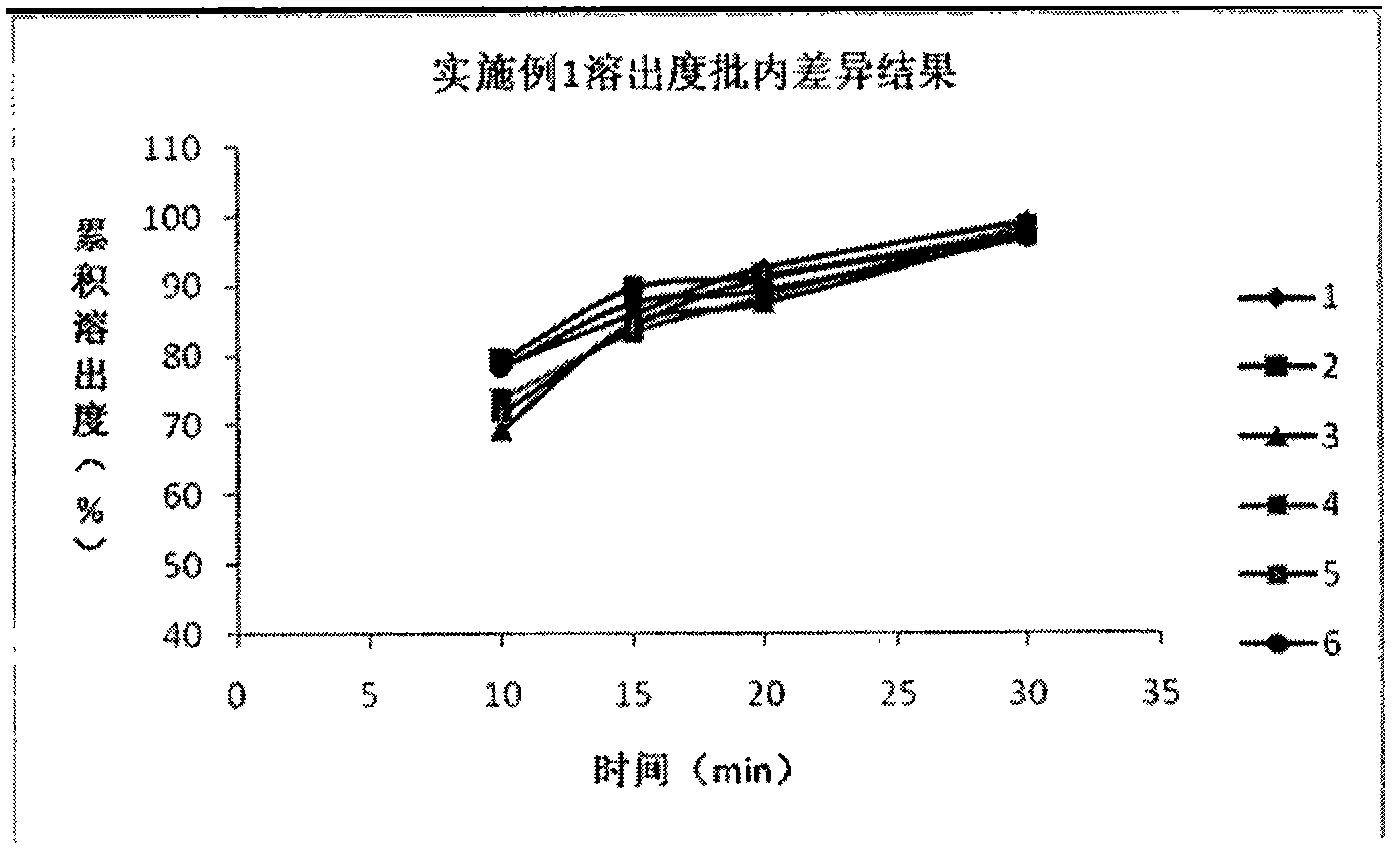
The invention relates to a method for detecting and evaluating in-vitro dissolution of an enteric preparation. The method is performed according to standards of Chinese Pharmacopoeia and comprises thesteps as follows: conducting a dissolution test in a simulated gastric fluid dissolution medium; then, transferring the enteric preparation in water or weakly acid dissolution medium for a dissolution test; besides, directly performing a dissolution test on the preparation in water or the weakly acid dissolution medium; comparing the difference between dissolution results of preparation subjectedto or not subjected to dissolution treatment in the simulated gastric fluid dissolution medium to determine the reliability of the enteric performance of the preparation. The method can provide datasupport for selection of multi-source reference preparations in generic drug consistency evaluation and provide reference for prescription screening and bioequivalence risk evaluation. More importantly, the method is used for detecting and evaluating in-vitro dissolution of the enteric preparation, so that the incidence rate of adverse events of clinical medication can be reduced.
Owner:BEIJING INST FOR DRUG CONTROL
Preparation method for telmisartan tablets
InactiveCN111700866APrescription process optimizationHigh similarityOrganic active ingredientsPharmaceutical non-active ingredientsAqueous ethanolMannitol
The invention relates to a preparation method for telmisartan tablets. The preparation method comprises the following steps: S1, weighing telmisartan, sodium hydroxide and meglumine to prepare an aqueous solution or an aqueous ethanol; S2, adding weighed povidone and sieved mannitol into a fluidized bed to make the povidone and mannitol in a fluidized state, spraying the mixed solution prepared instep S1, performing spray granulation, and performing granule finishing after drying; and S3, adding the finished granules to the weighed magnesium stearate and sodium stearyl fumarate, and performing tabletting after uniform mixing to obtain plain tablets, wherein the hardness of the plain tablets is controlled to be 7-11kgf. The preparation method optimizes the prescription technique of the telmisartan tablets, and improves the similarity of multiple dissolution curves of own products and reference preparations.
Owner:CHONGQING CONQUER PHARML
Valaciclovir hydrochloride tablet and preparation method thereof
ActiveCN110279667AQuality improvementStable batchInorganic non-active ingredientsAntiviralsAdhesiveReference preparation
The invention provides a valaciclovir hydrochloride tablet and a preparation method thereof. The valaciclovir hydrochloride tablet is prepared from valaciclovir hydrochloride particles, a filling agent, a disintegrating agent, an adhesive, a lubricant A and a lubricant B, wherein the valaciclovir hydrochloride particles are prepared from valaciclovir, magnesium oxide, the lubricant A and the lubricant B. The preparation method comprises the following steps: preparing the valaciclovir hydrochloride particles by adopting a dry granulation technology; then mixing the valaciclovir hydrochloride particles with other auxiliary materials; and tabletting. The magnesium oxide as an alkaline oxidant is added for preparing the valaciclovir hydrochloride particles, so that the release rate of a medicine can be effectively adjusted, and the in-vitro dissolution behavior of the medicine is enabled to be similar to that of a reference preparation in multi-medium; and in addition, the inter-batch quality and the within-batch quality of the valaciclovir hydrochloride tablet are stabilized.
Owner:珠海润都制药股份有限公司
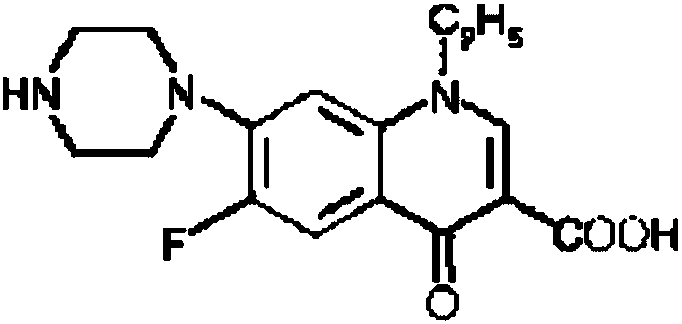
Oral norfloxacin capsule and preparation method thereof
InactiveCN109620814ADetermination of curative effectQuality improvementAntibacterial agentsOrganic active ingredientsMedicineNorfloxacin
The invention relates to an oral norfloxacin capsule and a preparation method thereof and belongs to the field of pharmaceutical preparations. The oral norfloxacin capsule contains norfloxacin, sodiumcarboxymethyl starch and a pharmaceutically acceptable auxiliary material, wherein the mass ratio of the norfloxacin to the sodium carboxymethyl starch is 10-30 to 1. A norfloxacin raw material has the particle diameter D50 of 100 microns or below, and D90 of 240 microns or below, preferably D50 of 60 microns or below and D90 of 180 microns or below. The particle size of the sodium carboxymethylstarch is D50 of 40 microns or below and D90 of 120 microns or below, preferably D50 of 30 microns or below and D90 of 75 microns or below. The key process for the preparation of the contents is thatnorfloxacin and sodium carboxymethyl starch are premixed and then are mixed with other pharmaceutically acceptable auxiliary materials. The norfloxacin-containing capsule is consistent with norfloxacin tablet reference preparations produced by Japan Xinglin joint-stock company on the market at present in in-vitro dissolution.
Owner:WUHAN TONGJI MODERN PHARMA TECH CO LTD
Simultaneous measurement of ligustrazine and aspirin in blood plasma by LC-MS
PendingCN112345683AThe method is sensitive and efficientLow and accurate detection concentrationComponent separationMetaboliteBlood plasma
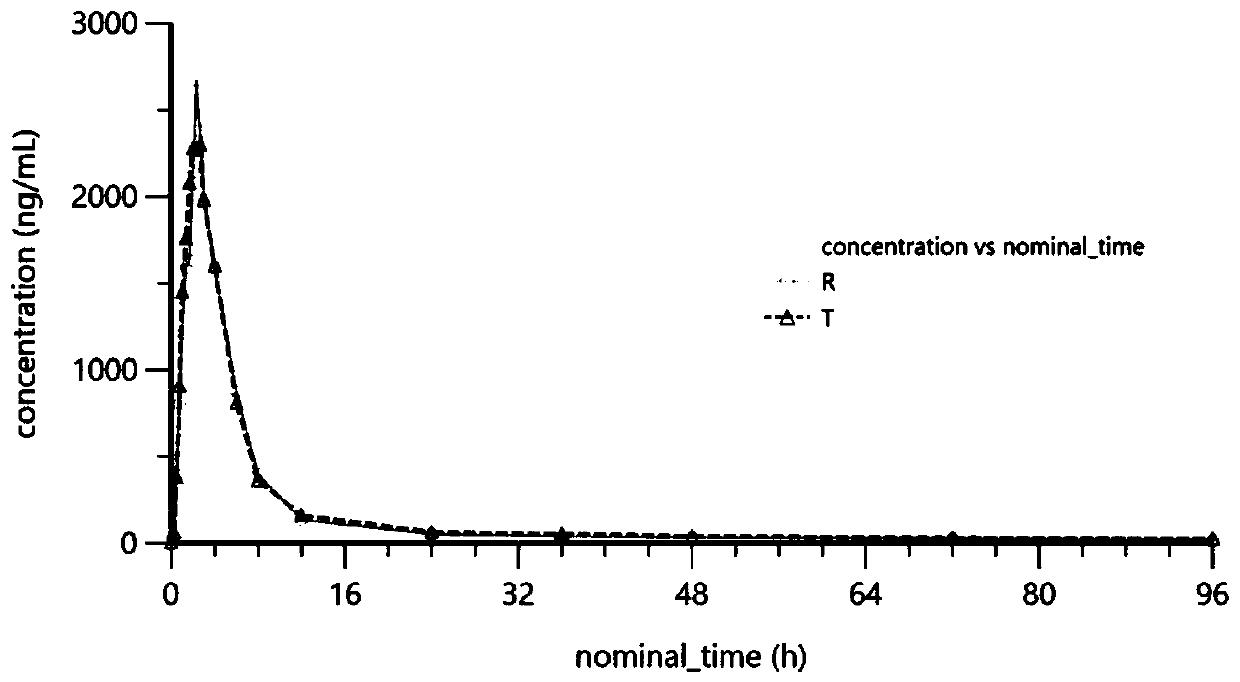
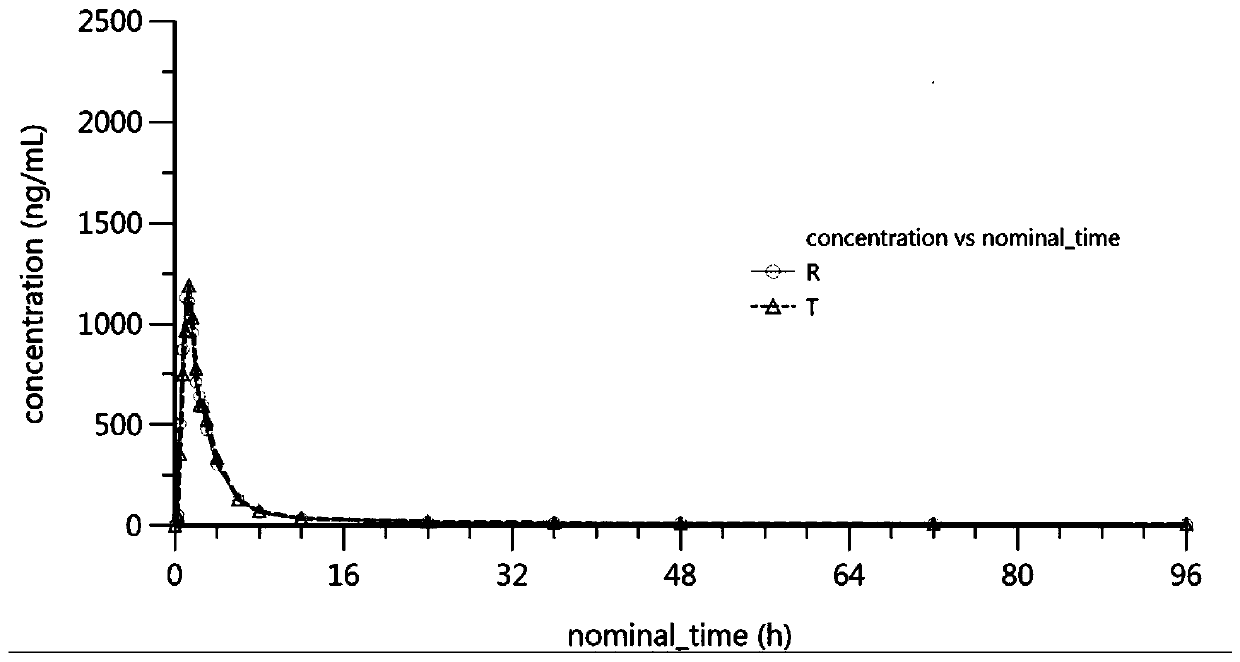
The invention provides simultaneous measurement of ligustrazine and aspirin in blood plasma by an LC-MS, and relates to the technical field of medicines. A chromatographic column filled with octadecylbonded silica gel is included, the flow rate of a liquid phase is 0.6 ml / min to 1.4 ml / min, and a mobile phase is prepared from methanol and a formic acid aqueous solution according to a certain proportion; a liquid chromatography-mass spectrometry tandem isocratic elution method is used for separating and analyzing ligustrazine, aspirin and in-vivo metabolites of ligustrazine and aspirin after aplasma sample is treated, and the method is sensitive, efficient, low and accurate in detection concentration, simple and easy to operate, capable of effectively detecting whether a tested sample hasbioequivalence with a reference preparation or not and high in sensitivity; a single component can be detected, a corresponding atrioventricular model can be established on the basis of the detectedblood concentration, and indexes such as key parameters Cmax, Tmax, T1 / 2 and AUC of the atrioventricular model are solved, so that whether bioequivalence exists between preparations or not is deduced.
Owner:LIAONING YAOLIAN PHARMA
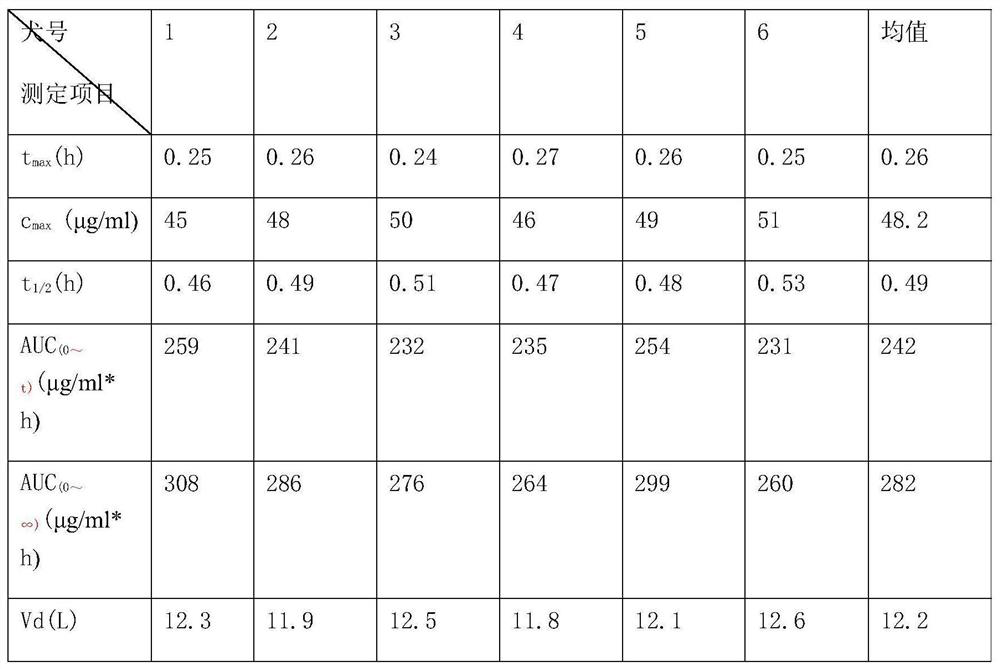
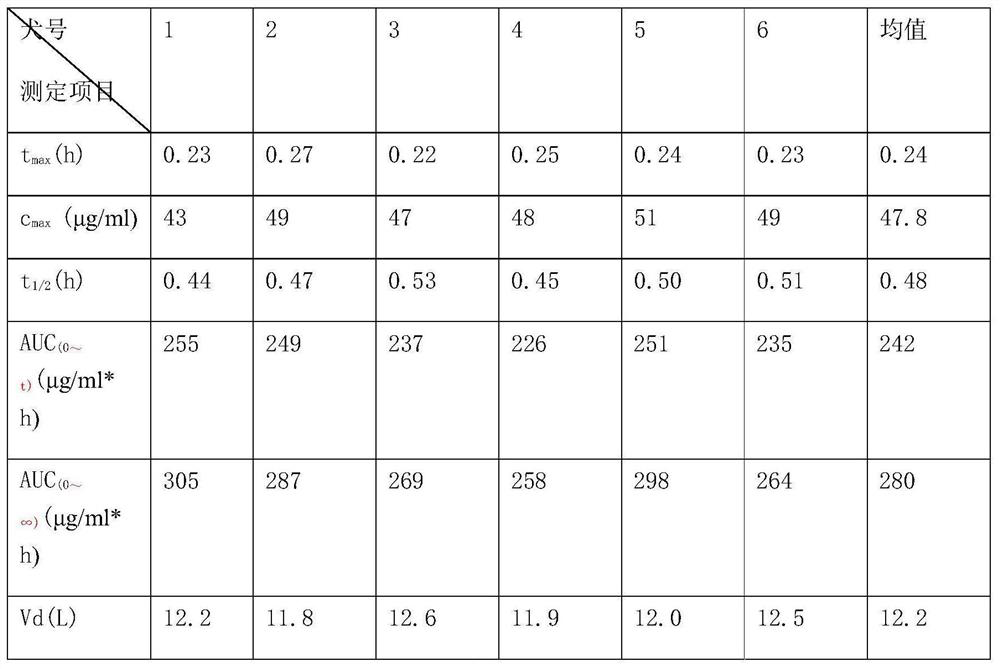
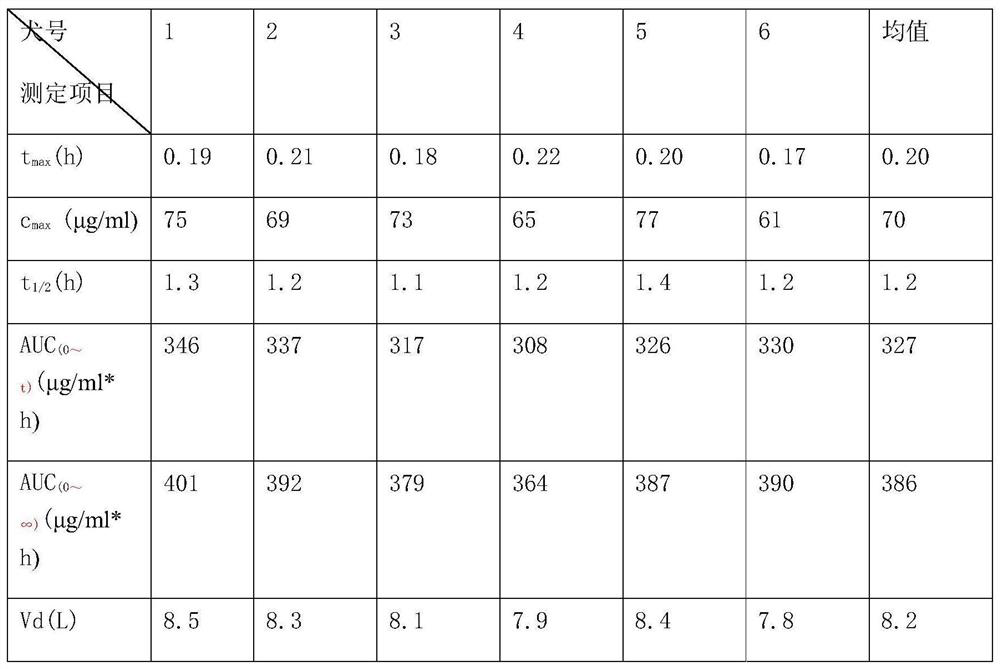
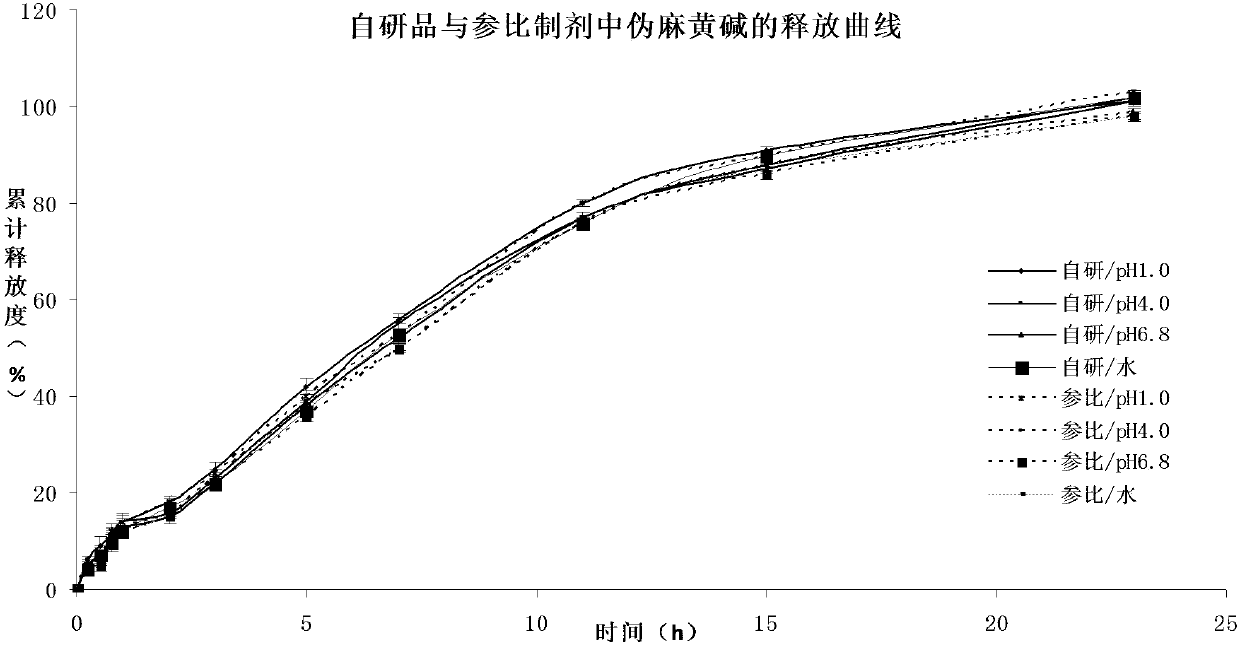
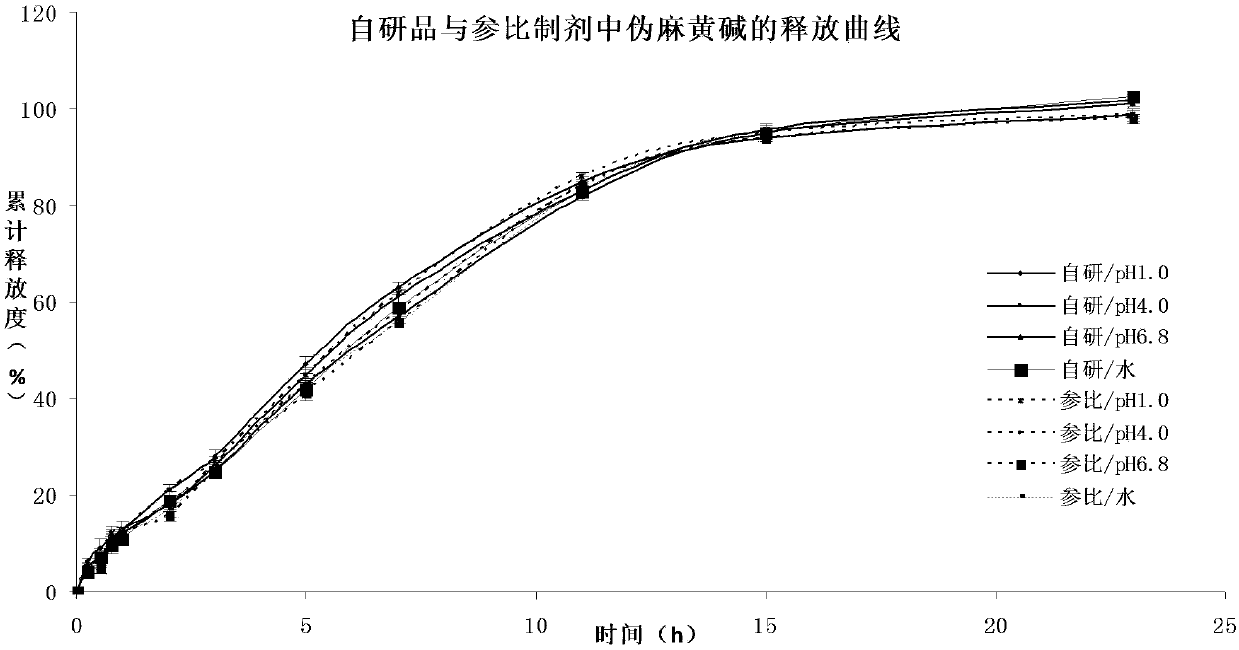
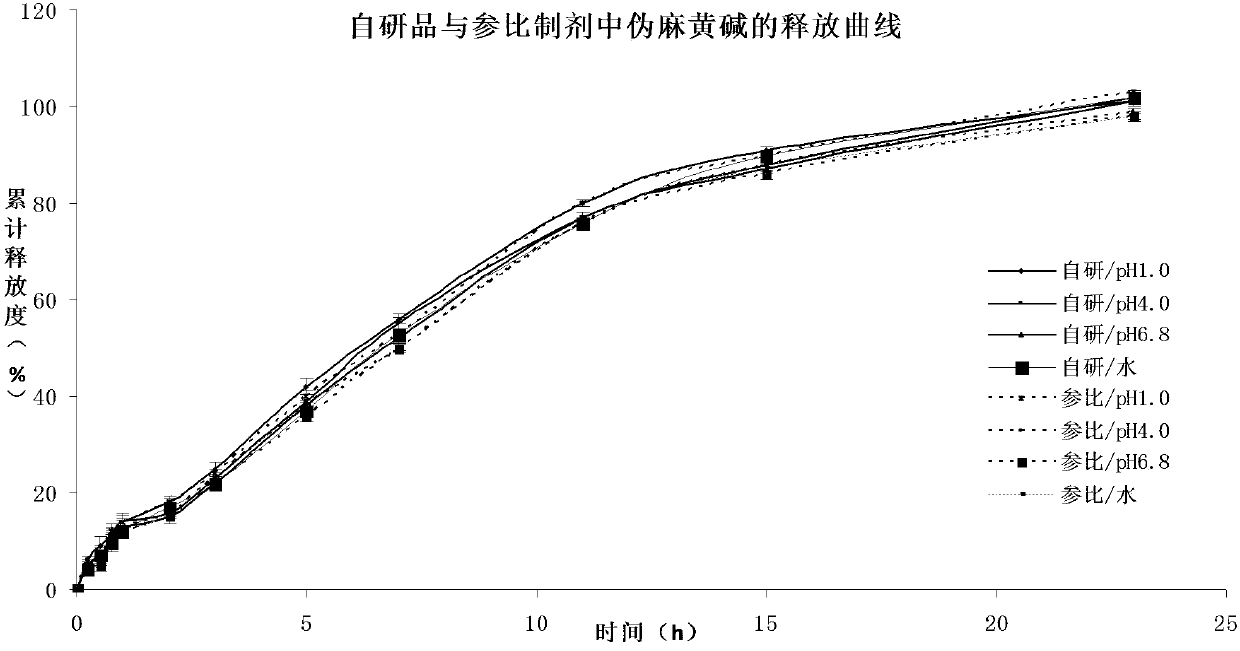
Pseudoephedrine slow release pellets and preparation method thereof
ActiveCN109966265AImprove stabilityImprove bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsLipid formationHydrophilic polymers
The invention provides pseudoephedrine slow release pellets and a preparation method thereof. The pseudoephedrine slow release pellets are prepared through the steps of preparing pseudoephedrine containing pellet cores by an extruding and rounding method, and sequentially coating a slow release layer on the pellet cores through a hot melting and coating technique, and coating materials do not needany solvents. Compositions of lipid materials, hydrophilic polymers having surface activity and the like are prepared into the pseudoephedrine slow release pellets through the hot melting and coatingtechnique, the advantages of the hot melting and coating technique being safe, environmentally-friendly, energy-saving, high-efficiency and capable of increasing medicine stability and biological availability can be exerted, the medicines can be released as needed and can be released completely, a release curve and a reference preparation have consistency, and large-scale production can be realized.
Owner:GUANGZHOU PHARMACEUTICAL INDUSTRIAL RESEARCH INSTITUTE
Terbinafine hydrochloride tablets and preparation method thereof
The invention discloses terbinafine hydrochloride tablets and a preparation method thereof. The terbinafine hydrochloride tablets consist of terbinafine hydrochloride and pharmaceutic adjuvants, wherein the pharmaceutic adjuvants comprise a filling agent, a disintegrating agent, an adhesive and a lubricating agent. By adopting a wet granulation process, the terbinafine hydrochloride tablet is simple in preparation process, easy to operate, controllable in quality, good in reproducibility and suitable for industrial large-scale production, and the obtained terbinafine hydrochloride tablet is smooth in surface, high in dissolution rate, small in dissolution difference among batches, high in in in-vivo bioavailability, bioequivalent to a reference preparation in human body, and consistent with the reference preparation in quality.
Owner:NANJING CHENGONG PHARM CO LTD
Preparation method of glimepiride tablets
InactiveCN111135150APrescription process optimizationHigh similarityMetabolism disorderSulfonylurea active ingredientsAqueous ethanolMagnesium stearate
The invention relates to a preparation method of glimepiride tablets. The preparation method of the glimepiride tablets comprises the following steps: taking micronized glimepiride as a raw material;mixing the micronized glimepiride with auxiliary materials with relatively good solubilization effects, namely lactose and povidone, for a long period of time; and then, adding a certain amount of water or an aqueous ethanol solution as a moistening agent. According to the preparation method of the glimepiride tablets, the time of shearing and mixing is increased, so that the solubilization effects are promoted; and magnesium stearate, which has relatively little influence on dissolution, is added for performing mixed tablet compression, so that the final dissolution rate of the main drug is ensured with similarity of several dissolution curves between the self-made product and the reference preparation improved.
Owner:CHONGQING CONQUER PHARML
Norfloxacin capsule and preparation method thereof
The invention discloses a norfloxacin capsule and a preparation method thereof. The norfloxacin capsule is prepared from, by weight, 10-20 parts of ambroxol hydrochloride, 20-80 parts of lactose, 10-60 parts of corn starch, 1-2 parts of silicon dioxide and 0.5-1 part of magnesium stearate. An ambroxol hydrochloride table composition obtained is controllable in quality and has the advantages of quick dissolution as compared with domestic market-selling tablets and the similarity in dissolution with a reference preparation.
Owner:合肥远志医药科技开发有限公司
Tinidazole tablet consistency evaluation method
PendingCN112730639AReduce failure rateImprove stabilityComponent separationTinidazolePharmaceutical drug
The invention discloses a tinidazole tablet consistency evaluation method. The method comprises the following steps: before starting a BE test and an in-vitro dissolution curve, pre-evaluating a tinidazole tablet, evaluating whether the measured tinidazole tablet has consistency or not, and then carrying out the BE test and in-vitro dissolution curve verification so that the failure rate of the BE test is reduced, the time and the cost are saved, and the production efficiency is improved. Through the pre-evaluation process, the stability of the tinidazole tablet can be determined, and the basic composition and the characteristic structure of a tinidazole medicine and a reference preparation Fasigyn are consistent so that the consistency of the medicine effects of the two medicines is judged, and the success rate of the BE test and an in-vitro dissolution curve test is ensured. The method is used for solving technical problems that in an existing tinidazole tablet consistency evaluation method, testing is performed only through the BE test and the in-vitro dissolution curve, a large amount of time and cost need to be consumed, if test results are inconsistent, testing needs to be performed again, and a research and development progress is seriously affected.
Owner:HAINAN HAILI PHARMACEUTICAL CO LTD
Calcium acetate pharmaceutical composition and preparation method and application thereof
PendingCN110279668AGuaranteed stabilitySolve the problem of inconsistent crystal formsMetabolism disorderPill deliveryTriturationDissolution
The invention provides a calcium acetate pharmaceutical composition and a preparation method and application thereof. According to the invention, calcium acetate of which the crystal form is inconsistent with that of the original trituration is used as a raw material for granulation, conversion of the crystal form of the calcium acetate is realized by adjusting granulation parameters and changing the concentration of ethanol granulation, storage time at a normal temperature in a sealed mode and the granule drying temperature, then the calcium acetate is mixed with pharmaceutical auxiliary materials allowed to be used pharmaceutically, tabletting is carried out, and the crystal form of the prepared calcium acetate tablets is consistent with that of a reference preparation (trade name: CALCIUM ACETATE), different dissolution behaviors, bioavailability and the like caused by inconsistent crystal forms are avoided, so that the stability and bioequivalence of the drug are ensured, and the calcium acetate pharmaceutical composition has high practical application value.
Owner:SHANDONG CHUANGXIN PHARMA RES & DEV
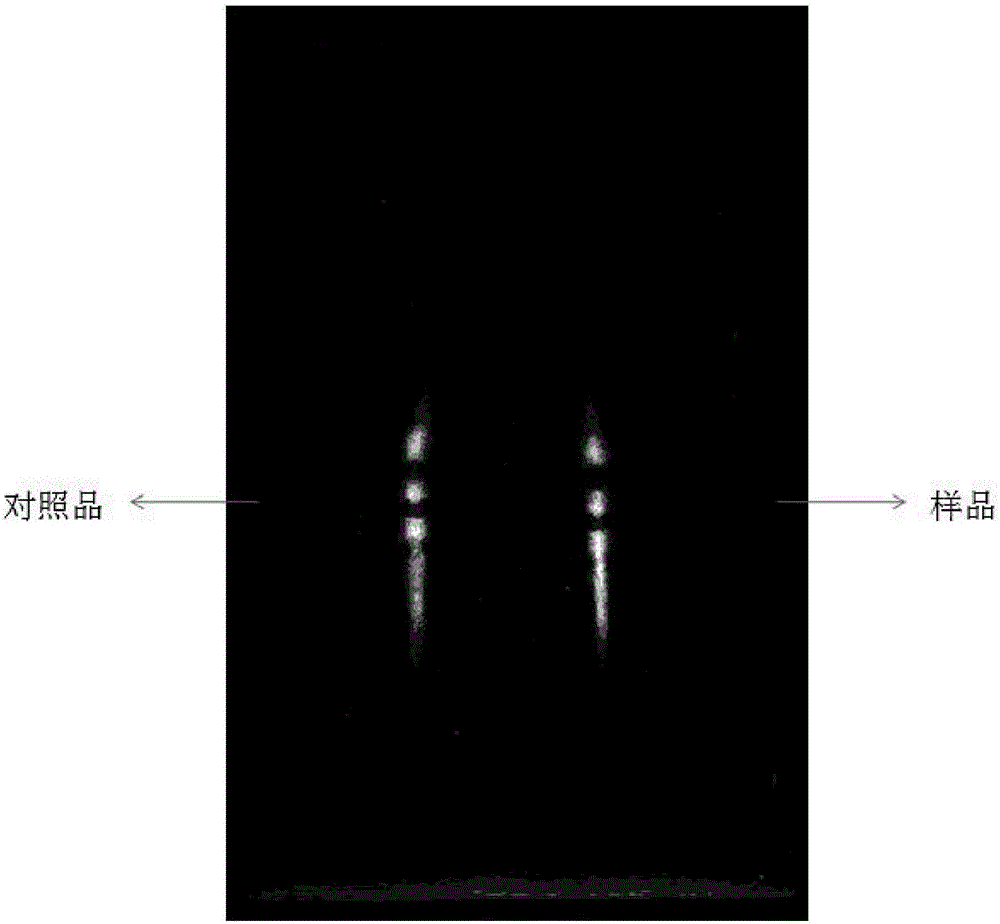
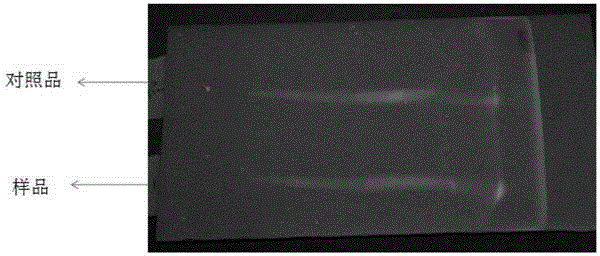
Identification method for effective components in ginkgo leaf preparation
The invention discloses an identification method for the effective components in a ginkgo leaf preparation. The identification method comprises: (1) preparing a testing product: taking a proper amount of a ginkgo leaf preparation to be detected, adding n-butanol, extracting, filtering, evaporating the filtrate, and adding ethanol to the residue to dissolve so as to be adopted as the testing product solution; (2) preparing a reference substance solution: taking a proper amount of a ginkgo leaf reference preparation, and preparing the reference substance solution according to the step (1); and (3) carrying out thin layer chromatography: respectively sucking 1-10 [mu]lt of the testing product solution and the reference substance solution, respectively spotting on the same silica gel G plate, developing with an ethyl acetate, isopropyl alcohol, methanol and water mixture, taking out the thin plate, carrying out air drying, spraying a 1-5% aluminum chloride ethanol solution, placing in a 365 nm ultraviolet light lamp, viewing the fluorescence, and observing the spot. According to the present invention, with the identification method, the good separation effects of various effective components are good, the spots are clear, the Rf value is moderate, various effective components can be accurately identified, and the qualitative analysis and quality control are easily achieved.
Owner:HANGZHOU CONBA PHARMA
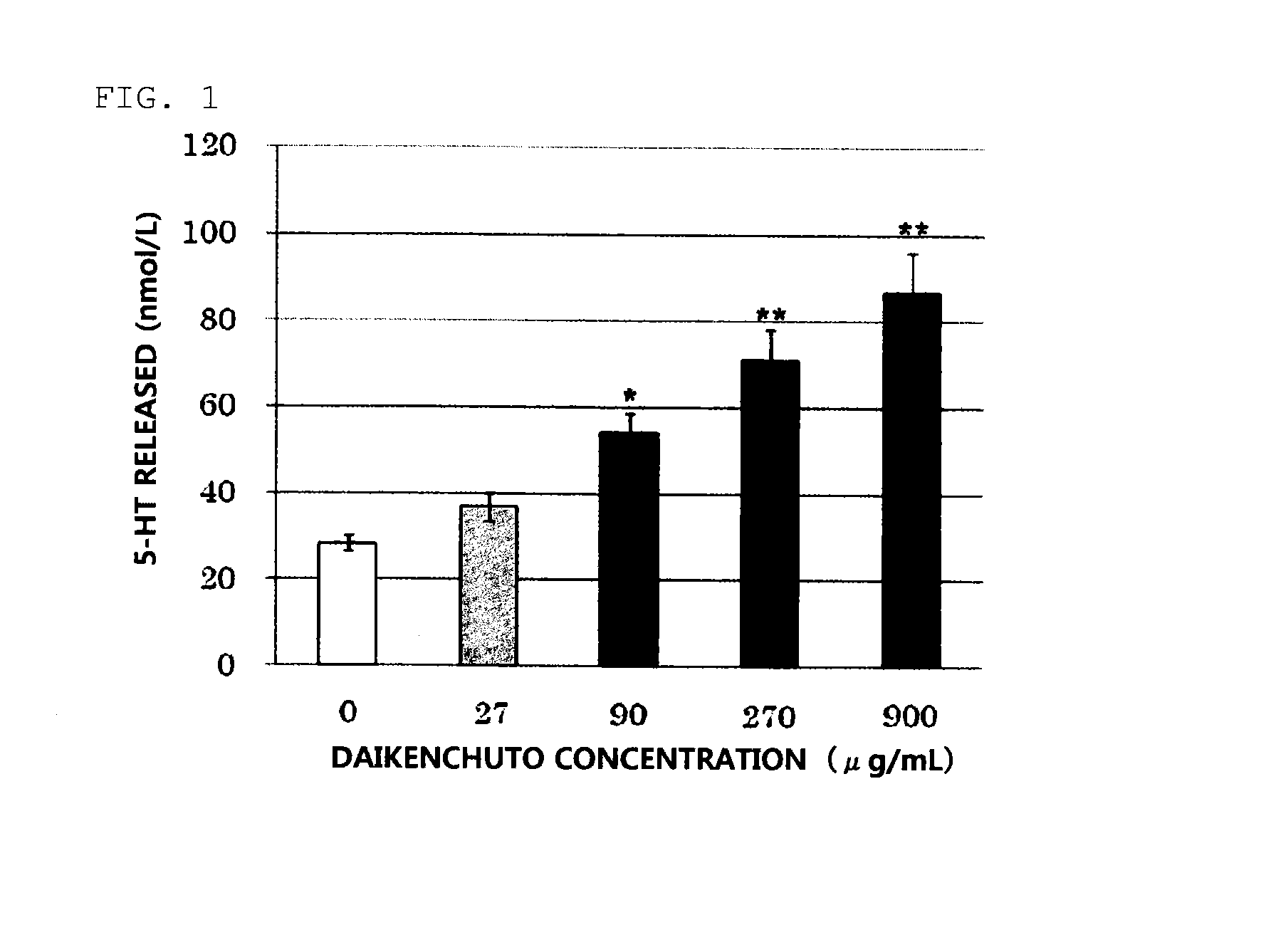
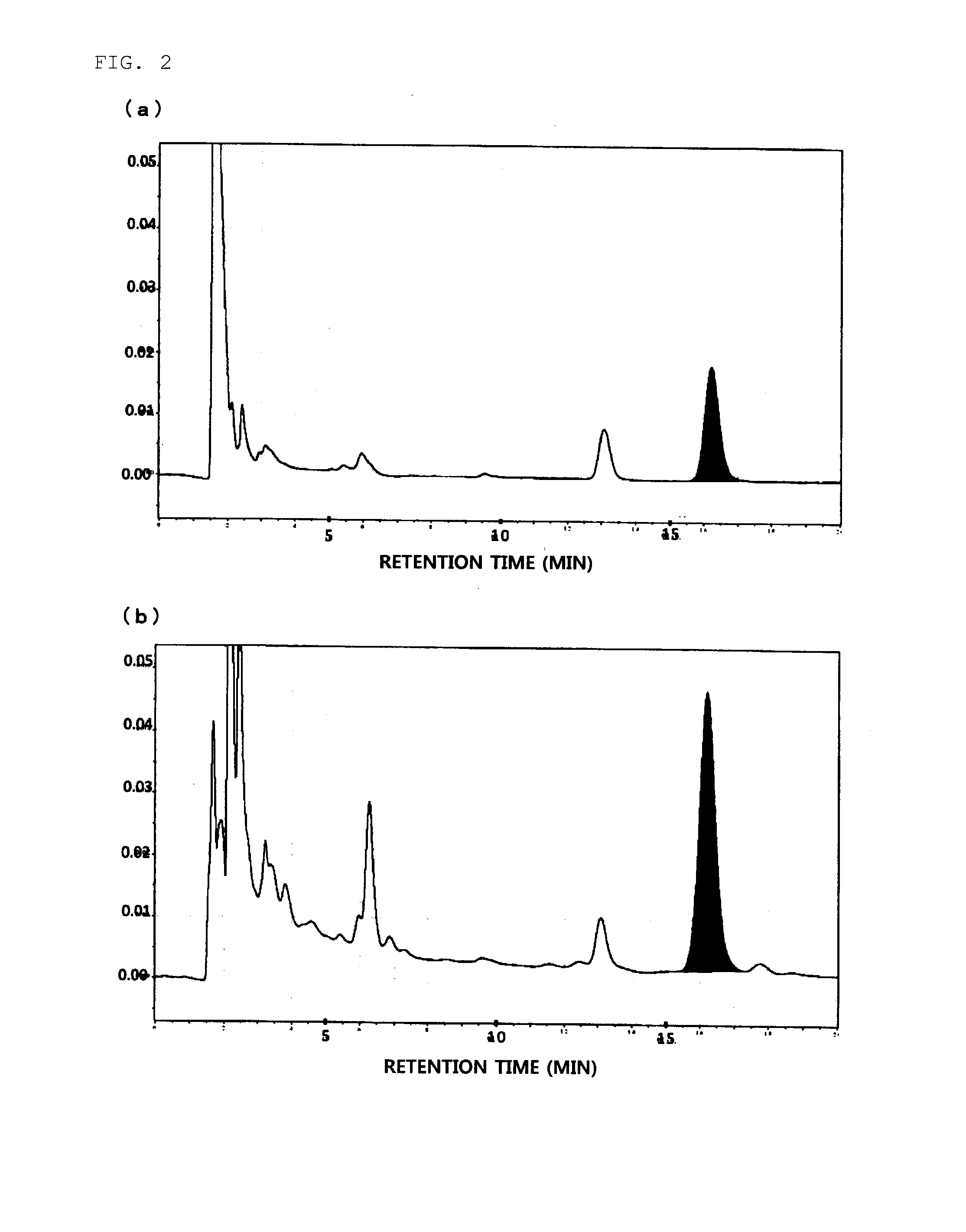
Daikenchuto bioassay method and quality management method using same
ActiveUS20130260401A1Highly accurate quality controlMicrobiological testing/measurementBiological material analysisSerotoninTest sample
The invention intends to provide a bioassay method using a simple in-vitro test for Daikinchuto, and further to provide a more highly accurate method for quality control of Daikenchuto using the same. These methods are a bioassay method for the pharmacological activity of Daikenchuto, characterized in that a test sample containing Daikenchuto is added to cultured serotonin-producing cells, and the serotonin content in the culture supernatant is subsequently measured; and a quality control method for Daikenchuto preparations in which the pharmacological activity of a test preparation and a reference preparation for which the pharmacological effect as Daikenchuto has been clinically confirmed are evaluated under the same conditions, and the equivalence of the reference preparation and testing preparation is evaluated.
Owner:TSUMURA
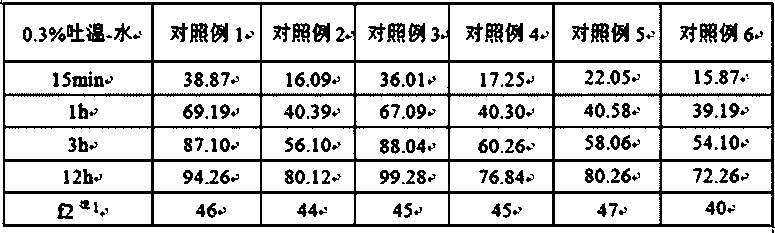
Method for detecting dissolution curve of simvastatin tablet
ActiveCN108414656AImprove discriminationModerately differentiated qualityComponent separationGeneric drugDrug product
The invention relates to a method for detecting the dissolution curve of a simvastatin tablet. The method comprises the following steps: (1) preparing a dissolution medium: preparing the dissolution medium at a dissolution temperature of 40 DEG C or less by using Tween-80 as a medium; 2) taking a simvastatin reference substance, and preparing a reference substance solution; (3) preparing a samplesolution by using a dissolvability detection technology; (4) detecting the dissolution quantity by high performance liquid chromatography, wherein the sample introduction temperature of a sample chamber is 4-5 DEG C; (5) repeating step (3) and step (4) twice or more; and (6) evaluating: calculating a similarity factor f2 by using a similarity factor technology, and comparing the similarity betweenthe dissolution curve of a generic preparation and the dissolution curve of a reference preparation by using the similarity factor f2. The method is scientific, durable and reproducible, can be usedfor evaluating the quality consistency evaluation of a generic drug and an original drug, and also can provide a guarantee for the consistency of the inter-batch quality of the drug.
Owner:四川省食品药品检验检测院
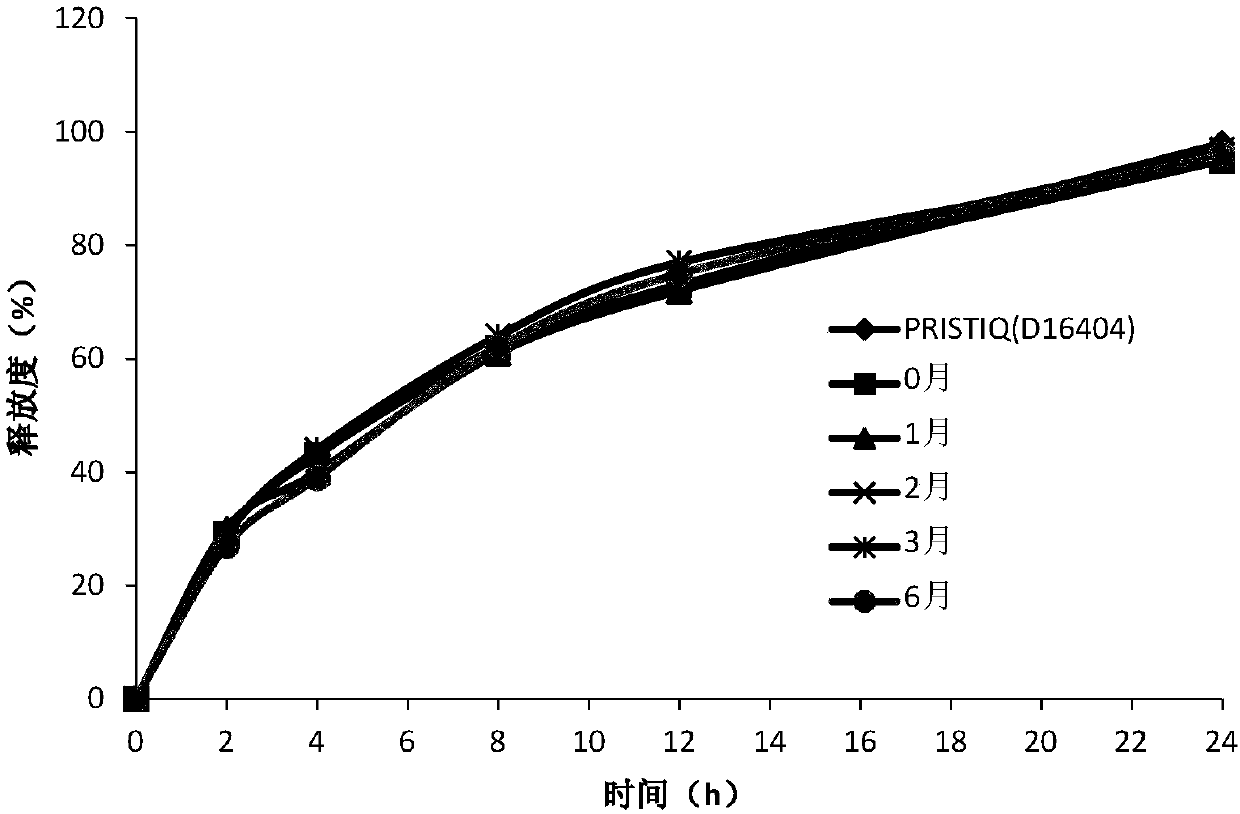
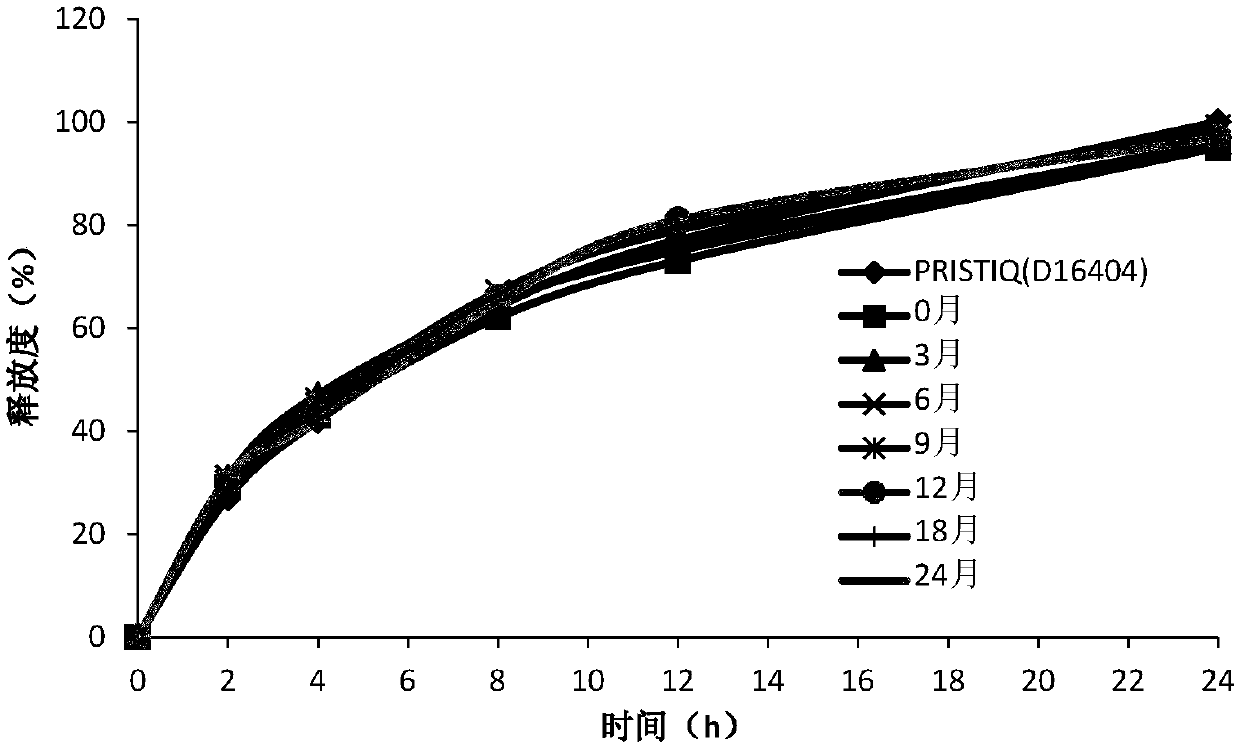
Desvenlafaxine hydrochloride pharmaceutical composition and preparation method thereof
InactiveCN109771380AConsistent in vitro release profileConsistent reference productOrganic active ingredientsNervous disorderFiller ExcipientDesvenlafaxine
The invention relates to a desvenlafaxine hydrochloride pharmaceutical composition and a preparation method thereof. The desvenlafaxine hydrochloride pharmaceutical composition contains desvenlafaxinehydrochloride, a gel matrix material, a filler, a lubricant and an anti-adhesive agent. The pharmaceutical composition is prepared by wet granulation and tabletting. The in-vitro release curves of the desvenlafaxine hydrochloride pharmaceutical composition provided by the present invention is substantially consistent with that of a reference preparation, and the desvenlafaxine hydrochloride pharmaceutical composition has good stability. The preparation process is simple and easy to operate, and the preparation process is smooth and easy to industrialize.
Owner:连云港恒运药业有限公司
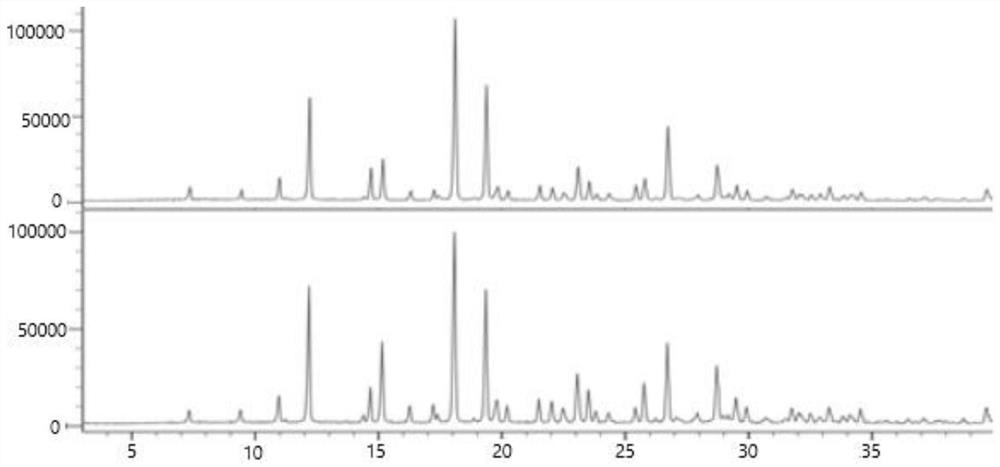
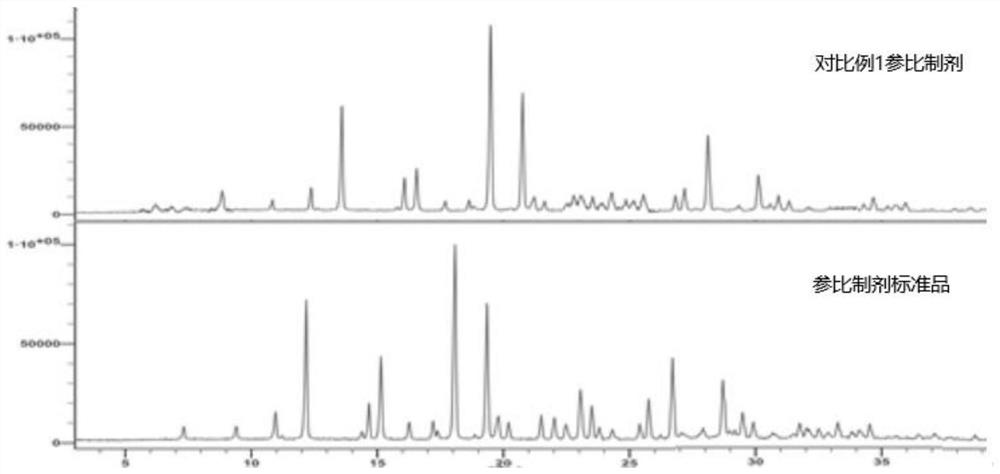
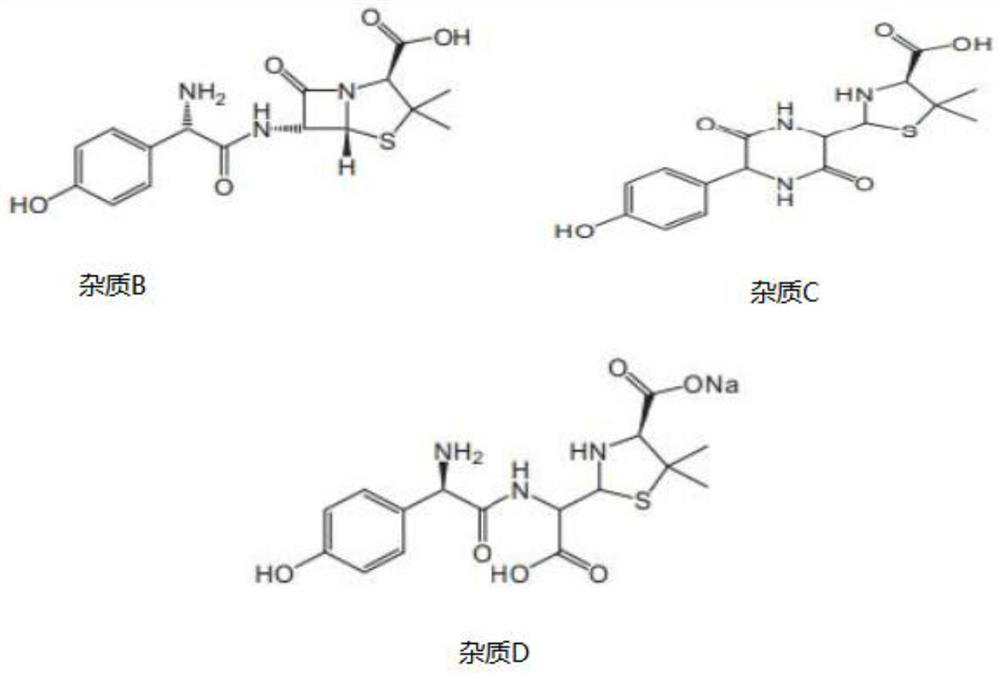
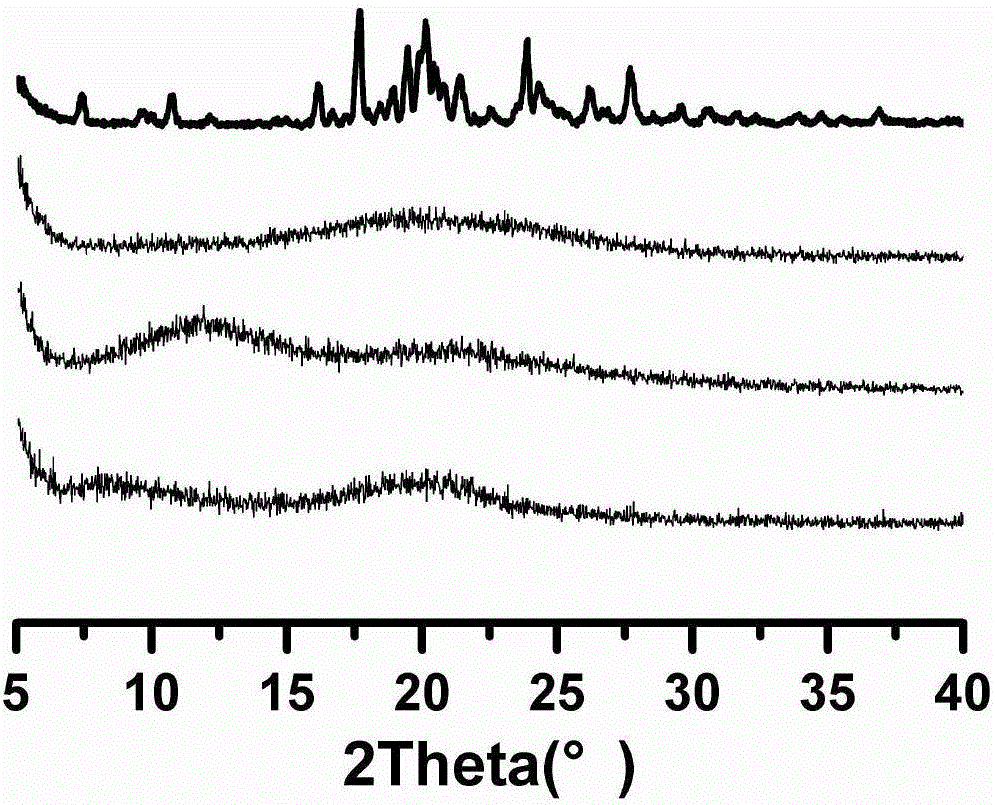
Consistency evaluation and detection method for amoxicillin capsules
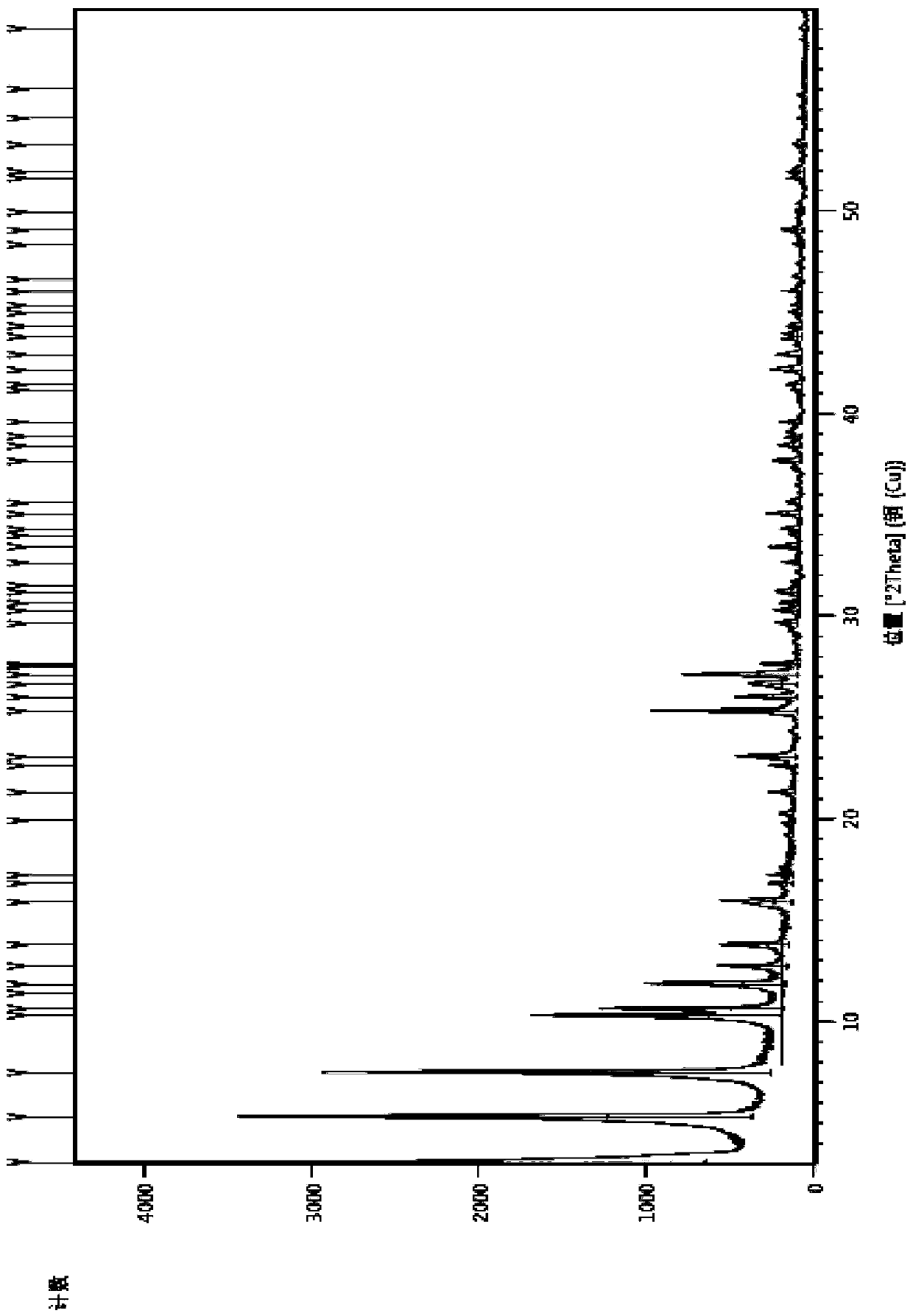
PendingCN112986289ASensitive detection of crystal form characteristic peaksImprove accuracyComponent separationMaterial analysis using radiation diffractionMedicinePhysical chemistry
The invention discloses a consistency evaluation and detection method for amoxicillin capsules. The method systematically and comprehensively investigates and evaluates the consistency between the to-be-detected amoxicillin capsule and the reference preparation of the original research medicine by comparing crystal forms and impurities of the to-be-detected amoxicillin capsule and the reference preparation. The corresponding scanning speed and intensity are selected according to the crystal form characteristics of the to-be-detected amoxicillin capsule, the detection of the crystal form characteristic peaks of the to-be-detected amoxicillin capsule and the reference preparation is sensitive, and the crystal form diffraction angles of the two drugs are accurately detected. Gradient elution is carried out by adopting a solution of a combination of a monopotassium phosphate solution and one or more of an acetonitrile solution, a formamide solution or an isopropanol solution as a mobile phase, so that the elution effect is better, and the accuracy and the sensitivity of a detection result are improved. The method is used for solving the technical problems that an existing amoxicillin capsule consistency evaluation detection method is low in accuracy and sensitivity and incomplete in detection.
Owner:HAINAN HAILI PHARMACEUTICAL CO LTD
Dirithromycin enteric-coated tablet enteric coating, preparation method thereof and dirithromycin enteric-coated tablet
ActiveCN109893511AImprove stabilityUniform and stable releaseAntibacterial agentsOrganic active ingredientsDirithromycinMedicine
The invention provides a dirithromycin enteric-coated tablet enteric coating. The dirithromycin enteric-coated tablet enteric coating is prepared from the following components in parts by weight: 60-70% of methacrylic acid-acrylate copolymer aqueous dispersion, 2-5% of titanium dioxide, 15-35% of triethyl citrate and 0-0.5% of talcum powder. The invention further provides a preparation method andprocess of the dirithromycin enteric-coated tablet enteric coating. The characteristic of uniform and stable release is achieved while stability of a dirithromycin enteric preparation is improved, thedirithromycin enteric-coated tablet enteric coating achieves the better similarity to a reference preparation, namely Dynabac in dissolution behavior, thus the domestic dirithromycin enteric preparation is broken through, and foreign post-marketing drugs are effectively substituted.
Owner:湖北舒邦药业有限公司
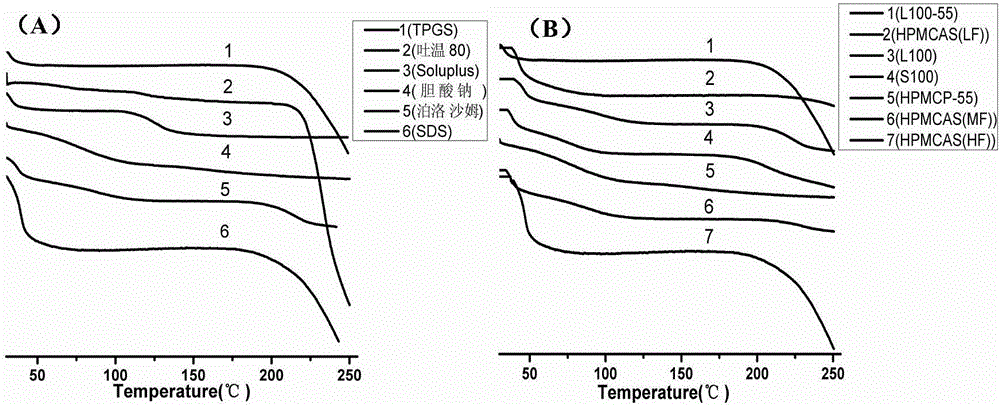
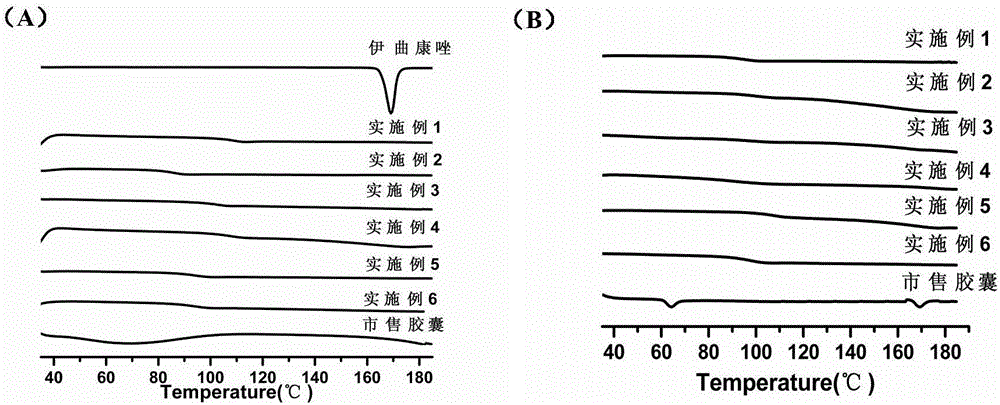
Itraconazole enteric solid dispersion and preparation method and application thereof
ActiveCN106619521AIncrease concentrationInhibition of recrystallizationOrganic active ingredientsPowder deliveryItraconazoleDissolution
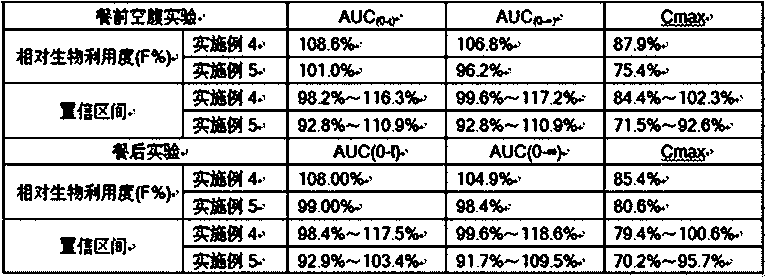
The invention discloses itraconazole enteric solid dispersion and a preparation method and application thereof; the solid dispersion is prepared from itraconazole acting as an active ingredient, an enteric macromolecular carrier and a surfactant; the solid dispersion is prepared by means of hot-melt extrusion technology, and itraconazole is present in the solid dispersion in an amorphous form; experiments on in-vivo bioavailability for Beagle dogs indicate that the itraconazole enteric solid dispersion has relative bioavailability of 180% relative to a reference preparation (commercially available capsules); the enteric macromolecular carrier and the surfactant in the solid dispersion can improve dissolution degree of itraconazole, and can also inhibit the recrystallization of itraconazole in stomach and intestines, thereby improving bioavailability and reducing adverse effects.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
A nifedipine sustained-release tablet composition
ActiveCN109172534ACmax is lower than the reference preparationCmax is higher than the reference preparationOrganic active ingredientsPharmaceutical non-active ingredientsSustained Release TabletNifedipine
The invention relates to a nifedipine sustained-release tablet composition and a preparation method thereof. The nifedipine sustained-release tablet composition of the invention comprises nifedipine,tween-80, lactose, microcrystalline cellulose, corn starch, and that D90 of nifedipine is 95-135 [mu] m, the mass ratio of lactose to nifedipine is 1.8: 1-2.2: 1, the dosage of Tween is 6-9%. As thattechnical scheme of the invention is reasonably adjust in composition, the process and equipment limit caused by the grinding fineness are avoided, and the tablet composition with low Cmax than the reference preparation and high AUC than the reference preparation is prepared.
Owner:DISHA PHARMA GRP
Formula and technology for improving strong wet-absorbing performance and dissolving-out behavior of levocarnitine tablets
ActiveCN104511018AConsistent qualityOrganic active ingredientsMetabolism disorderMANNITOL/SORBITOLMedicine
The invention discloses a formula and technology for improving the strong wet-absorbing performance and dissolving-out behavior of levocarnitine tablets. The API levocarnitine with a strong wet-absorbing performance and an auxiliary material namely mannitol, which can prevent the wet-absorbing property, are evenly mixed according to a ratio of 0.9:1-3.4:1; then an adhesive agent, a disintegrating agent, a flow aid, and a lubricant are added into the mixture and then evenly mixed, and the powder are directly pressed into tablets. Through the technical scheme mentioned above, solved are the problems that the levocarnitine absorbs water during the pressing process, the materials are adhesive, and the tablets crack during the production process. Furthermore, the dissolving-out curve of the prepared levocarnitine tablet is similar to the four dissolving-out curves of a reference preparation CARNITINE@ tablet, and thus the quality of the prepared levocarnitine tablet is identical with that of the reference preparation.
Owner:BEIJING CHENG JI PHARMA
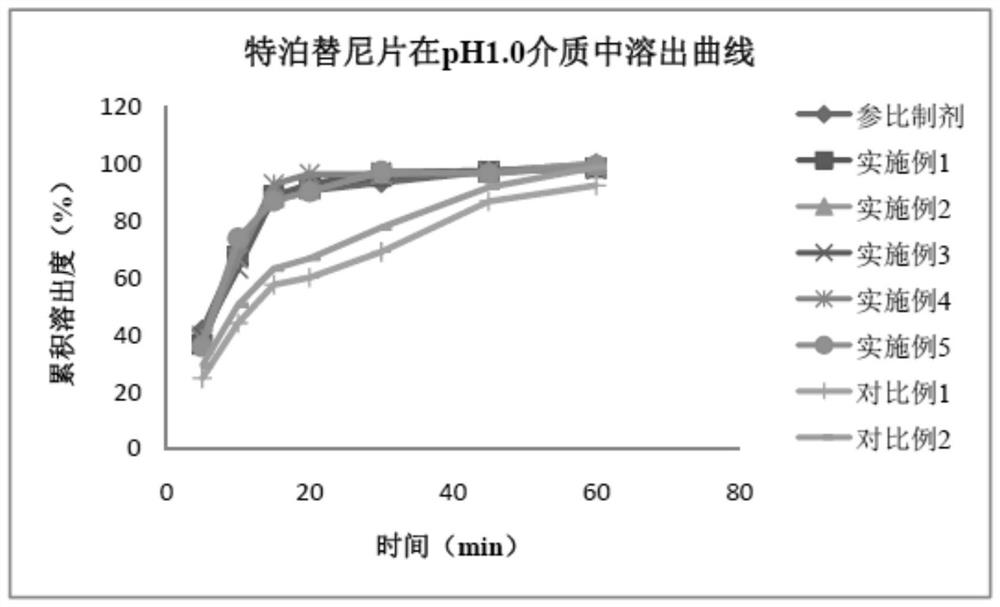
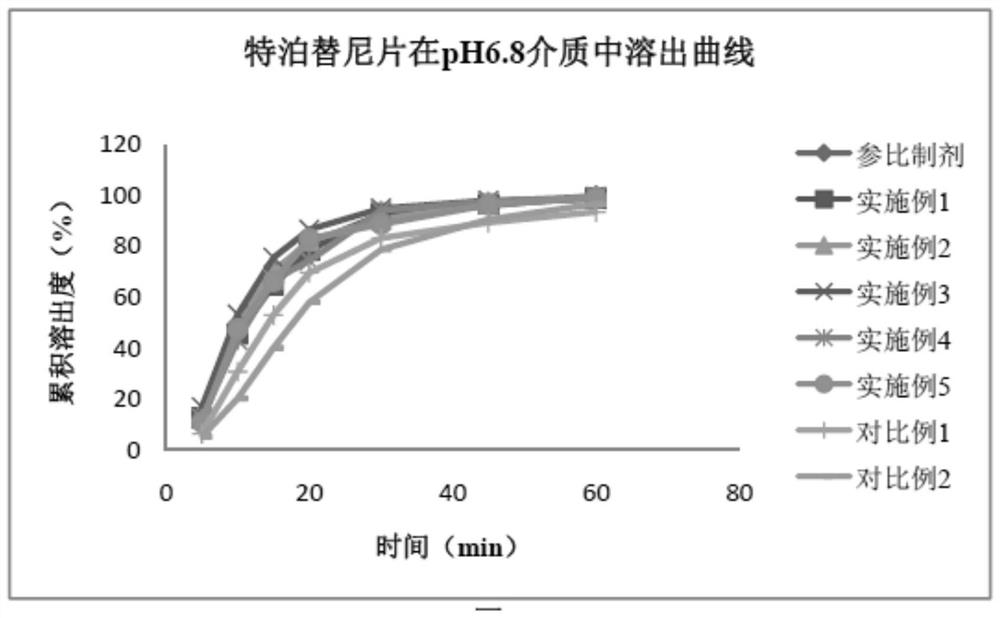
Tepotinib tablet and preparation method thereof
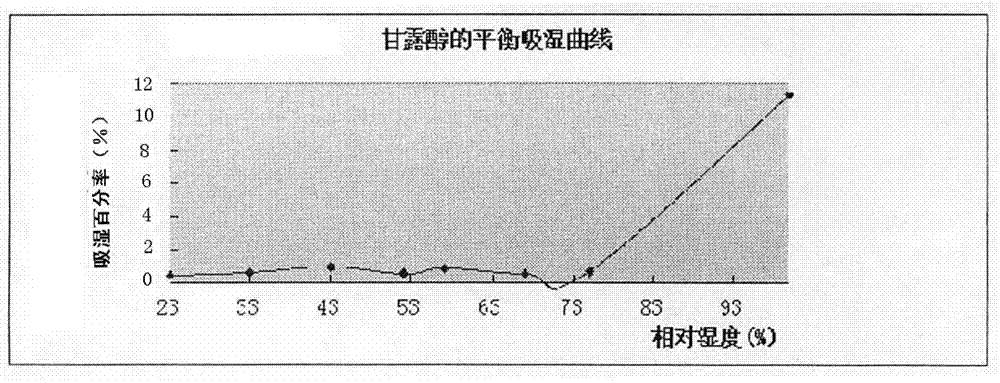
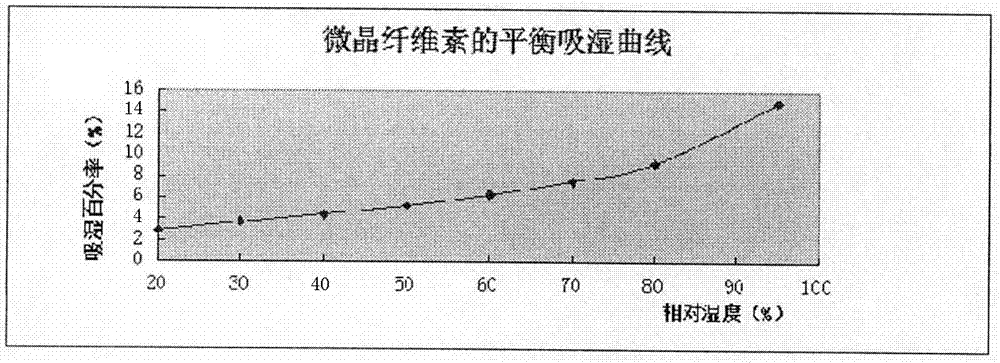
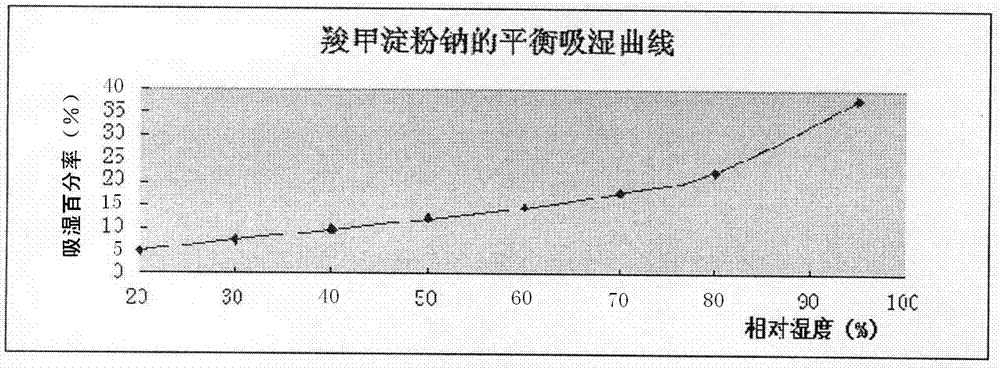
PendingCN112137979ADetermination of curative effectQuality improvementOrganic active ingredientsPharmaceutical non-active ingredientsCoated tabletsImmediate release
The present invention relates to the technical field of pharmaceutical preparations and particularly to a tepotinib tablet and a preparation method thereof. The tepotinib tablet comprises a medicine tablet core and a coating layer, and the medicine tablet core is prepared from the following components in parts by weight: 1 part of a tepotinib hydrochloride hydrate, 1.5-3 parts of a filler, 0.06-0.2 part of a disintegrating agent, 0.01-0.1 part of a flow aid and 0.01-0.1 part of a lubricating agent; wherein the filler, the disintegrating agent and the lubricating agent are added internally andexternally. The tepotinib tablet is a quick-release film-coated tablet and a detection result of an in-vitro dissolution curve of the tepotinib tablet in a medium with a pH value of 1.0-6.8 is similarto that of a reference preparation. The present invention also provides the preparation method of the tepotinib tablet.
Owner:REYOUNG PHARMA
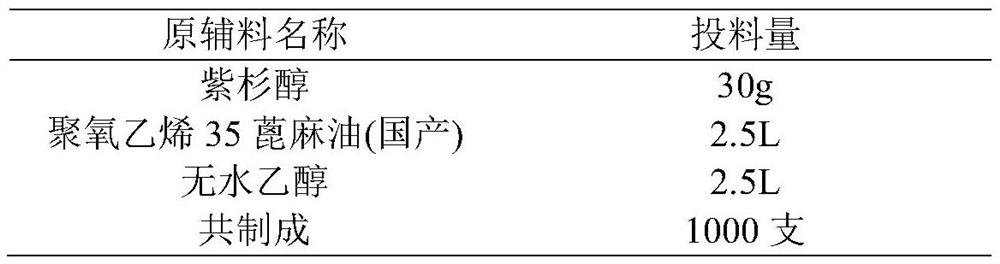
Preparation method of paclitaxel injection
ActiveCN114344251AImprove complianceImprove securityOrganic active ingredientsPharmaceutical delivery mechanismPolyoxyethylene castor oilPaclitaxel Injection
According to the preparation method of the paclitaxel injection with the stable property, the blank auxiliary materials are pretreated, carboxylate anions in the auxiliary materials are reduced, degradation of main components is inhibited, and the stability of the paclitaxel injection in the storage period is improved; meanwhile, introduction of a pH regulator is avoided, the pH value of the obtained preparation is 4-6, in the pH value range of infusion tolerable by the human body, adverse reactions can be reduced, and the compliance and safety of the medicine in the clinical use process are improved. Besides, finished products which are prepared from domestic and imported polyoxyethylene 35 castor oil and have obvious difference in quality can achieve similar stability, are consistent with related substances of a reference preparation in level and are good in stability, and the safety of the products in the using process can be effectively guaranteed.
Owner:BEIJING XINLINGXIAN MEDICAL TECH DEV CO LTD
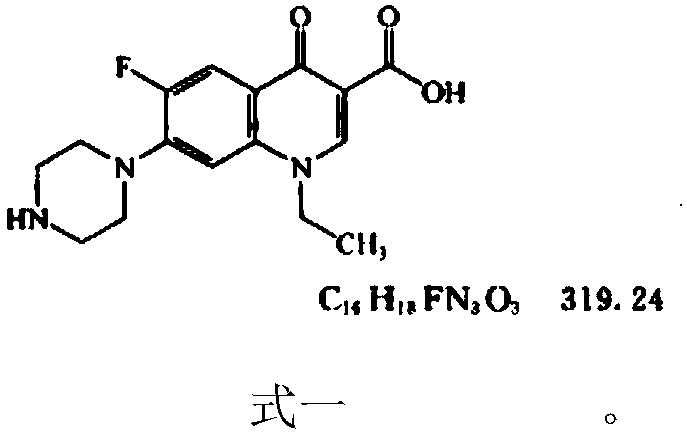
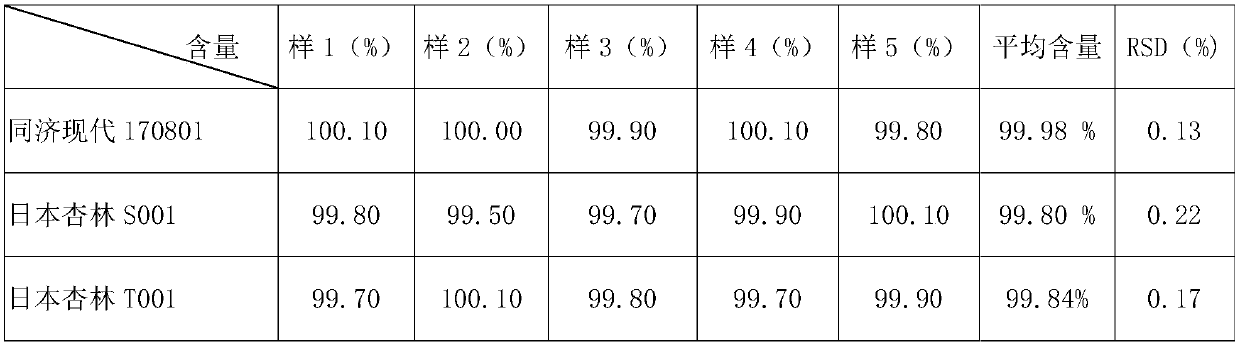
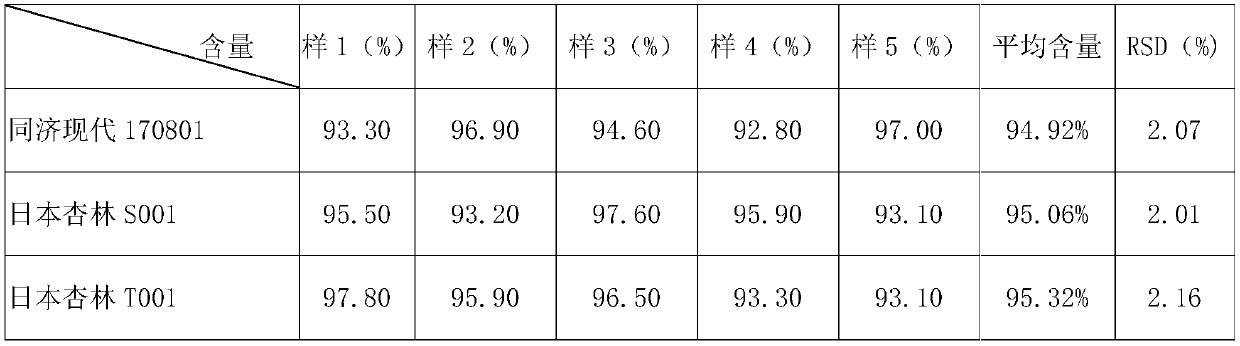
Determination method of norfloxacin content in norfloxacin capsule
InactiveCN109655556AThe measurement result is accurateImprove uniformityComponent separationTest samplePhosphoric acid
The invention relates to a determination method of norfloxacin content in a norfloxacin capsule, belongs to the field of drug analysis. The determination method comprises development and verificationof an analysis method. The determination method comprises the following specific steps: when a test sample solution is prepared, dissolving and diluting with a solvent comprising 0.025M phosphoric acid solution and acetonitrile (with the ratio of 87: 13). Compared with a ChP2015 pharmacopoeia method, the method provided by the invention has the advantages that content detection is carried out on aself-made sample and two batches of reference preparations, and results show that the content of the two batches of reference preparations and the self-made sample is higher in detection accuracy, the sample is more fully dissolved, and the uniformity of the determination results is good.
Owner:WUHAN TONGJI MODERN PHARMA TECH CO LTD
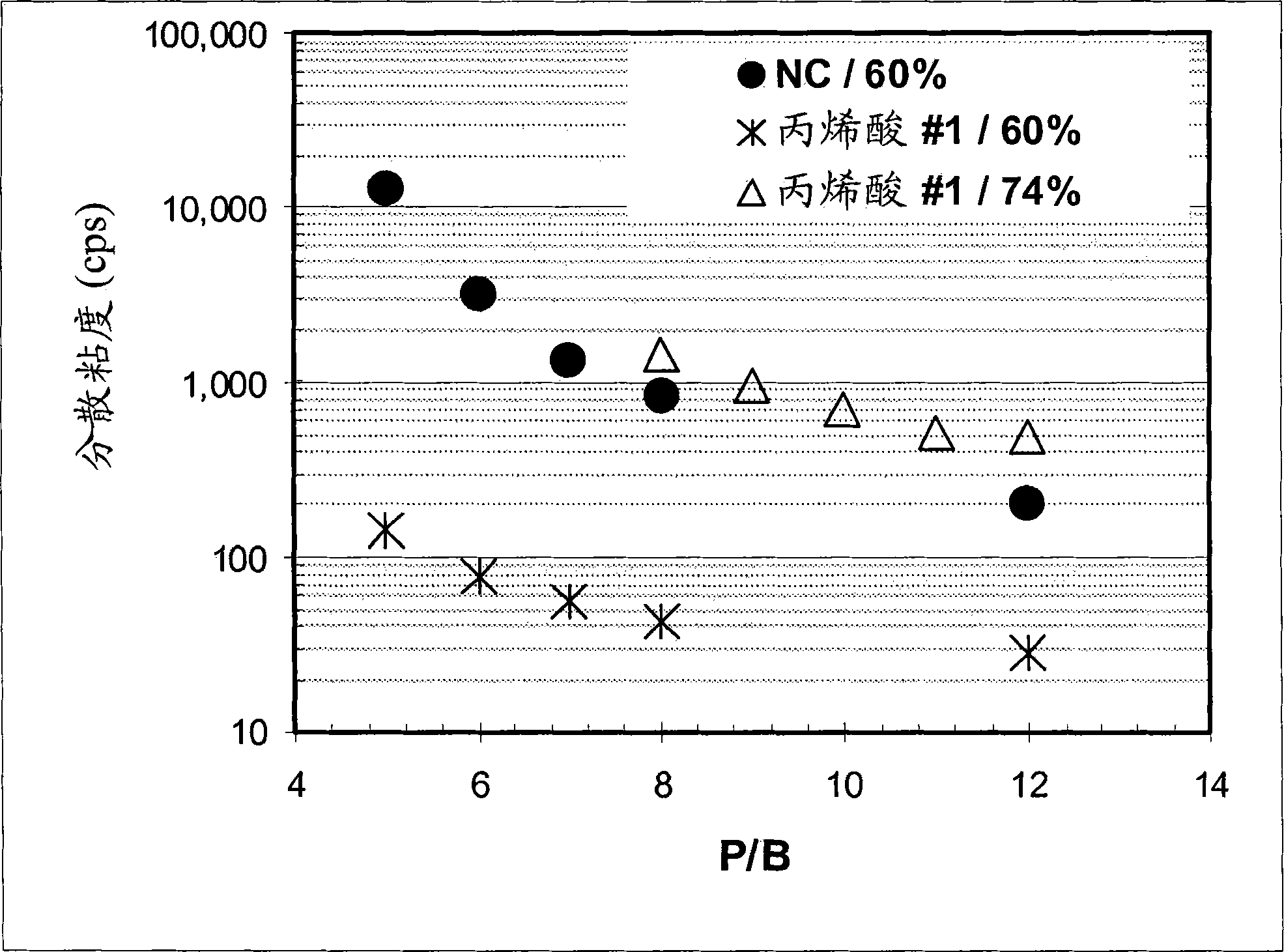
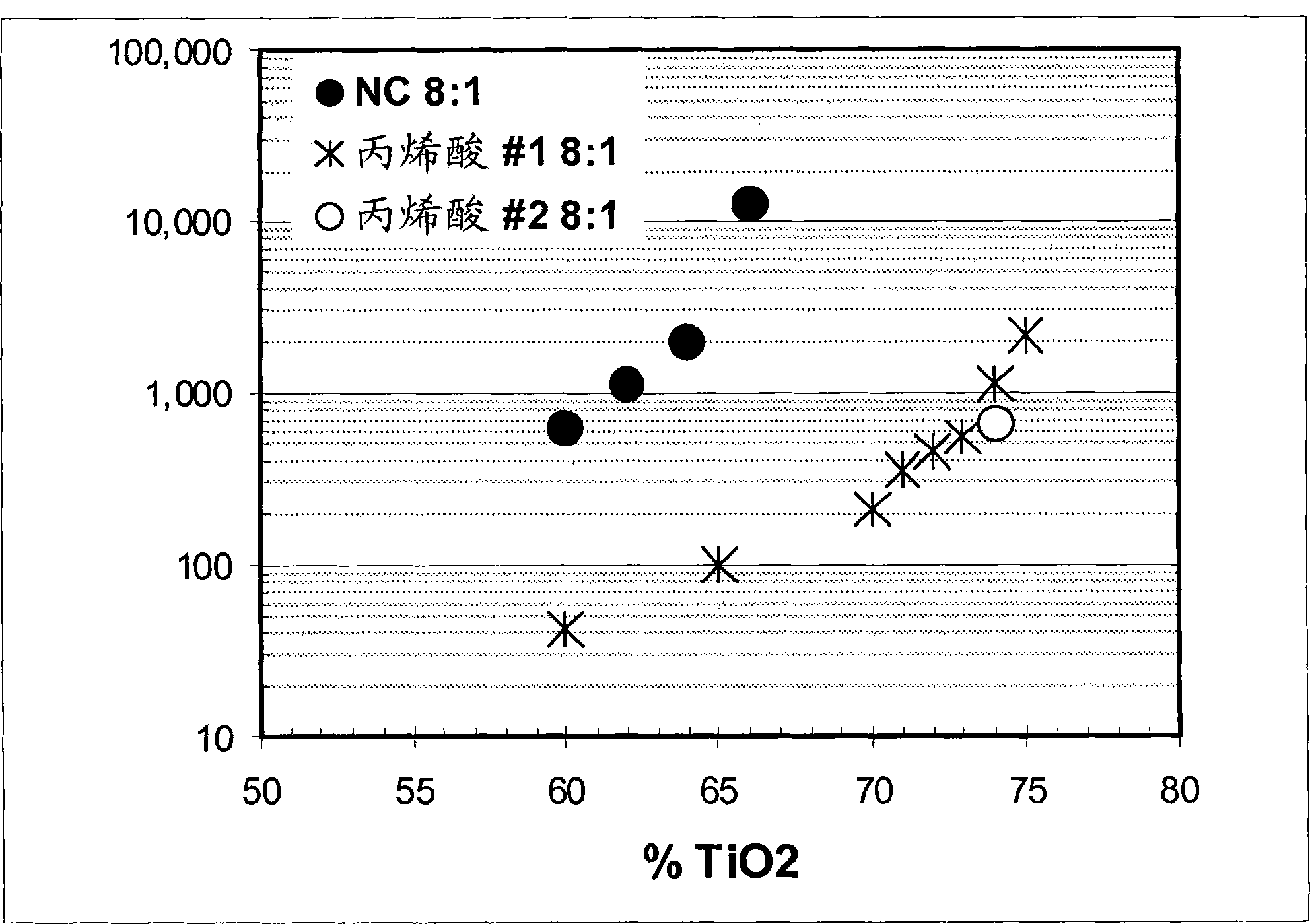
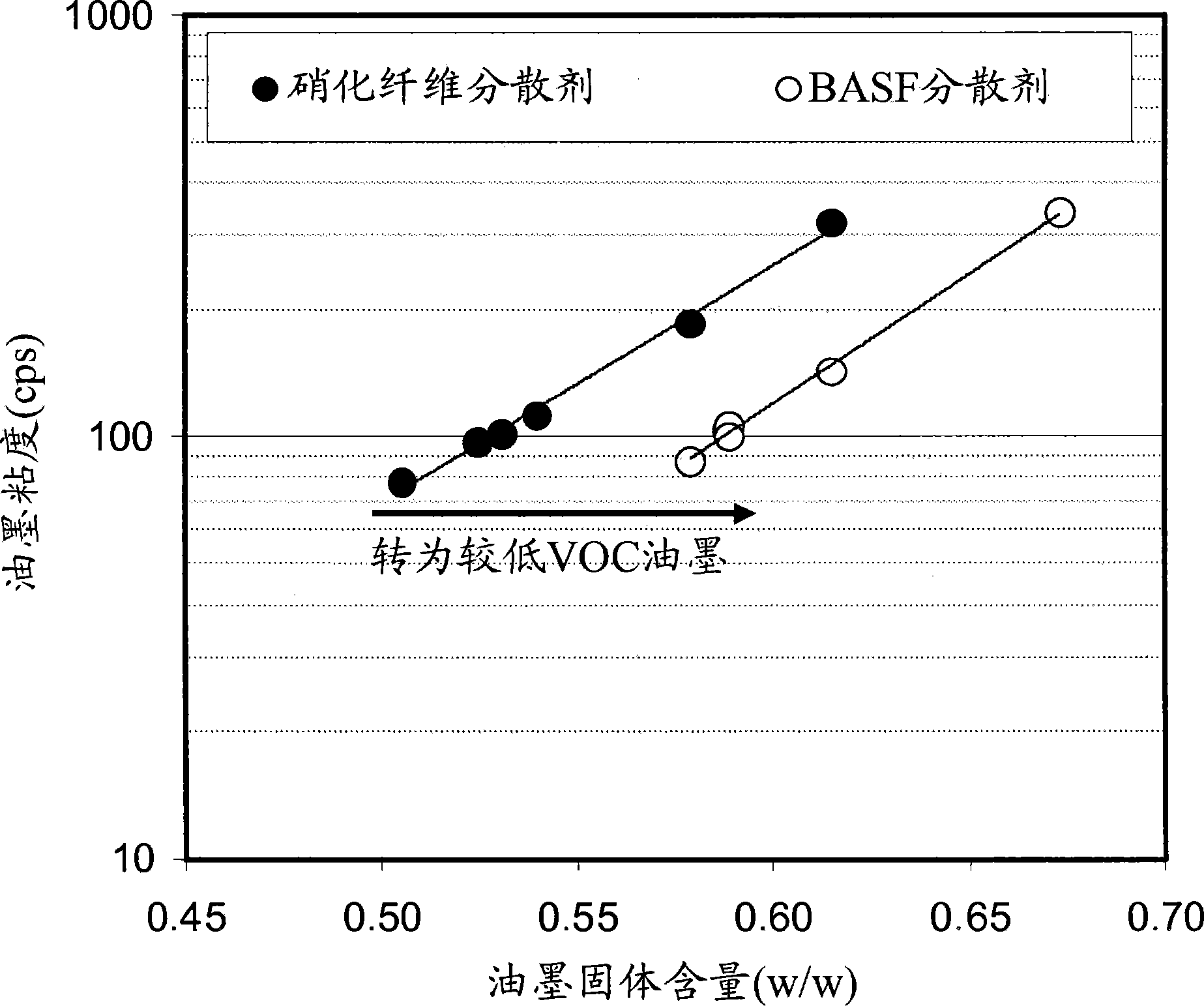
Low voc solvent-borne printing inks
The styrene-acrylic dispersants used in the pigment dispersions were less viscous than benchmark formulations prepared under otherwise identical conditions, such as nitrocellulose, dimer acid-based polyamides, and thermoplastic polyurethanes. Compared to traditional dispersants, the lower viscosity allows: (a) at lower solvent loads; or if solvent loads are to be maintained, (b) higher pigment and solids contents from styrene- Acrylics make dispersions and inks of similar viscosity.
Owner:BASF CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com