Itraconazole enteric solid dispersion and preparation method and application thereof
A technology of solid dispersion and itraconazole intestine, applied in the field of medicine, can solve problems such as insignificant crystallization effect, and achieve the effects of no solvent residue, simple preparation method and excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
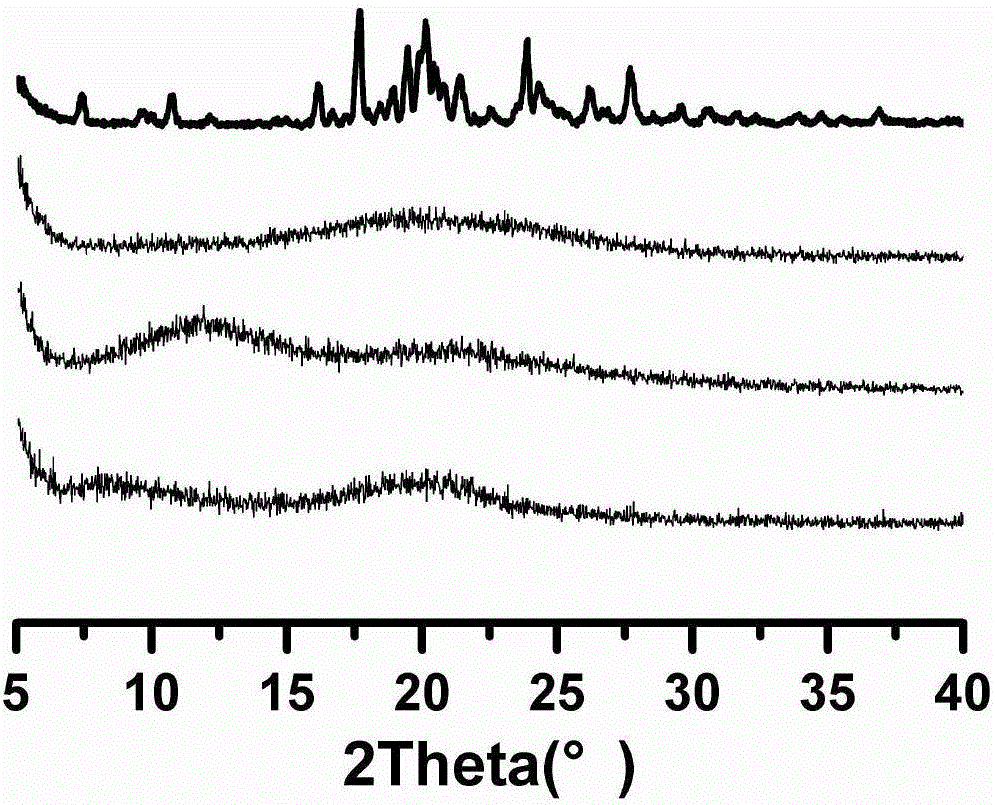
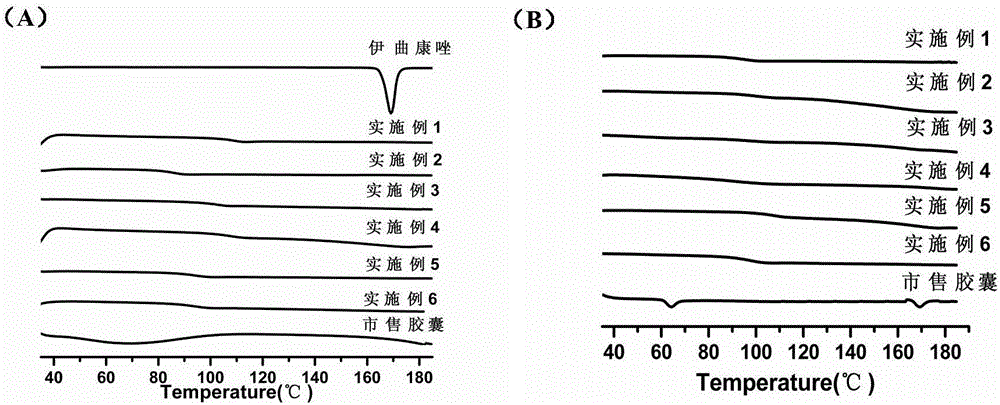
[0043] Example 1 Itraconazole enteric solid dispersion
[0044] An itraconazole enteric solid dispersion of this embodiment is prepared from the following components in weight percentage: 10% itraconazole, 80% enteric polymer carrier material, and 10% surfactant, wherein The enteric polymer carrier material is hypromellose phthalate, and the surfactant is poloxamer F188.
[0045] The preparation method of this itraconazole enteric-coated solid dispersion is as follows:
[0046] 1) Weigh 1.2g of itraconazole, 9.6g of hypromellose phthalate, and 1.2g of poloxamer F188, grind and mix them uniformly to make a physical mixture;
[0047] 2) Set the extrusion temperature of the twin-screw hot-melt extruder to 160°C, start the screw after reaching the preset temperature, and the screw speed is 150 rpm, and add the prepared physical mixture to the twin-screw hot-melt extruder In the process, the mixture is extruded in strips through the screw;
[0048] 3) The hot-melt extruded strip...
Embodiment 2
[0050] Example 2 Itraconazole enteric solid dispersion
[0051] A kind of itraconazole enteric solid dispersion of this embodiment is prepared from the following components by weight percentage: itraconazole 20%, enteric polymer carrier material 60%, surfactant 20%, wherein The enteric polymer carrier material is hypromellose acetate succinate (LF type), and the surfactant is sodium cholate.
[0052] The preparation method of the solid dispersion of this itraconazole is as follows:
[0053] 1) Weigh 2.4g of itraconazole, 7.2g of hypromellose acetate succinate (LF type), 2.4g of sodium cholate, grind and mix uniformly to make a physical mixture;
[0054] 2) Set the extrusion temperature of the twin-screw hot-melt extruder to 170°C, start the screw after reaching the preset temperature, and the screw speed is 100 rpm, and add the prepared physical mixture to the twin-screw hot-melt extruder In the process, the mixture is extruded in strips through the screw;
[0055] 3) The h...
Embodiment 3
[0056] Example 3 Itraconazole enteric solid dispersion
[0057] A kind of itraconazole enteric solid dispersion of this embodiment is prepared from the following components by weight percentage: itraconazole 36.4%, enteric polymer carrier material 50%, surfactant 13.6%, wherein The enteric polymer carrier material is acrylic resin (Eudragit L100-55), and the surfactant is Tween 80.
[0058] The preparation method of this itraconazole enteric-coated solid dispersion is as follows:
[0059] 1) Weigh 4.8g of itraconazole, 6.6g of acrylic resin (Eudragit L100-55), 1.8g of Tween 80, grind and mix evenly to make a physical mixture;
[0060] 2) Set the extrusion temperature of the twin-screw hot-melt extruder to 175°C, start the screw after reaching the preset temperature, and the screw speed is 120 rpm, and add the prepared physical mixture to the twin-screw hot-melt extruder In the process, the mixture is extruded in strips through the screw;
[0061] 3) The hot-melt extruded st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com