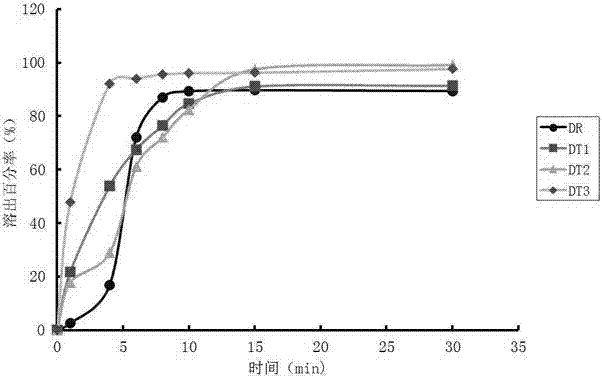
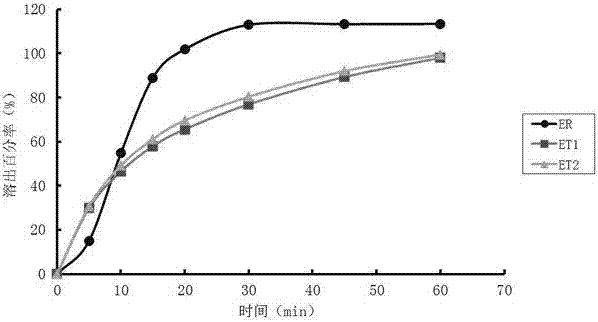
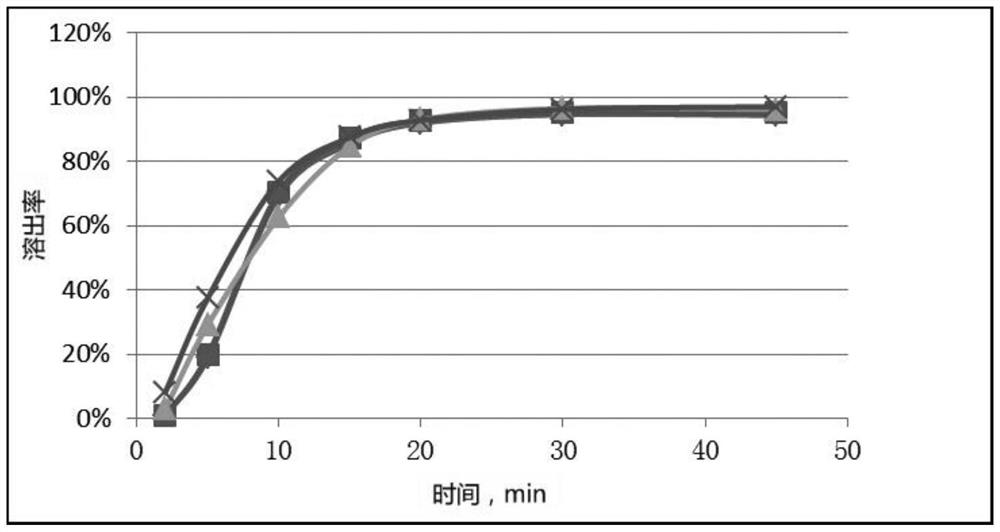
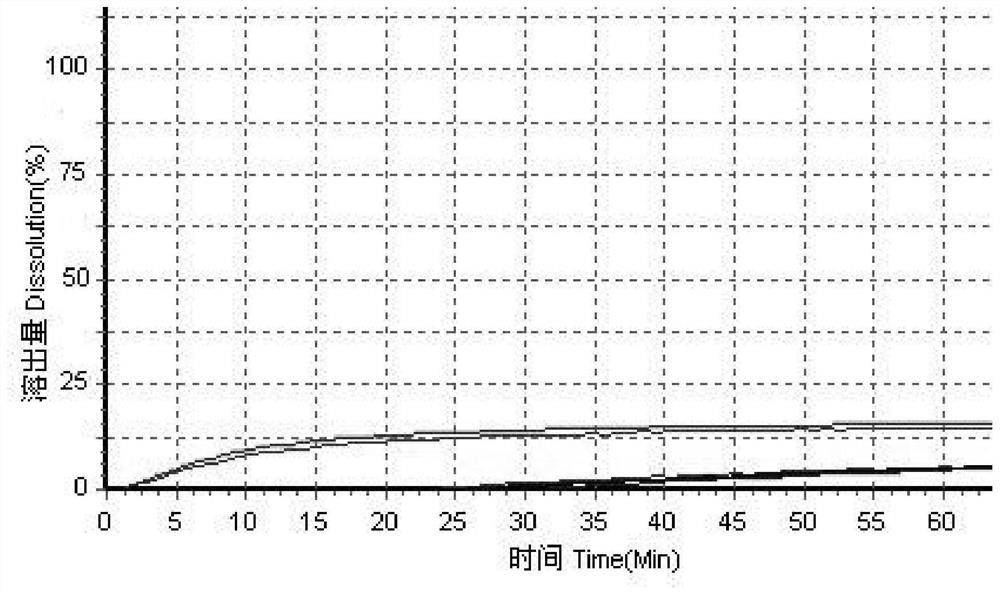
Patents
Literature
37 results about "Generic drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A generic drug is a pharmaceutical drug that contains the same chemical substance as a drug that was originally protected by chemical patents. Generic drugs are allowed for sale after the patents on the original drugs expire. Because the active chemical substance is the same, the medical profile of generics is believed to be equivalent in performance. A generic drug has the same active pharmaceutical ingredient (API) as the original, but it may differ in some characteristics such as the manufacturing process, formulation, excipients, color, taste, and packaging.
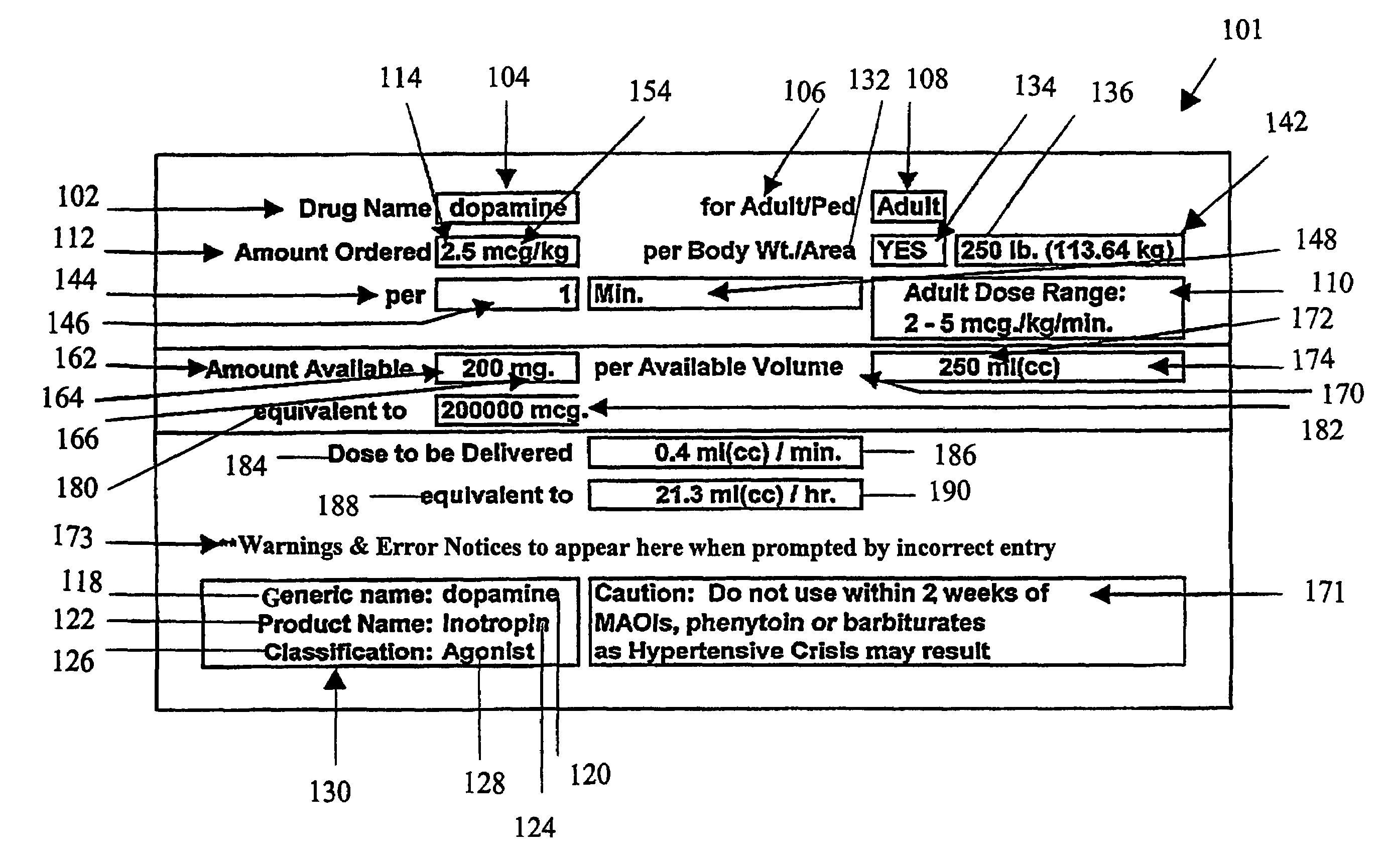
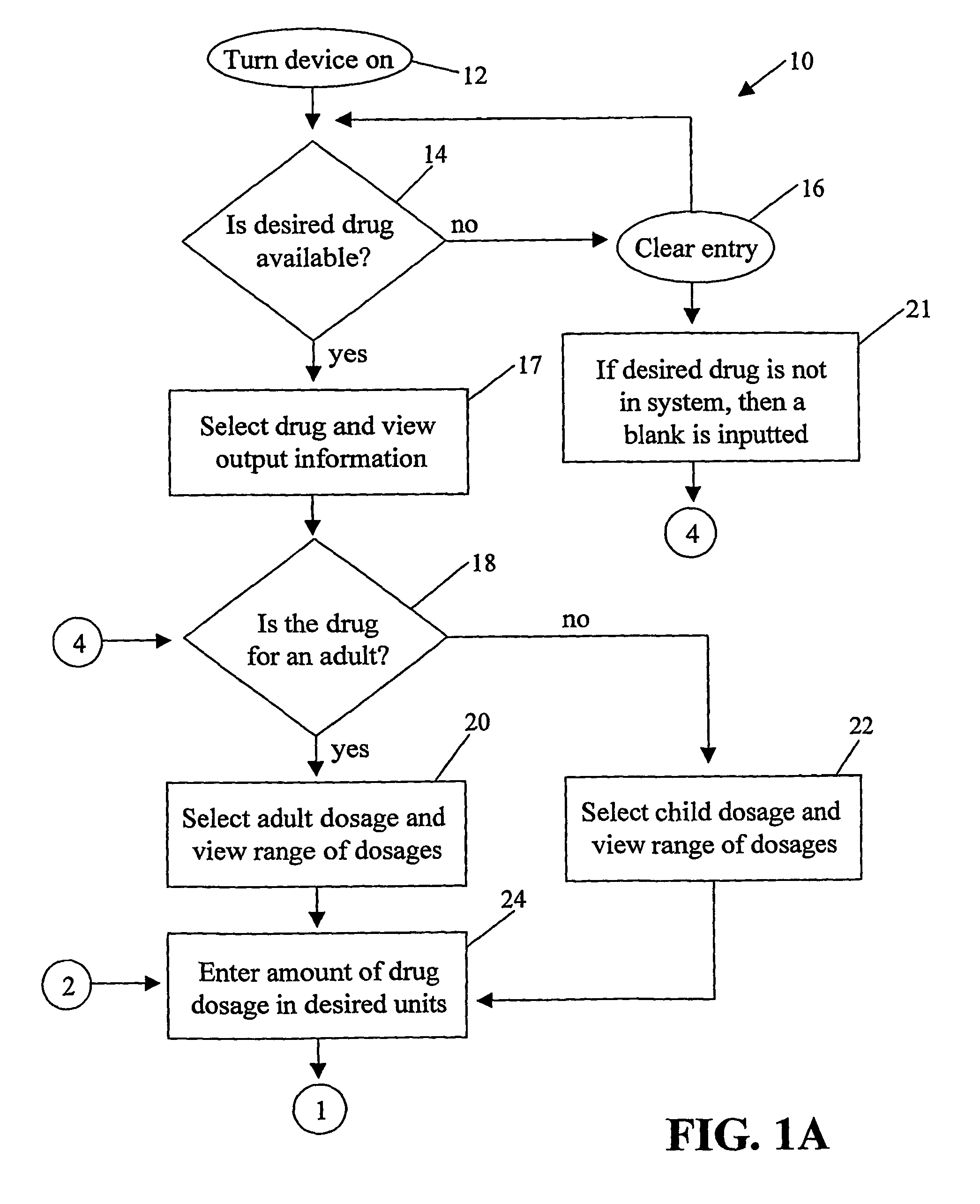
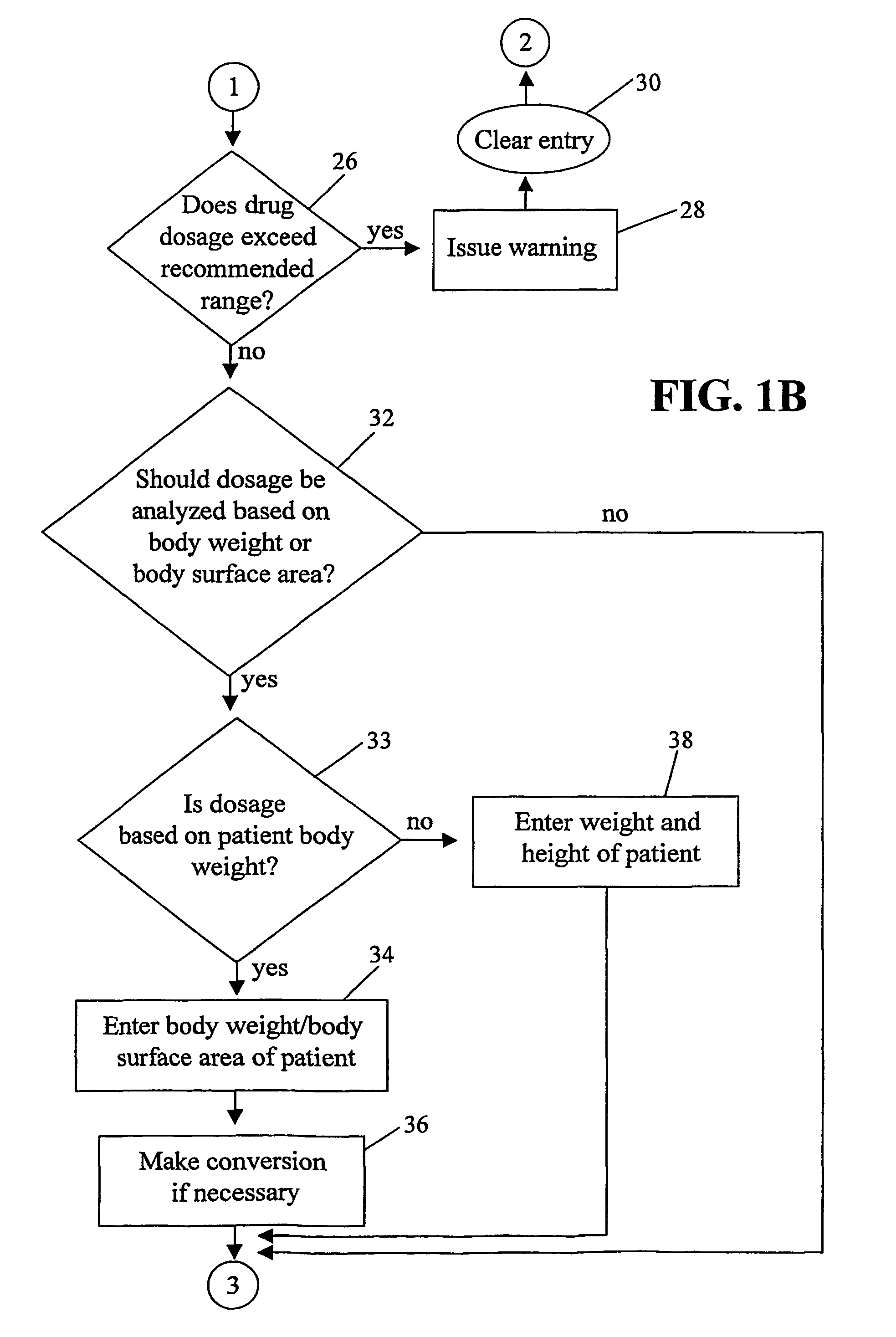
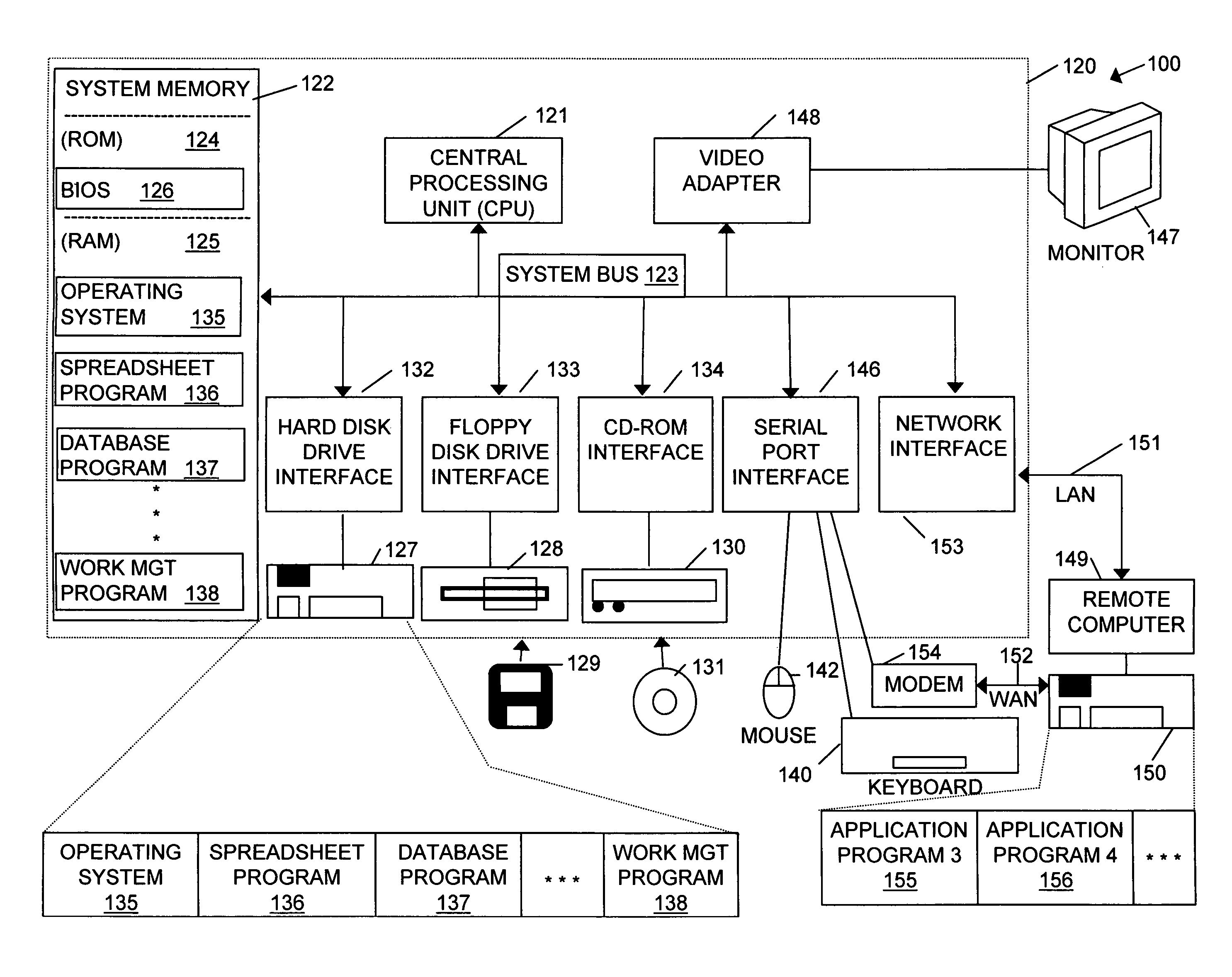
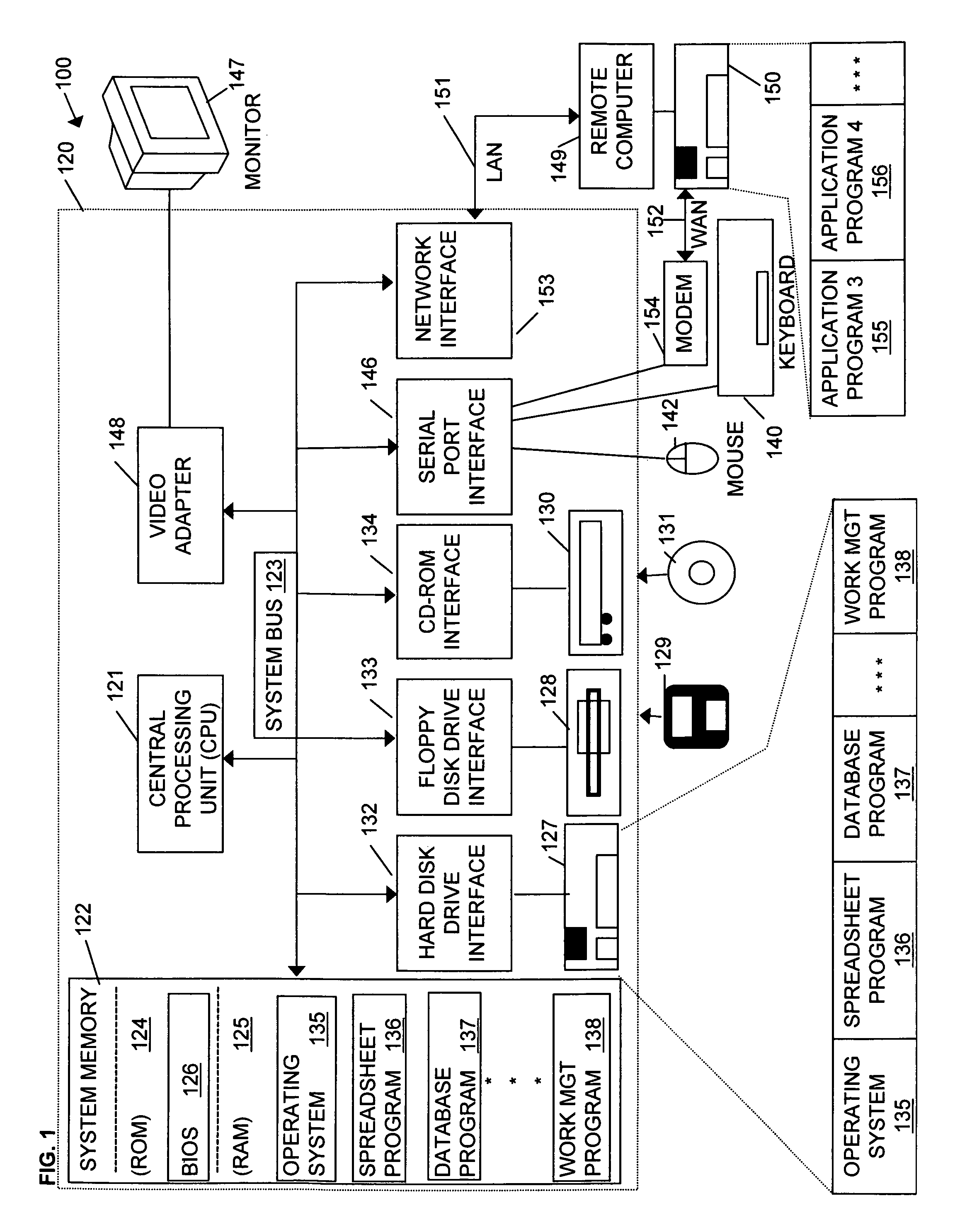
Handheld medication dosage calculator
InactiveUS6978286B2Reduce needDrug and medicationsDigital data processing detailsGeneric drugHand held
A handheld medication dosage calculator (100) and method for comparing an inputted, ordered medication dosage with a known medication dosage range in a database including an input device for inputting a desired drug name, indicating whether the drug is for a child or an adult, an amount of the drugs that are ordered, the body weight or body surface of the patient, an amount of drug available in standard packaging, and the available volume associated with the available drug and a computing mechanism for determining the dosage of the drug to be delivered. The handheld medication dosage calculator (100) provides warnings when the inputted amount of drug exceeds the dose range limits or is incorrect. The handheld medication dosage calculator (100) converts an inputted drug unit of measure into a desired unit of measure. The handheld medication dosage calculator (100) lists generic drug names, trademarked product names, drug classifications and cautionary drug warning information.
Owner:INFORMMED
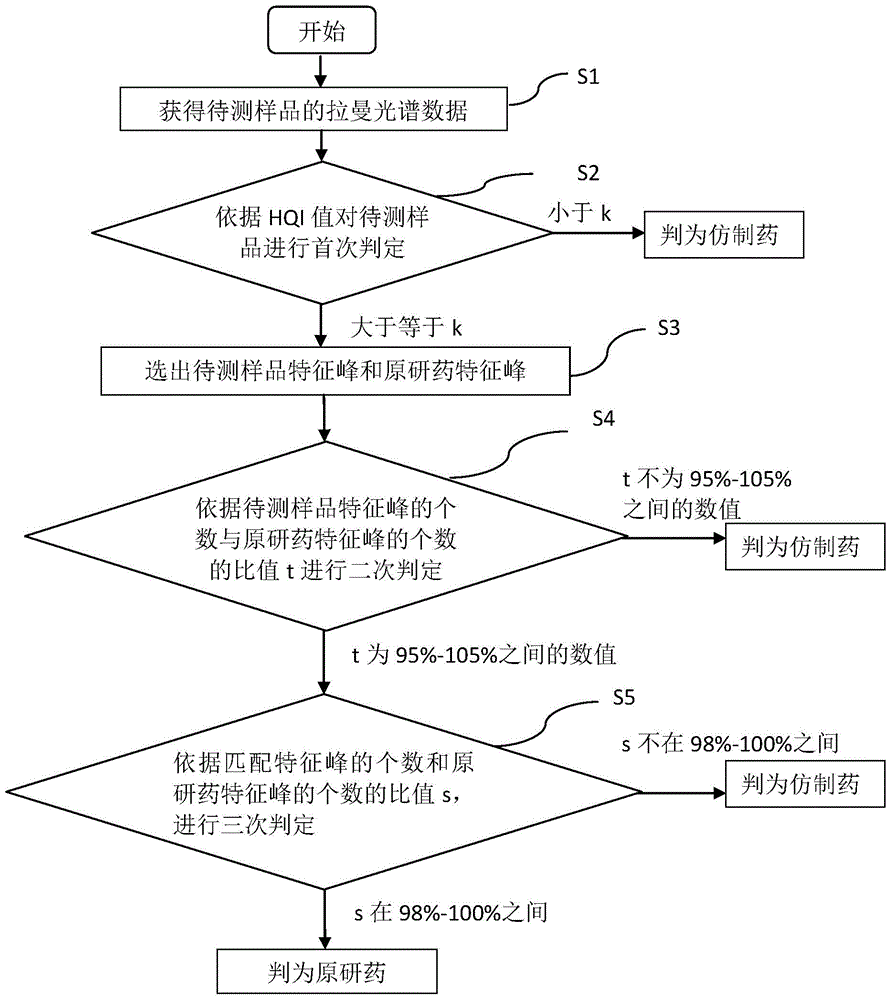
Detection method of generic drugs pretending original drugs
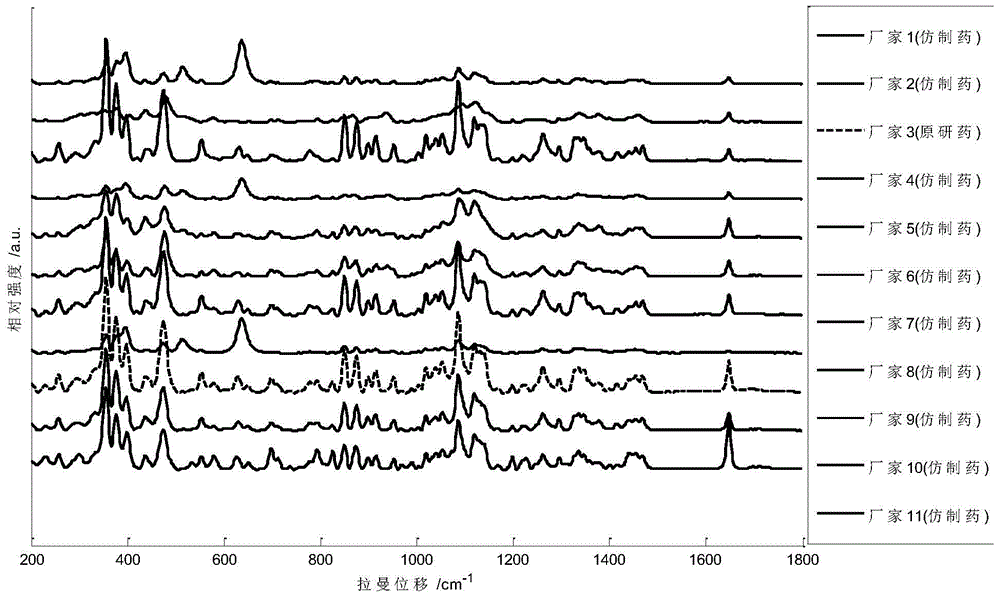
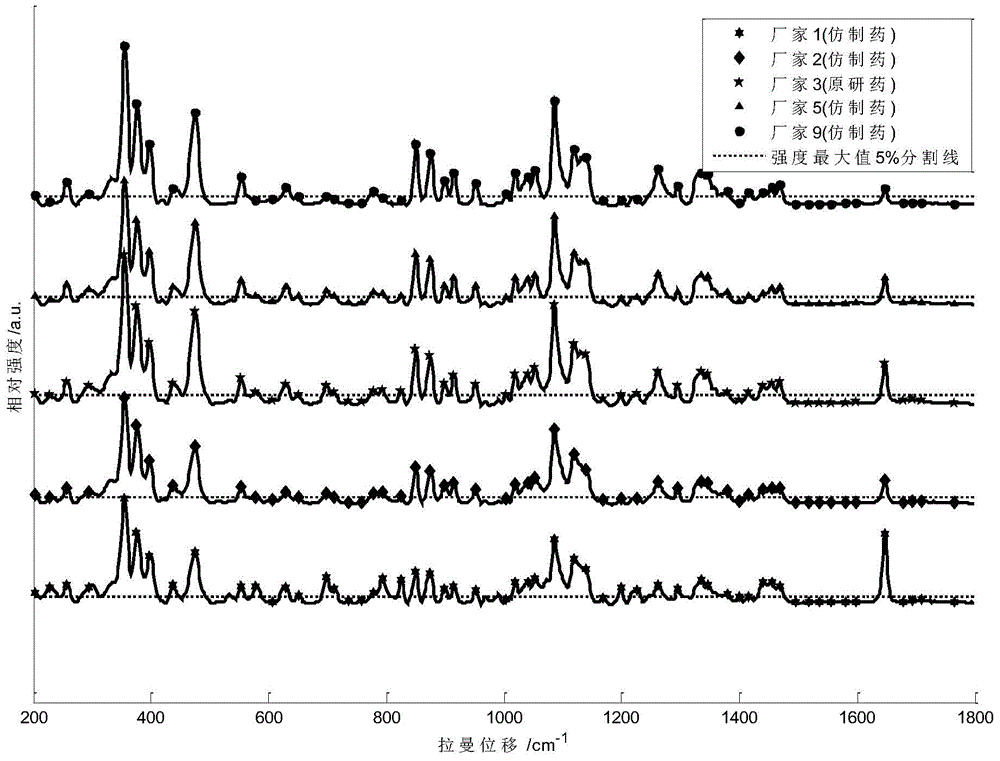
ActiveCN104949956AImprove accuracyImprove detection accuracyRaman scatteringGeneric drugComputer science
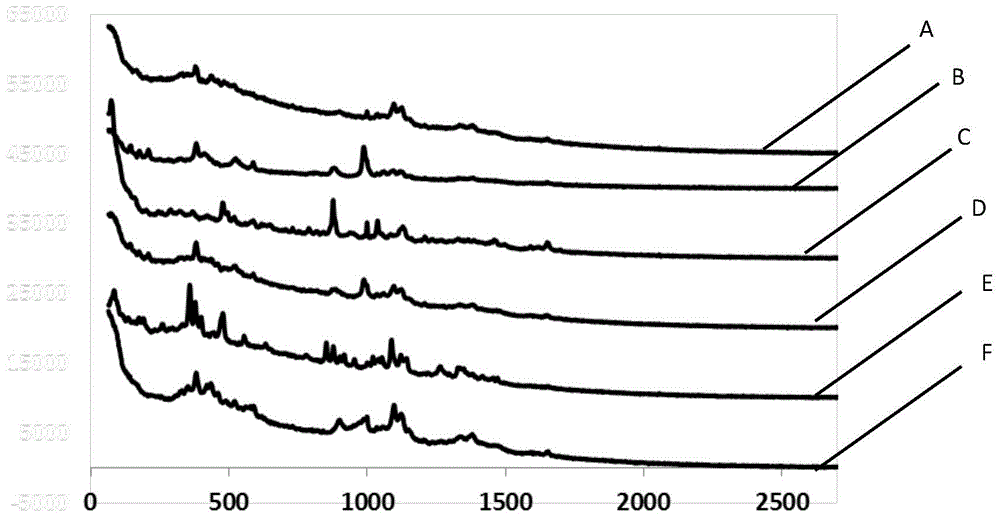
The invention provides a detection method of generic drugs pretending original drugs. The detection method comprises the following five steps: 1, acquiring Raman spectrum data of a sample to be detected; 2, calculating an HQI value of the sample to be detected according to standard Raman spectrum data of the original drugs and the Raman spectrum data of the sample to be detected, and carrying out first judgment on the sample to be detected according to the HQI value; 3, selecting characteristic peaks of the sample to be detected and characteristic peaks of the original drugs; 4, calculating a ratio t of the quantity of the characteristic peaks of the sample to be detected to the quantity of the characteristic peaks of the original drugs, and carrying out second judgment on the sample to be detected according to the t; and 5, calculating a ratio s of the quantity of matched characteristic peaks to the quantity of the characteristic peaks of the original drugs, and carrying out third judgment on the sample to be detected according to the s. The detection method provided by the technical scheme is high in accuracy and strong in reasonability; and the false positive rate of the judgment is remarkably reduced and the requirements of on-site rapid detection can be met.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
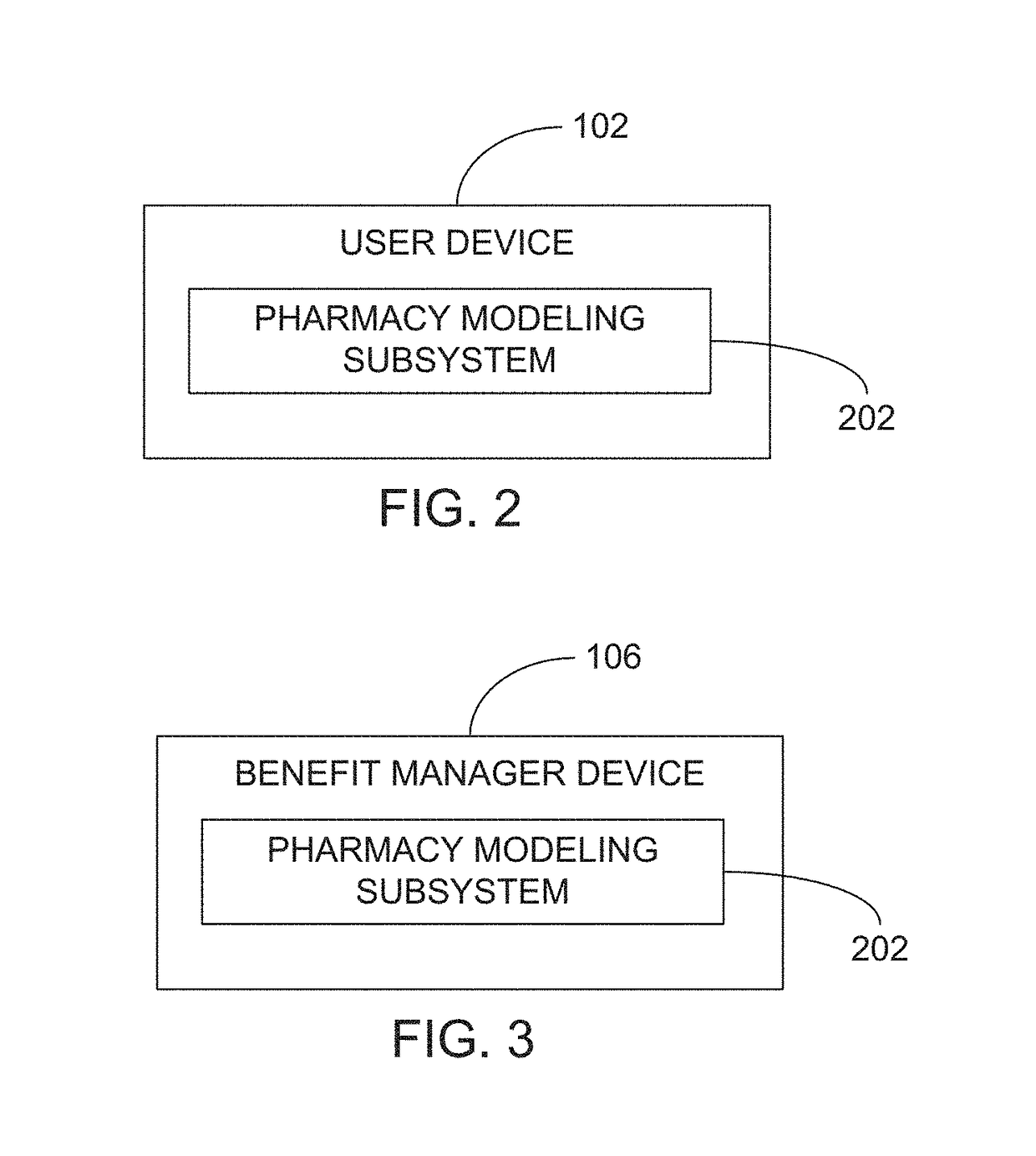
Methods and systems for pharmacy modeling
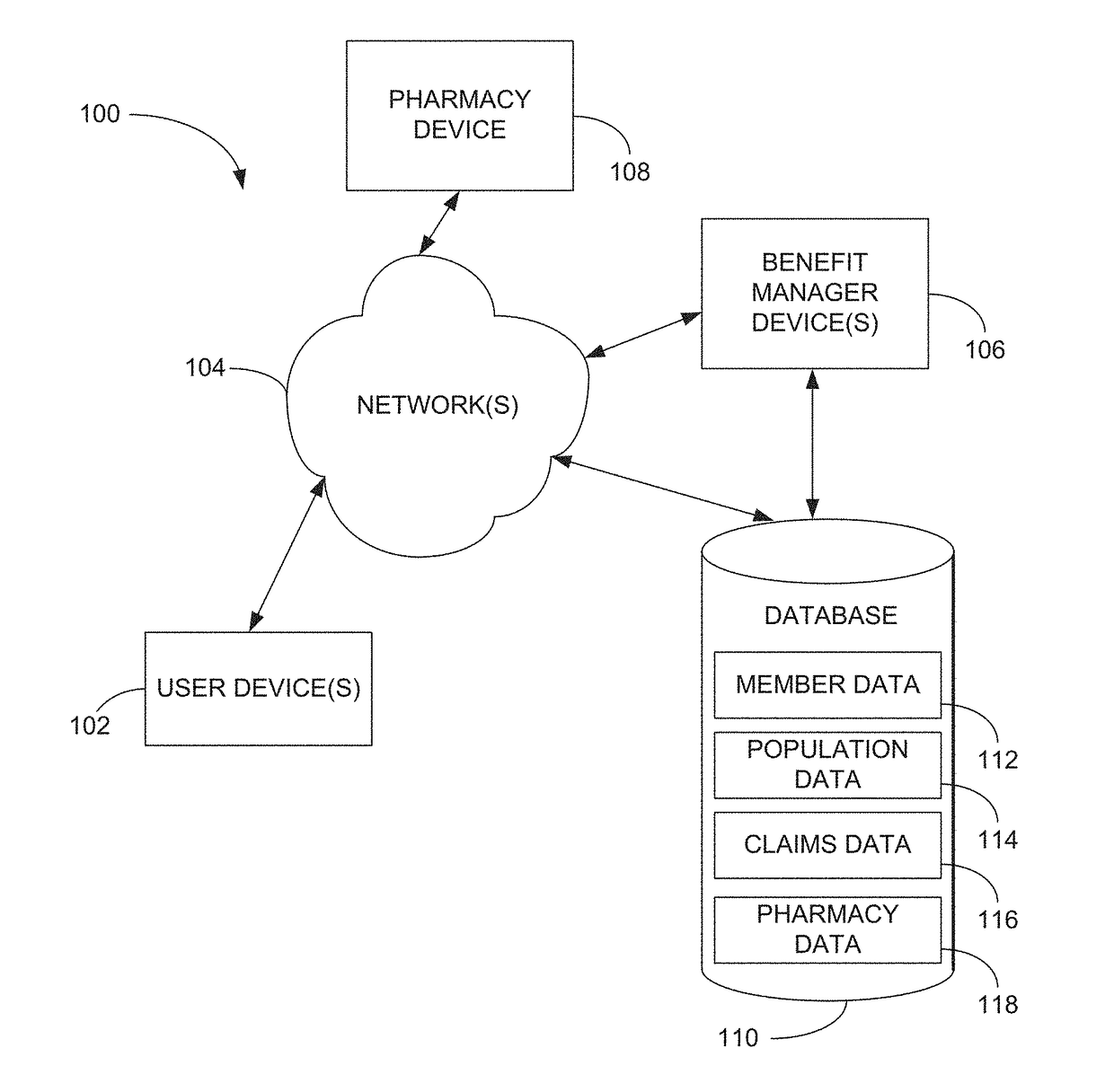
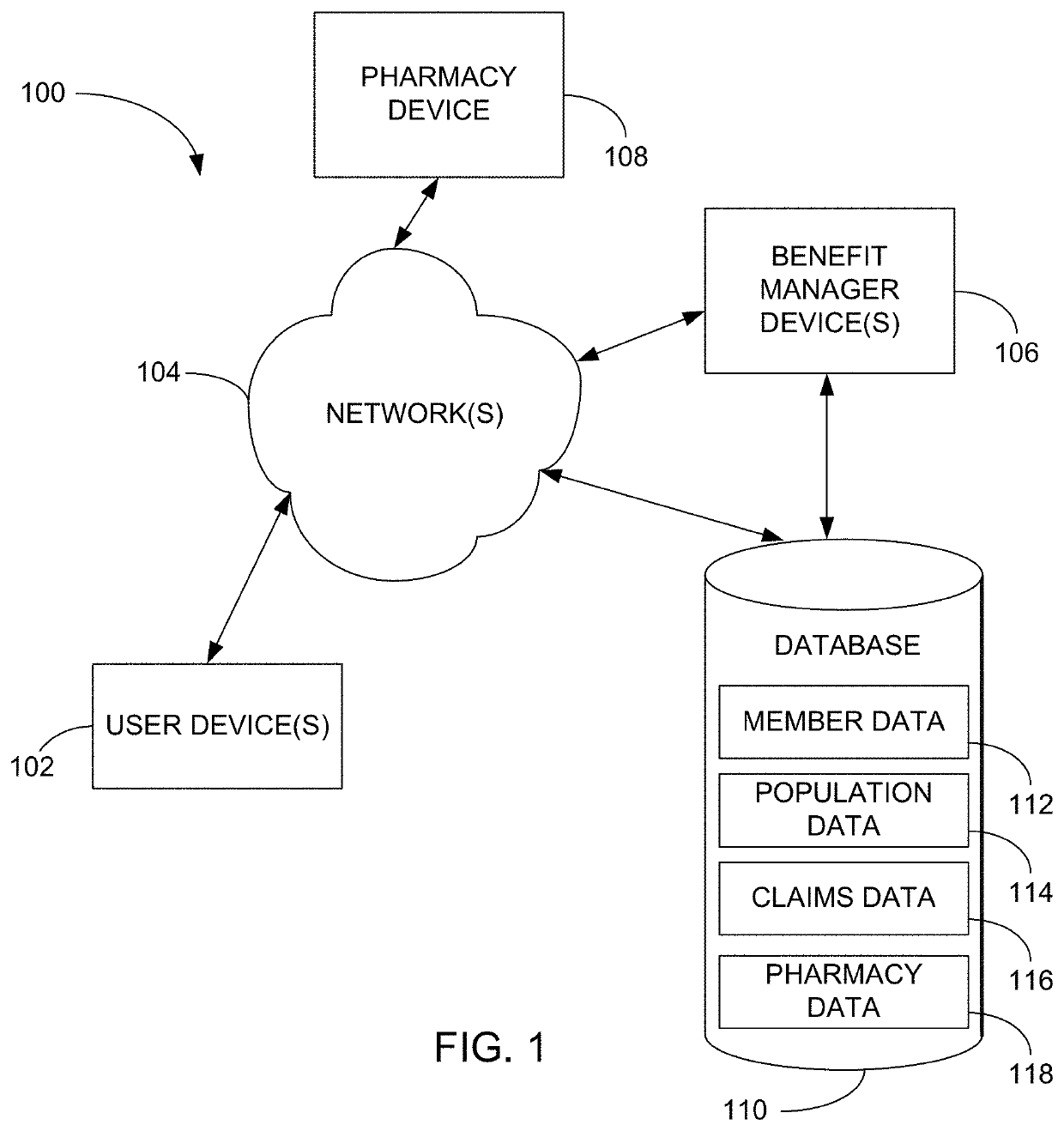
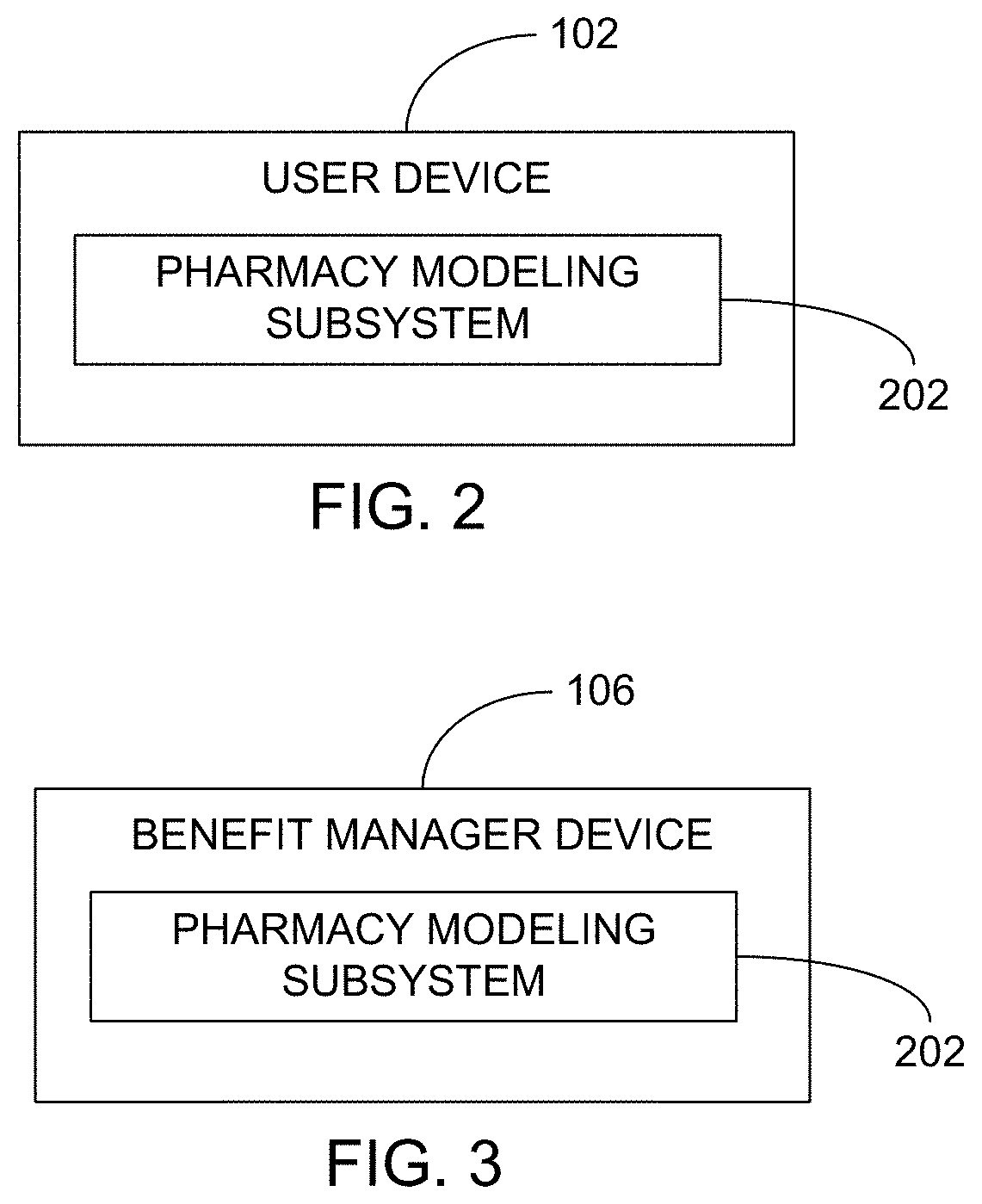
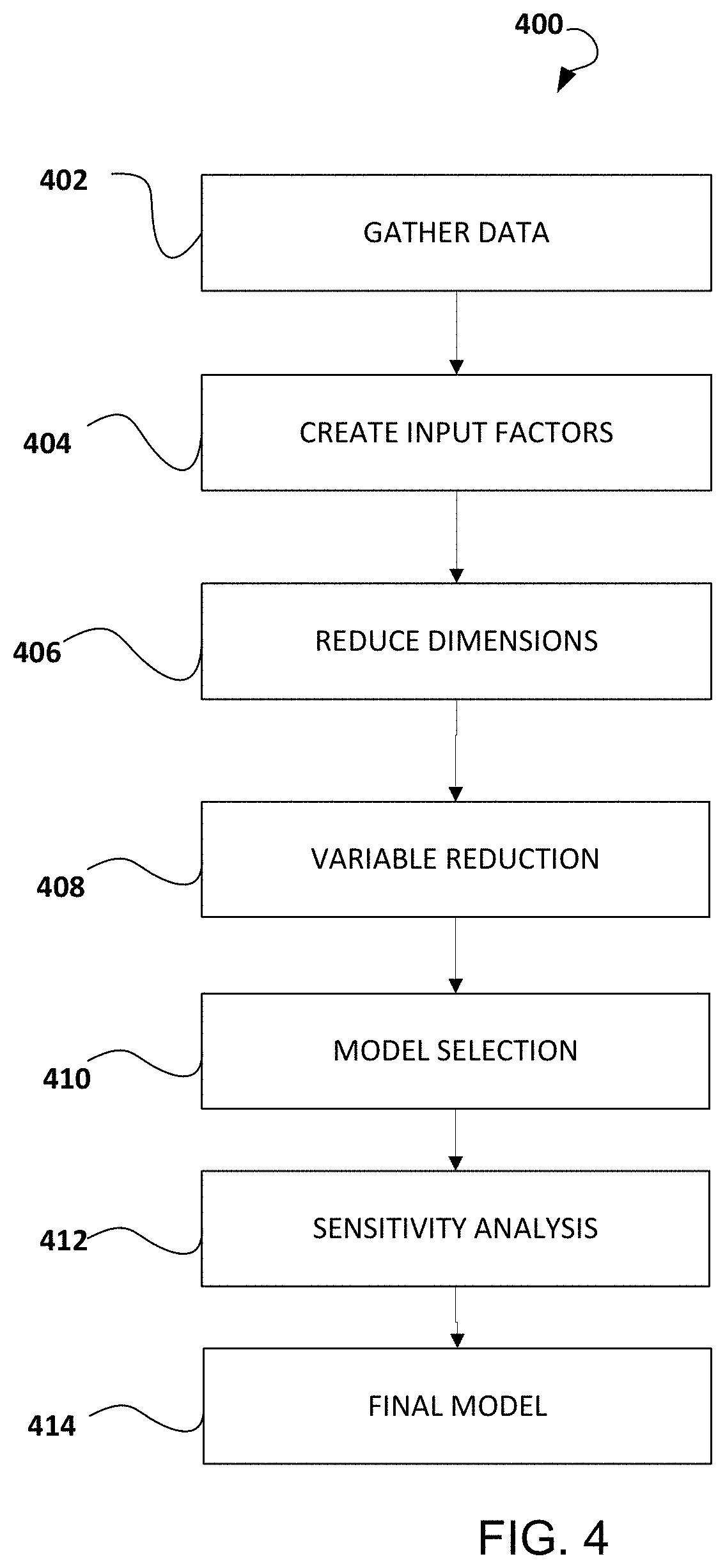
Methods and systems for pharmacy modeling are described. The risk adjusted pharmacy predictive model is created from member data, claims data, and population data. This model can be used to compare the actual pharmacy performance to an expected actual pharmacy performance value, which can be used to identify pharmacies at risk or not performing to an acceptable level. The model can be used for adherence and generic drug utilization ratings of pharmacies. The pharmacy can be judged on a therapy class by therapy class basis with factors that reflect the demographic, socio-economic, location, benefits attributes, etc. that actually affect the performance of the pharmacy and may assist in determining the quality of care by a pharmacy.
Owner:EXPRESS SCRIPTS STRATEGIC DEV INC
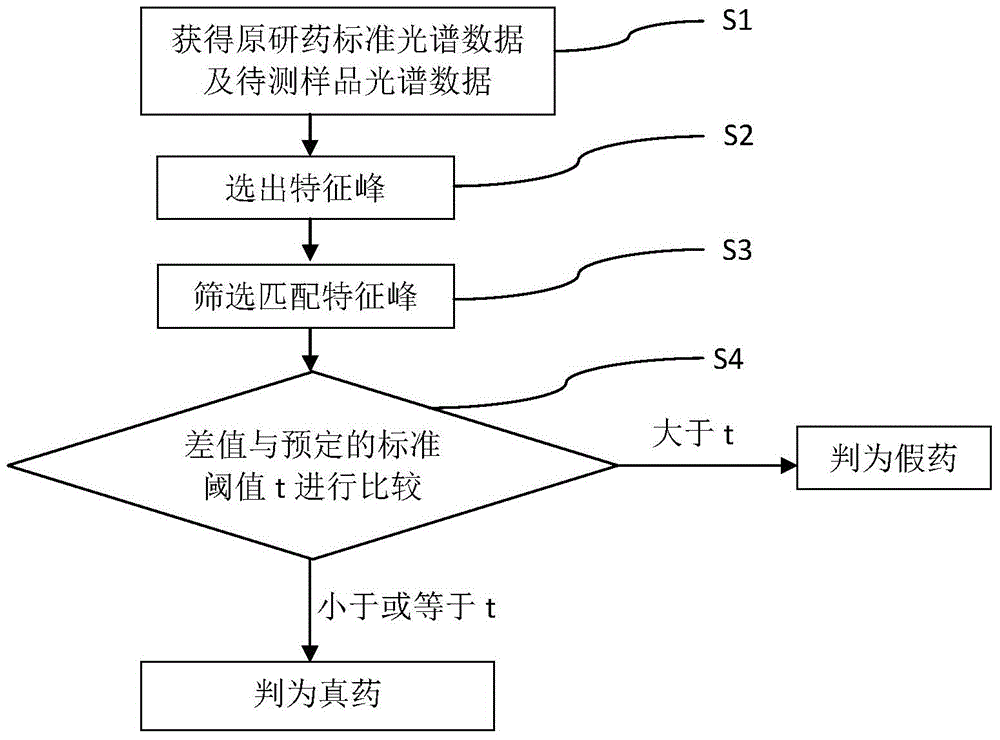
Method for detecting generic drug pretended to be reference listed drug
A method for detecting a generic drug pretended to be an reference listed drug comprises the following steps: step 1, obtaining reference listed drug standard spectral data and to-be-measured sample spectral data; step 2, choosing reference listed drug characteristic peaks and to-be-measured sample characteristic peaks; step three, screening matched characteristic peaks in the to-be-measured sample characteristic peaks conforming to matching conditions, and calculating the number of the matched characteristic peaks; and step four, comparing a difference value between the number of the reference listed drug characteristic peaks and the number of the matched characteristic peaks with a predetermined standard threshold t, when the difference value is less than or equal to t, judging a to-be-measured sample to be a real drug, and when the difference value is greater than t, judging the to-be-measured sample to be a fake drug, wherein t is an arbitrary integer value of 1-10. According to the method provided by the invention, the difference between the generic drug and the reference listed drug can be intuitively and comprehensively displayed, and a drug system with relatively low API content and with the other components in the drug and with relatively large interference on API characteristic peaks also can be effectively detected.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
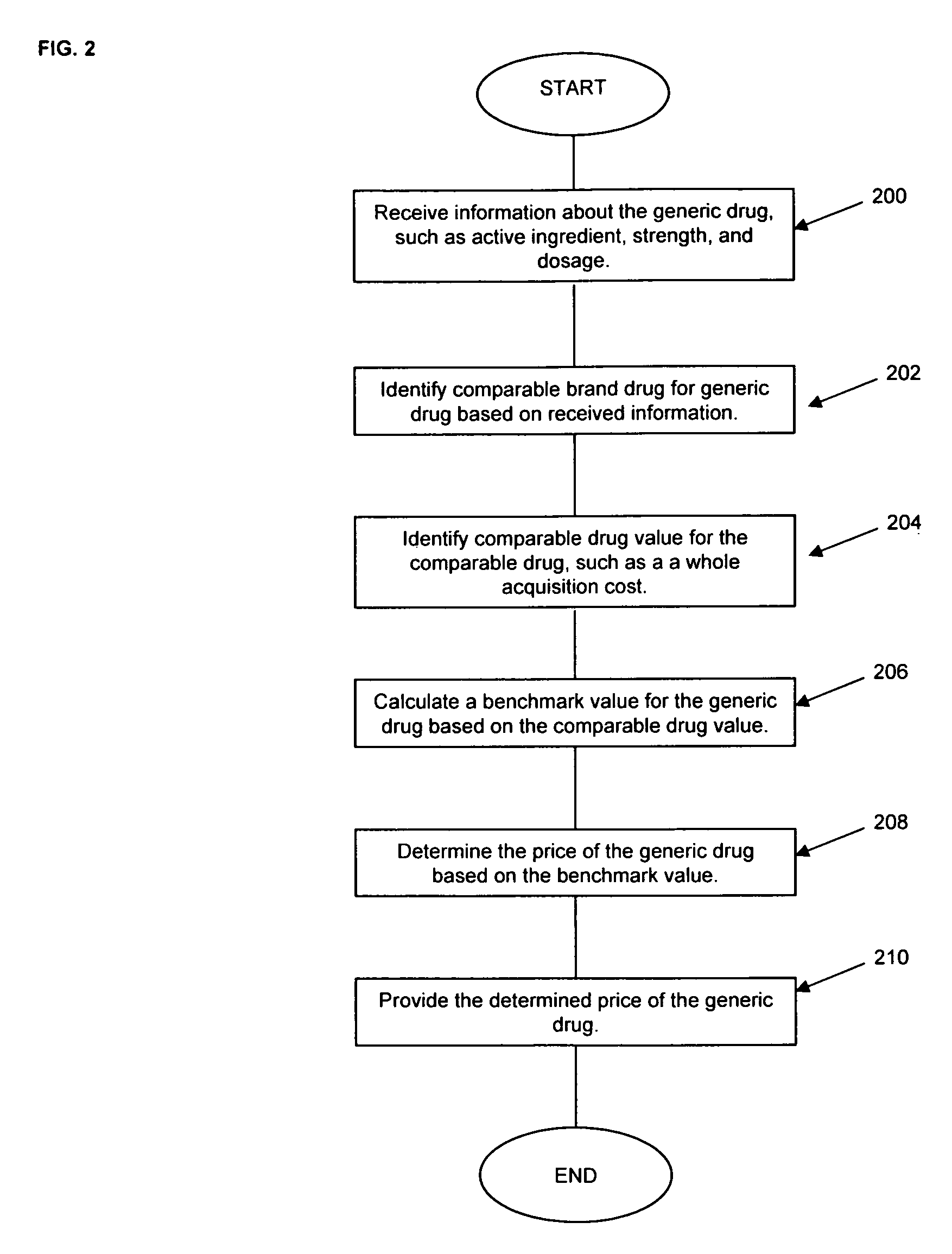
Pharmaceutical pricing method
This invention provides for innovative methods of pricing generic drugs. In a preferred embodiment, wholesale acquisition costs of identified comparable brand drugs for the generic drugs to be priced according to the invention are identified as comparable brand drug values. The comparable brand drug values are averaged to calculate a stable, predictable benchmark value to be used to determine the price of the generic drug, according to methods of this invention.
Owner:EXPRESS SCRIPTS STRATEGIC DEV INC
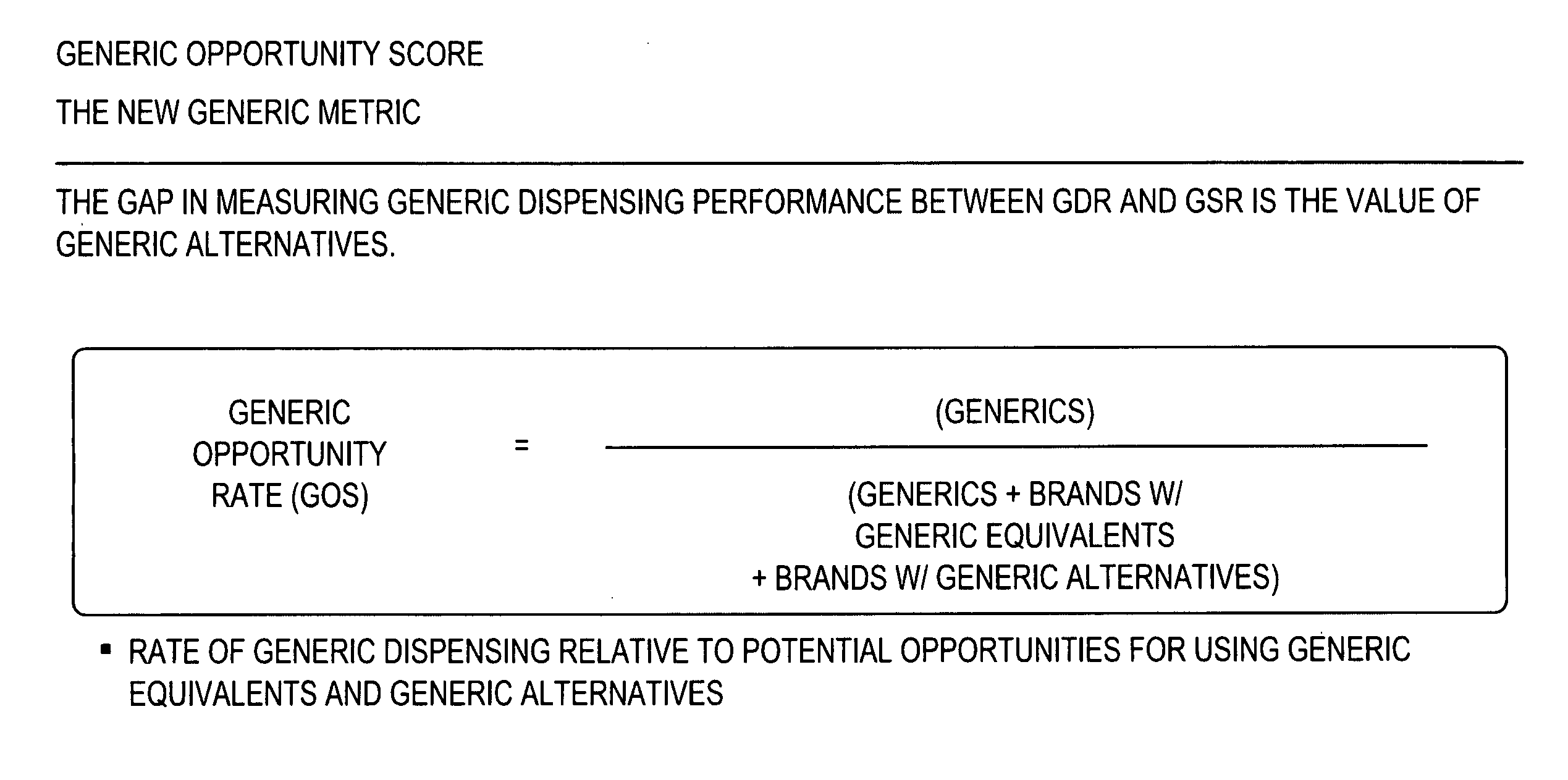
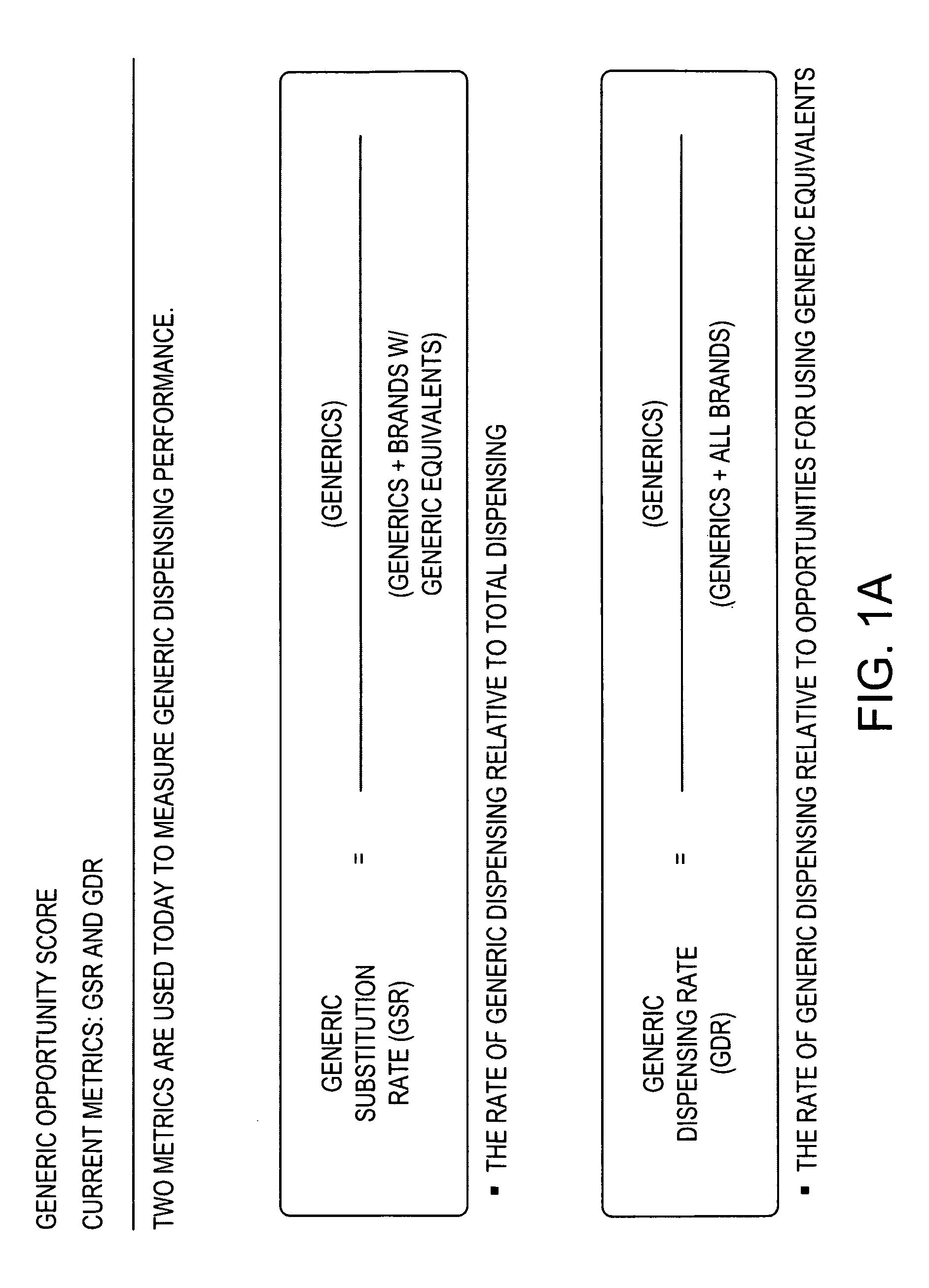
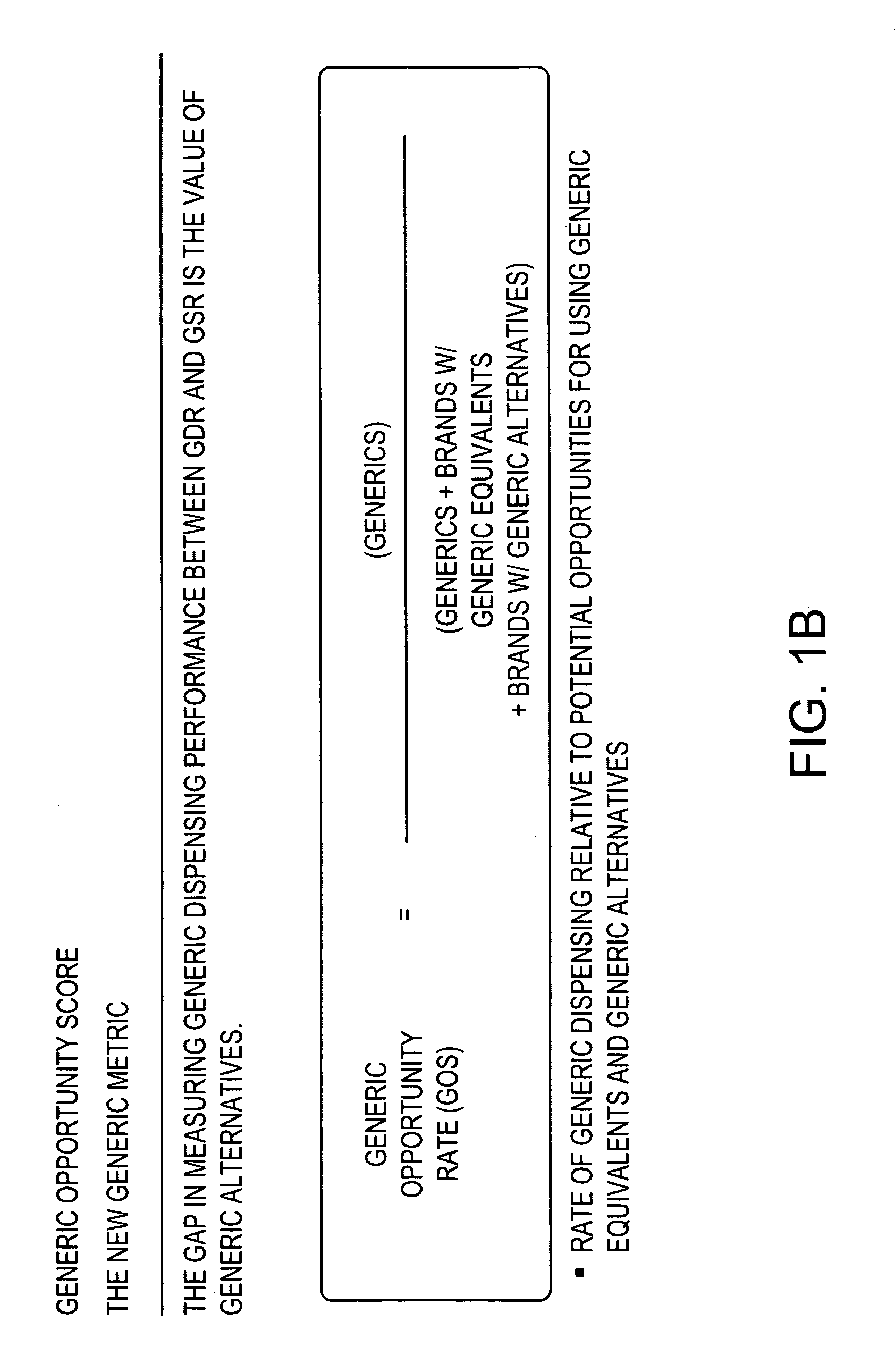
Methods and systems for generic opportunity scoring
A computer implemented method and / or computer system determines a Generic Opportunity Score (GOS) and / or a generic drug's performance demonstrating how a generic utilization can be better identified and improved relative to the total overall generic opportunity, which can provide savings information relative to a clinically appropriate generic alternative. In some embodiments, the process and / or system determines the GOS as the proportion of generic prescriptions dispensed relative to the maximum number of prescriptions that have a generic equivalent or a clinically-appropriate generic alternative. In some embodiments, the process determines the GOS as the number of generic claims dispensed over the total number of generic claims plus the brand claims that have a generic equivalent or generic alternative. In some embodiments, the data utilized will be provided through a prescription claims database, which can be segmented into three distinct groups: Generic Code Number (GCN), Brand / Generic Code, and Channel, i.e. retail or mail.
Owner:EXPRESS SCRIPTS STRATEGIC DEV INC
Method for detecting and evaluating in-vitro dissolution of enteric preparation
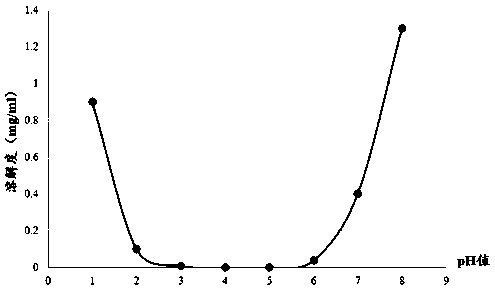
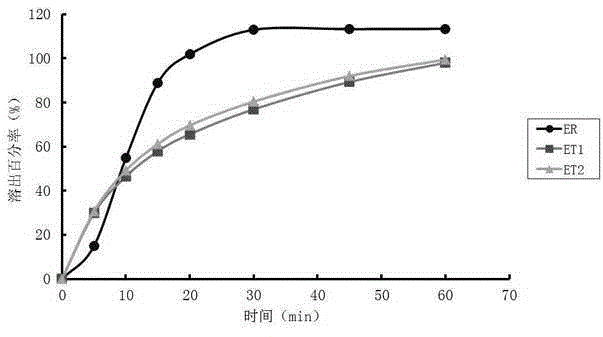
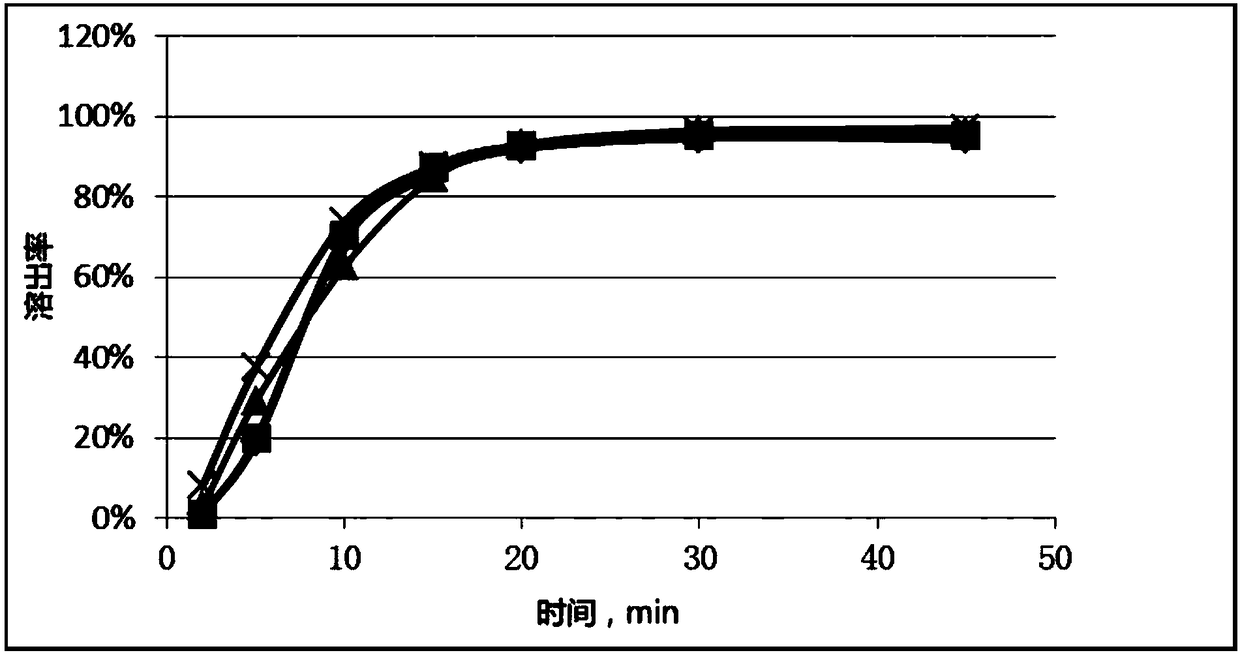
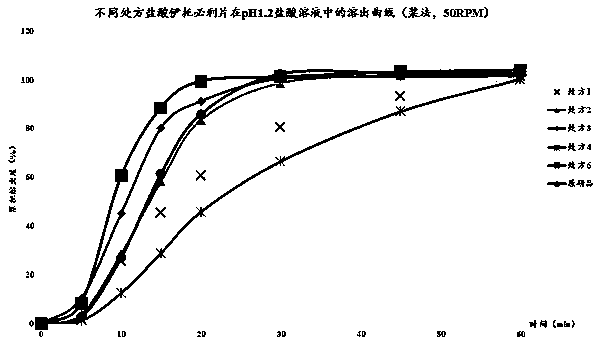
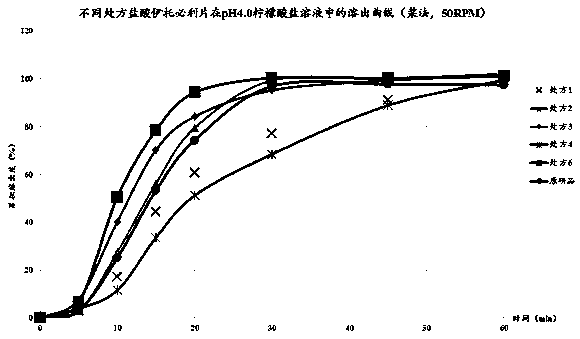
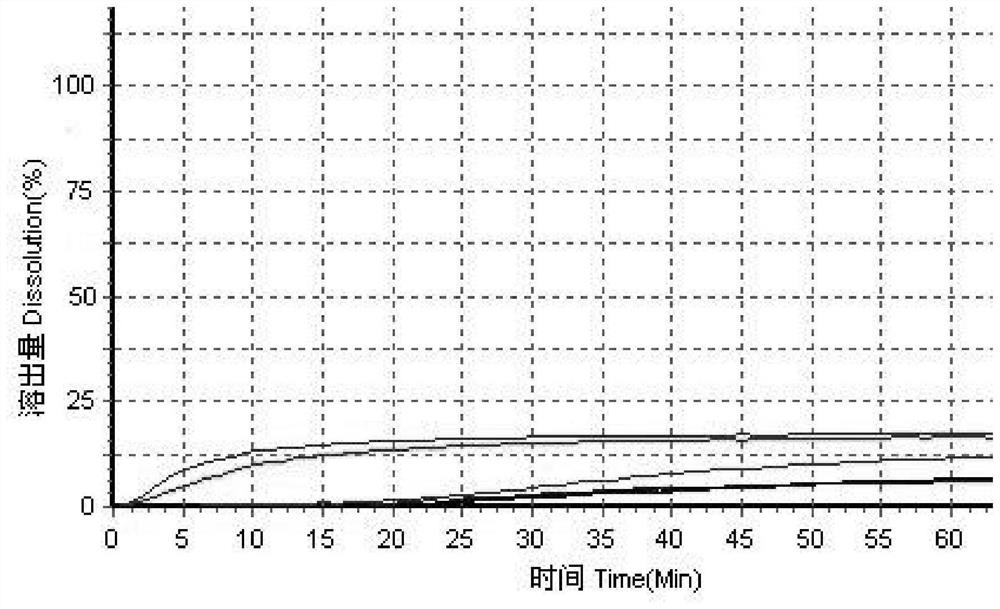
The invention relates to a method for detecting and evaluating in-vitro dissolution of an enteric preparation. The method is performed according to standards of Chinese Pharmacopoeia and comprises thesteps as follows: conducting a dissolution test in a simulated gastric fluid dissolution medium; then, transferring the enteric preparation in water or weakly acid dissolution medium for a dissolution test; besides, directly performing a dissolution test on the preparation in water or the weakly acid dissolution medium; comparing the difference between dissolution results of preparation subjectedto or not subjected to dissolution treatment in the simulated gastric fluid dissolution medium to determine the reliability of the enteric performance of the preparation. The method can provide datasupport for selection of multi-source reference preparations in generic drug consistency evaluation and provide reference for prescription screening and bioequivalence risk evaluation. More importantly, the method is used for detecting and evaluating in-vitro dissolution of the enteric preparation, so that the incidence rate of adverse events of clinical medication can be reduced.
Owner:BEIJING INST FOR DRUG CONTROL
Methods for determining liposome bioequivalence
This invention provides methods for determining liposome bioequivalence between a generic drug product and a reference brand-name product. Specific methods for determining bioequivalence between doxorubicin hydrochloric acid (HCl) liposome injection product (Doxil®) and a generic pegylated liposome doxorubicin product are disclosed herein.
Owner:ARONDO PHARMA
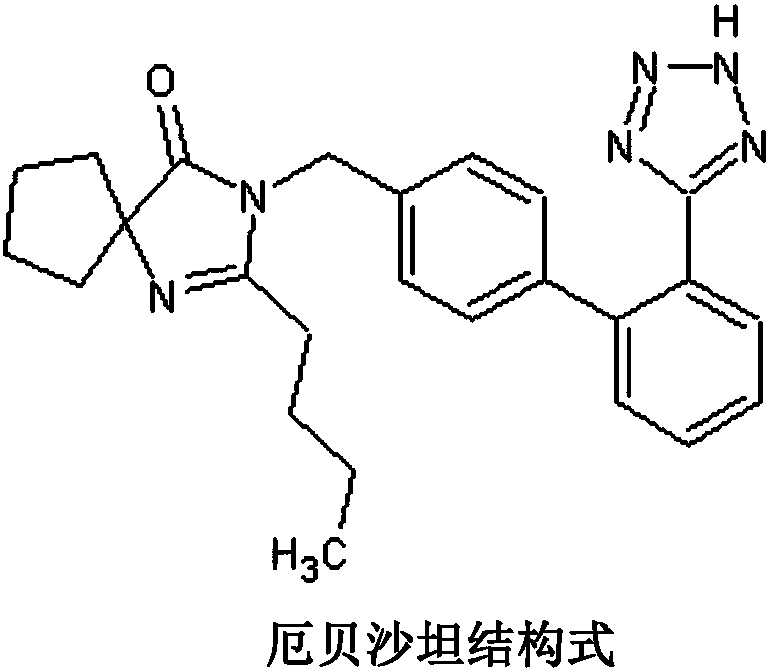
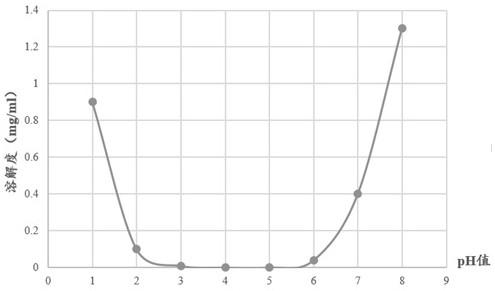
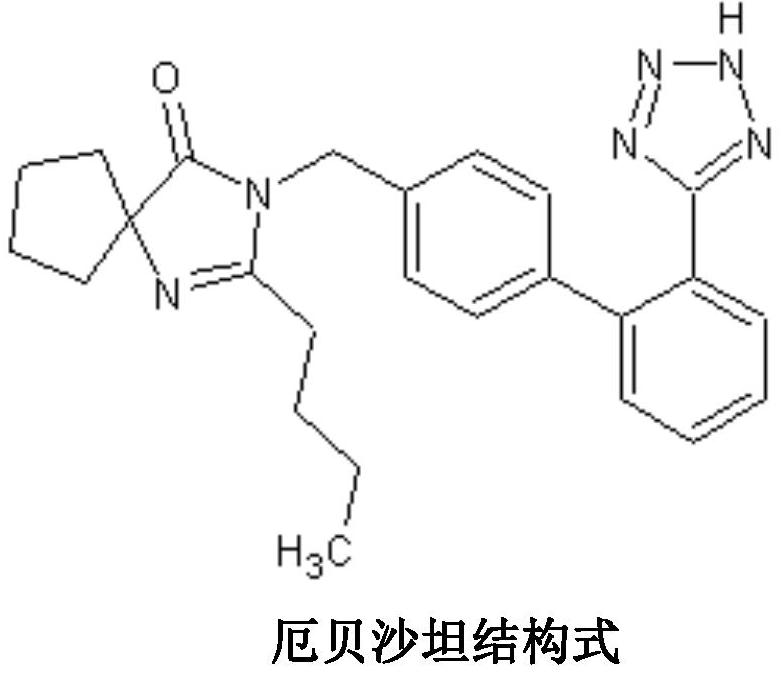
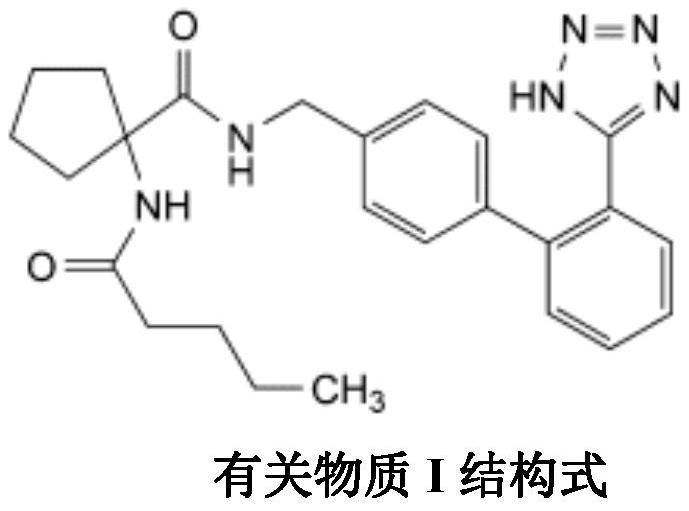
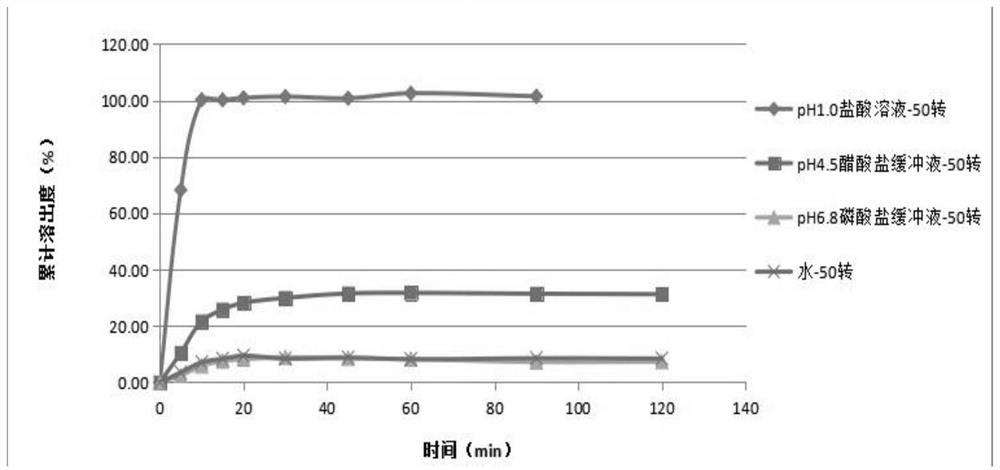
Irbesartan capsule and preparation method thereof
ActiveCN109157527AHigh Cmax valueQuick releaseOrganic active ingredientsPharmaceutical non-active ingredientsAdhesiveGeneric drug
The invention discloses irbesartan capsules and a preparation method thereof, and application in consistence evaluation on generic drugs. The preparation method comprises the following steps: uniformly mixing irbesartan, a filler, a disintegrant, a hydrogel matrix material, an acidifier and a surfactant according to a certain ratio, dissolving an adhesive into purified water, spraying into a fluidized bed pelletizing coating machine, carrying out primary pelletizing, drying, uniformly mixing a flow aid and a lubricant, and filling common gelatin capsules with the mixture, thereby obtaining theirbesartan capsules. In the acceleration testing and long-term testing and stability investigation process, properties, dissolution rates, related substances and the like of the irbesartan capsules produced by using the preparation process disclosed by the invention are not remarkably changed, and the irbesartan capsules have effects equivalent to those of a conventional product (the product nameis irbesartan tablet, the trade name is irbesartan, the specification is 0.15g, the producer is Sanofi Winthrop Industrie) on in-vivo organisms.
Owner:珠海润都制药股份有限公司
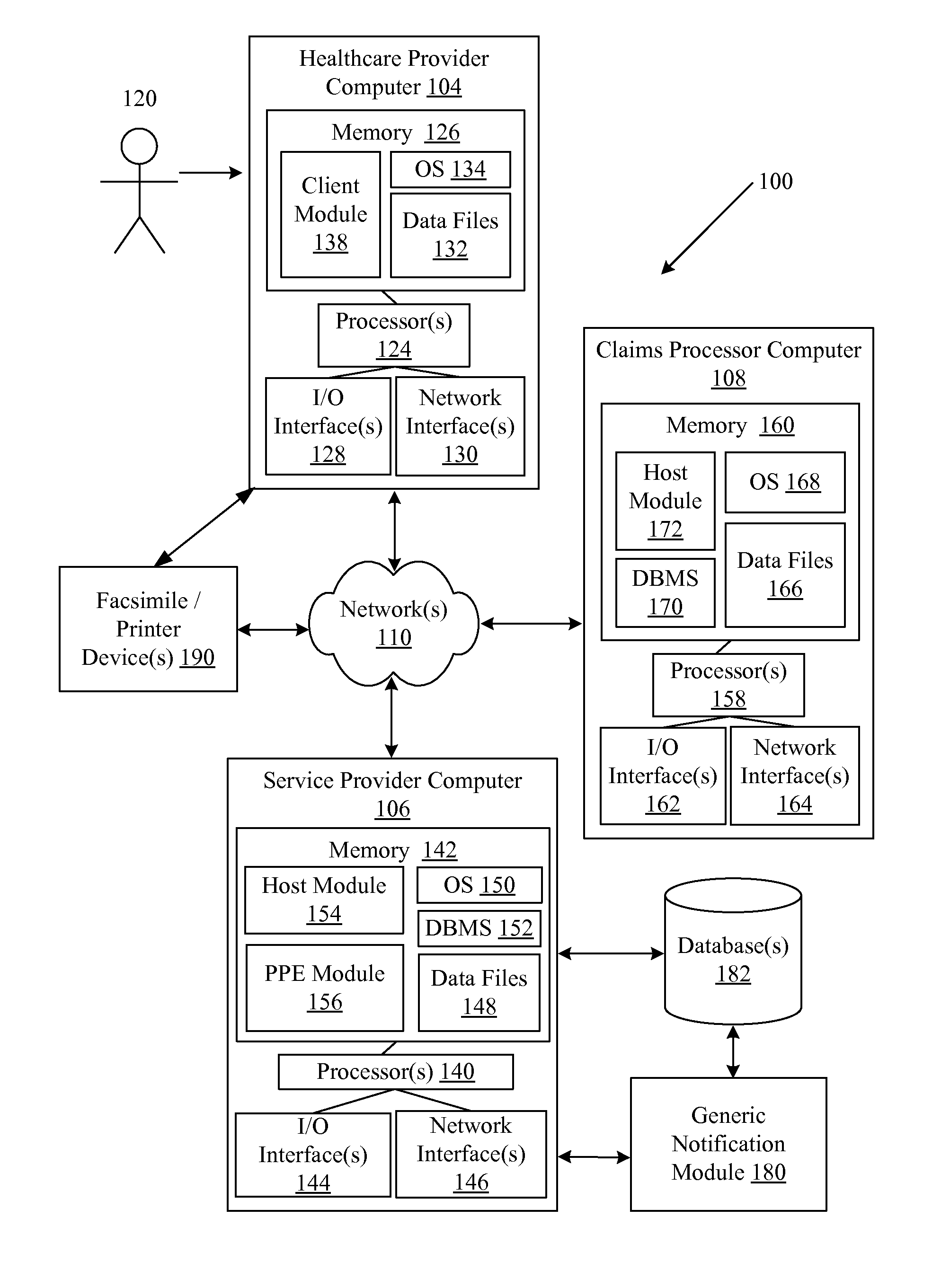
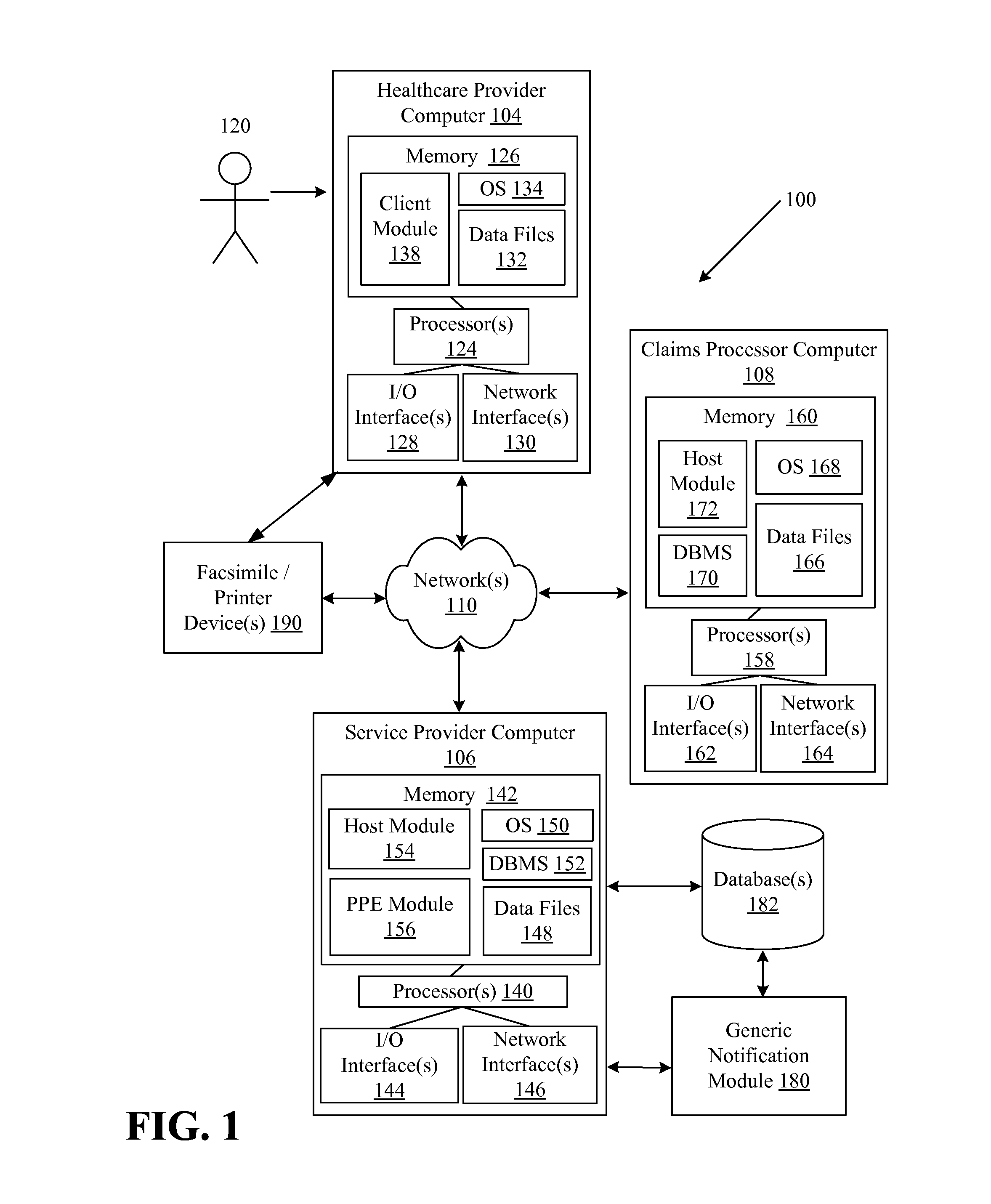
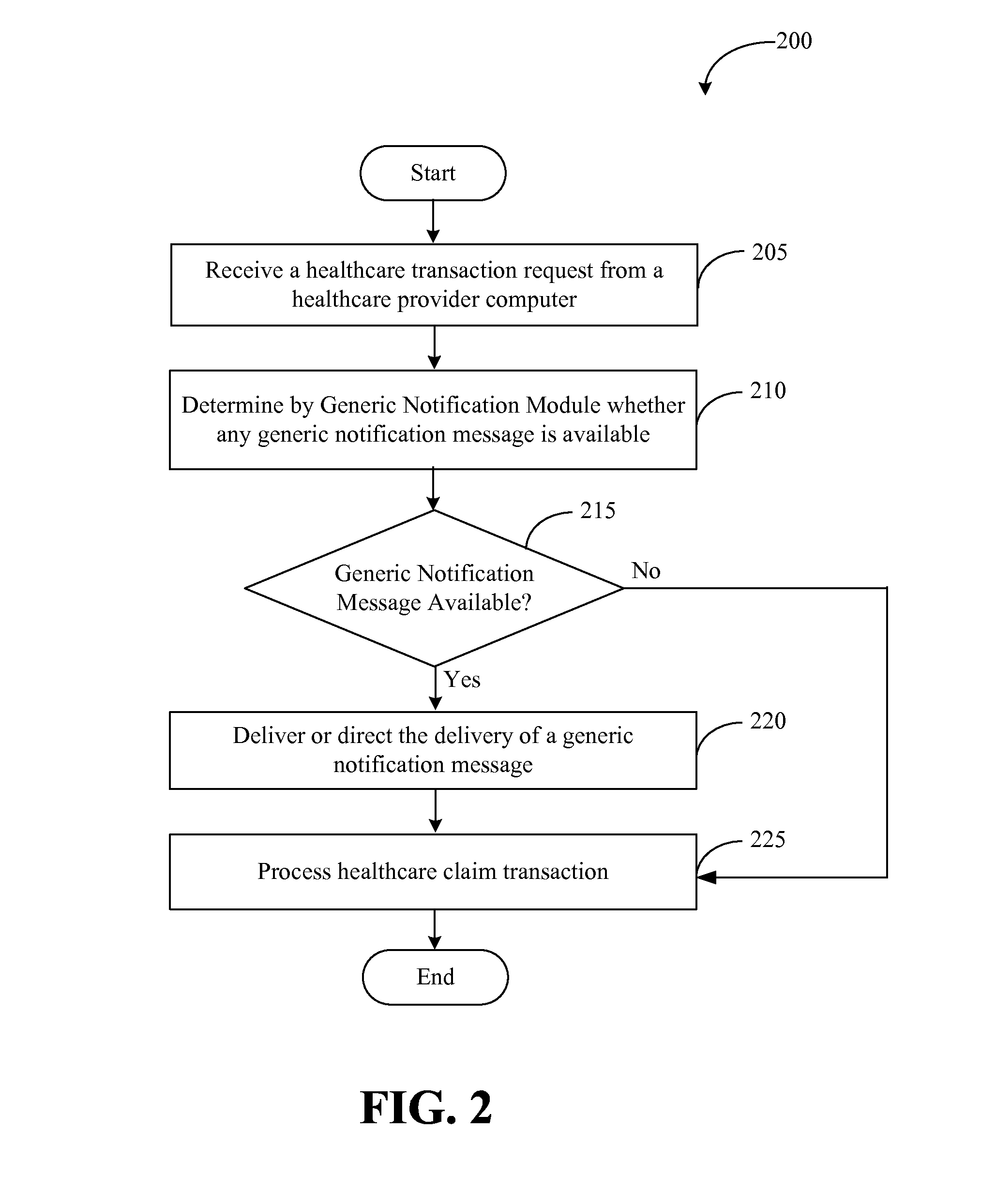
Systems and methods for providing notifications of availability of generic drugs or products
Systems and methods may provide notifications of availability of generic drugs or products. The systems and methods may include receiving, from a healthcare provider computer, a healthcare claim transaction request that identifies a product for a patient; determining that the product identified by the healthcare claim transaction request is a non-generic product or a branded product; determining that a generic equivalent for the product identified by the healthcare claim transaction request will become available on a future date; generating a generic notification message indicating the availability of generic equivalent for the product on the future date; and delivering or directing a delivery of the generic notification message.
Owner:MCKESSON CORPORATION
Preparation method of irbesartan capsule
ActiveCN107028912AHigh dissolution rateImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsGeneric drugMedicine
The invention discloses a preparation method of an irbesartan capsule and application in the evaluation of generic drug consistency. After Irbesartan is mixed with various auxiliary materials to prepare a soft material, granulation is performed through an extruding granulation method, granules are evenly mixed with a lubricant after drying, and the capsule is filled. The irbesartan capsule produced by adopting the preparation process is consistent to an original research product (product name: irbesartan tablets; trade name: Avapro; specification: 0.15 g; production manufacturer: Sanofi Winthrop Industrie) on the aspect of multiple in-vitro dissolution curves, related substances have no obvious change in the test acceleration and long-term test stability investigation process and are biologically equivalent to the original research product.
Owner:珠海润都制药股份有限公司
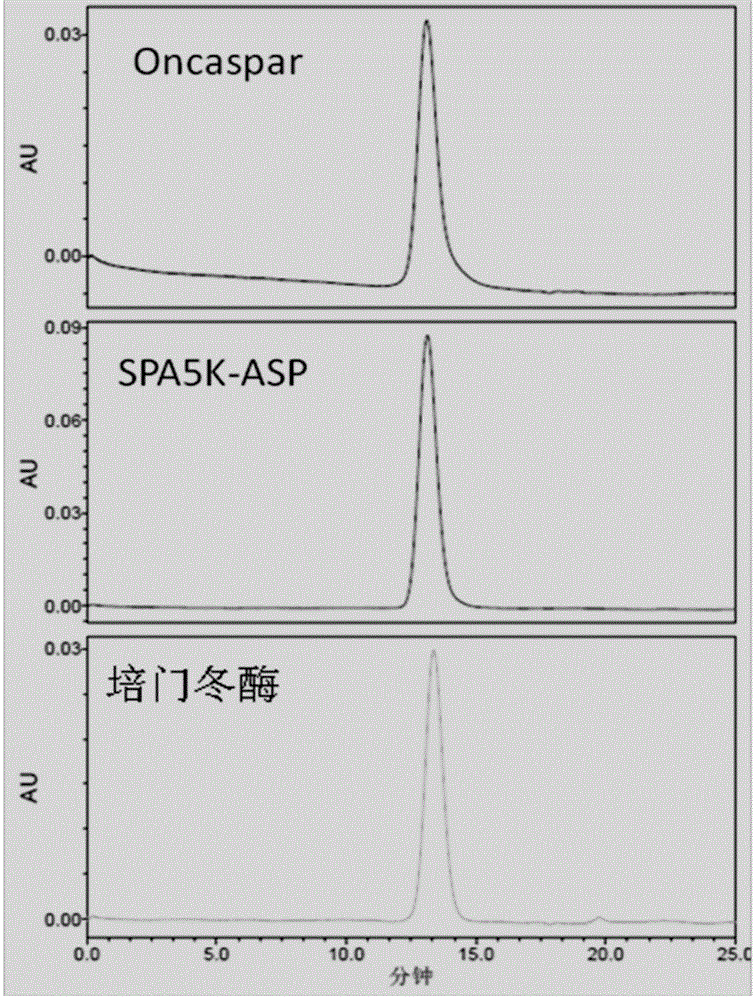
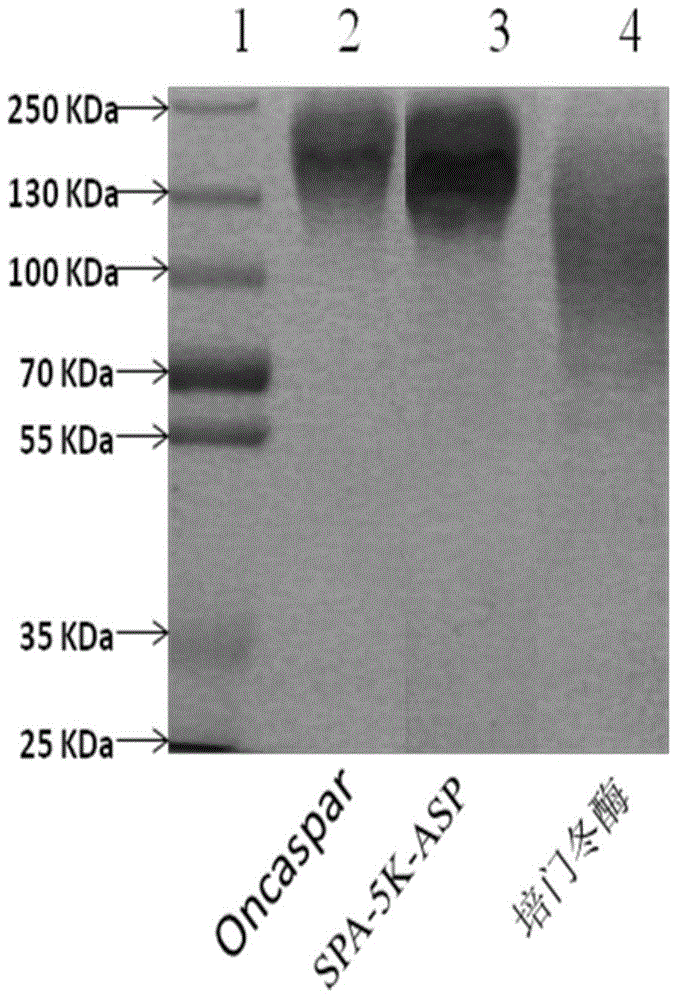
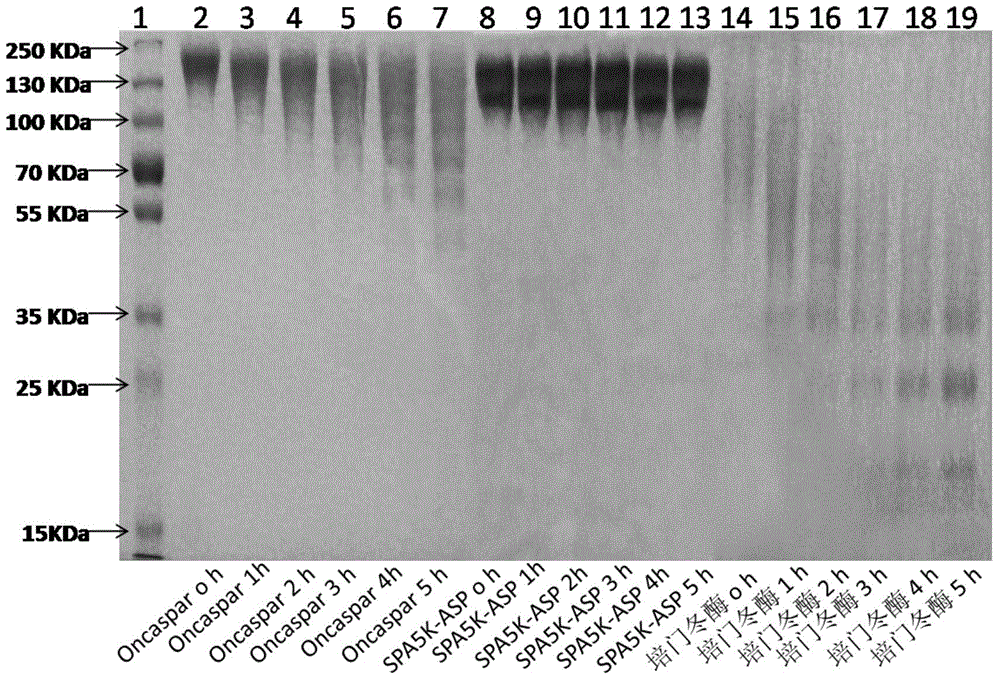
PEGylated asparaginase and applications thereof
PendingCN105802946ALow immunogenicityExtended half-lifePeptide/protein ingredientsHydrolasesPegylated asparaginaseGeneric drug
The invention discloses a PEGylated asparaginase as well as applications thereof to medicament preparation and clinic treatment. One molecule of asparaginase in the asparaginase modified by PEG is coupled to 13-45 molecules of PEG, and the PEG is straight-chain PEG whose average molecular weight is 2-20KDa. The PEGylated asparaginase is modified by PEG. The PEGylated asparaginase has the advantages of reduced immunogenicity, obviously prolonged half life, etc.; PEG and asparaginase are coupled more stable. Compared with branded drugs and generic drugs of commercially available PEGylated asparaginase, the PEGylated asparaginase has the advantages of more stable structure, firm combination of PEG, uneasy falling, higher homogeneity, and higher activity.
Owner:ZONHON BIOPHARMA INST
Detection method for generic drug quality
ActiveCN105372401AImprove accuracyHigh sensitivityTesting medicinal preparationsApplicability domainMedicine
The invention relates to the technical field of detection methods for drug quality, in particular to a detection method for the generic drug quality. The method comprises the steps that when two or more fitting parameters of a generic drug are out of a confidence interval of a brand-name drug, the quality of the generic drug is not similar to the quality of the brand-name drug; when three or more fitting parameters of the generic drug are within the confidence interval of the fitting parameters of the brand-name drug, the quality of the generic drug is similar to the quality of the brand-name drug. According to the detection method for the generic drug quality, the accuracy and sensitivity of a quality detection result can be improved, and therefore the quality of the generic drug can be detected; in addition, the detection method for the generic drug quality is applicable to quality detection of different drugs and drugs of different dosage forms, and therefore the application range of the detection method for the generic drug quality is enlarged.
Owner:中国人民解放军新疆军区联勤部药品仪器检验所
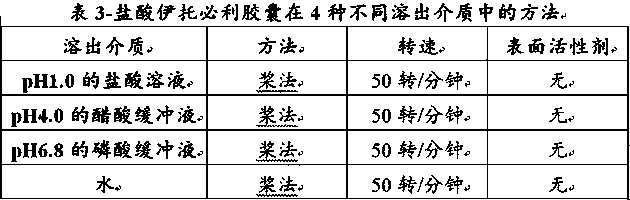
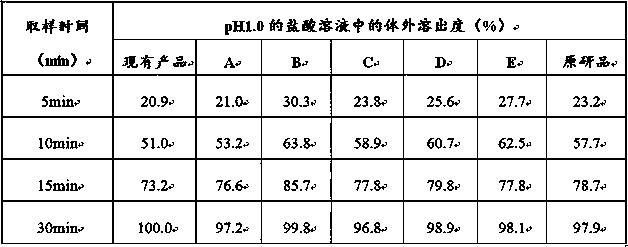
Itopride hydrochloride micro-tablet and preparation method thereof
ActiveCN109125277AIncrease postprandial bioavailabilitySolve the problem of inconsistent dissolution profiles in vitroOrganic active ingredientsDigestive systemGeneric drugAdditive ingredient
The invention discloses an itopride hydrochloride micro-tablet, a preparation method thereof and application thereof in consistency evaluation of generic drugs. According to the itopride hydrochloridemicro-tablet, the preparation method thereof and the application thereof in the consistency evaluation of the generic drugs, itopride hydrochloride and various ingredients are mixed, pelletized and dried, and then evenly mixed with an extra disintegrant and a lubricant, and the mixture is pressed into the micro-tablet of which the diameter is no more than 3MM. A capsule can be filled with the micro-tablet, multiple dissolution curves of a prepared itopride hydrochloride capsule in vitro are consistent with an original product (product name: itopride hydrochloride tablet; trade name: Weilisu;specification: 0.05g; certificate holding merchant: ABBOTT LABORATORIES (M) SDN. BHD), in the process of accelerated test and long-term test stability investigation, related substances have no significant change, and the micro-tablet has a bioequivalence trend with the original product after the meal.
Owner:珠海润都制药股份有限公司
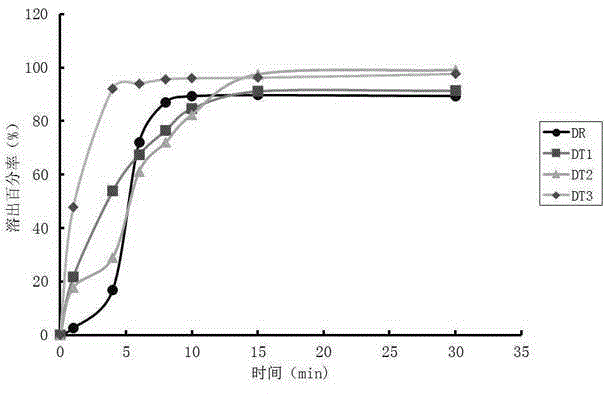
Method for detecting dissolution curve of simvastatin tablet
ActiveCN108414656AImprove discriminationModerately differentiated qualityComponent separationGeneric drugDrug product
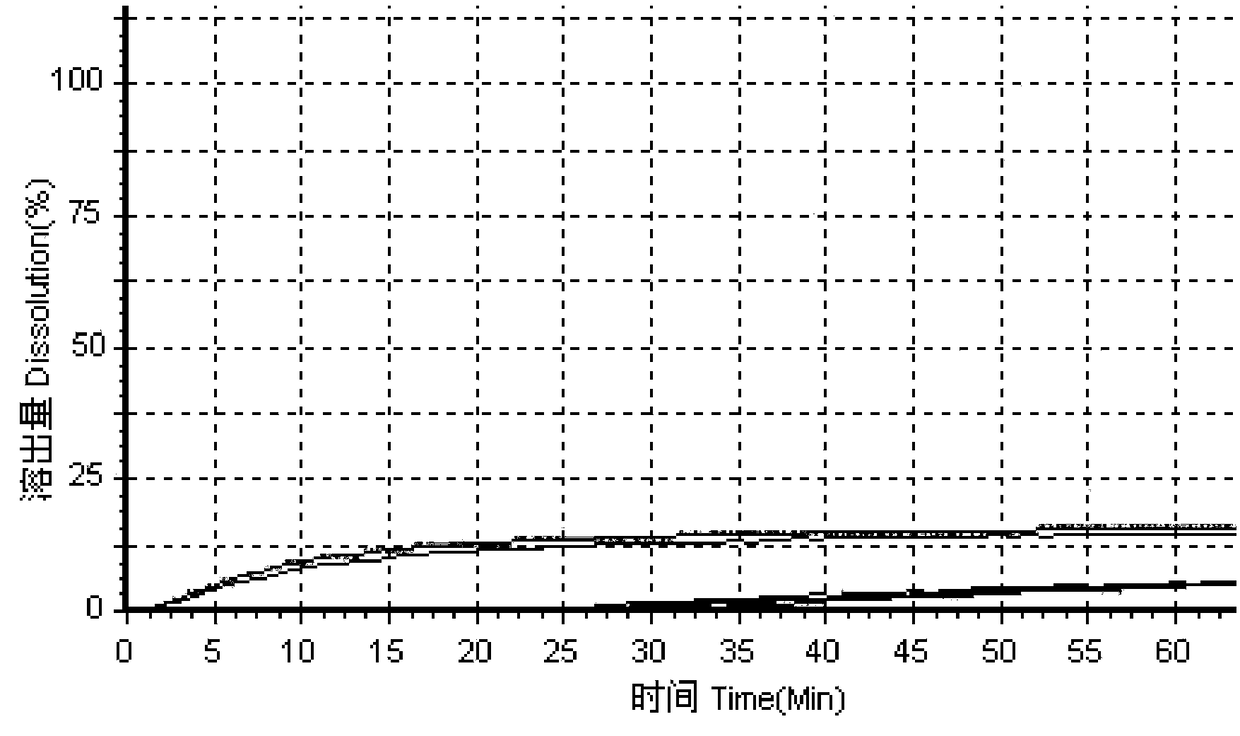
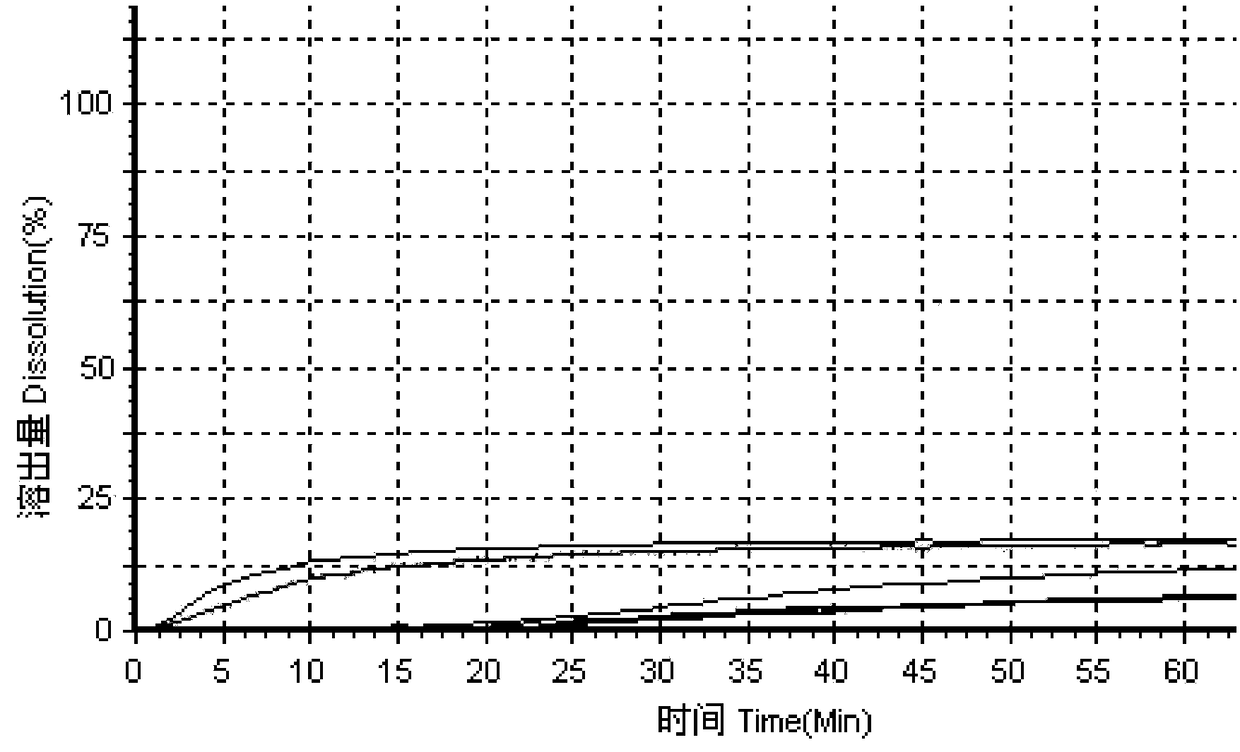
The invention relates to a method for detecting the dissolution curve of a simvastatin tablet. The method comprises the following steps: (1) preparing a dissolution medium: preparing the dissolution medium at a dissolution temperature of 40 DEG C or less by using Tween-80 as a medium; 2) taking a simvastatin reference substance, and preparing a reference substance solution; (3) preparing a samplesolution by using a dissolvability detection technology; (4) detecting the dissolution quantity by high performance liquid chromatography, wherein the sample introduction temperature of a sample chamber is 4-5 DEG C; (5) repeating step (3) and step (4) twice or more; and (6) evaluating: calculating a similarity factor f2 by using a similarity factor technology, and comparing the similarity betweenthe dissolution curve of a generic preparation and the dissolution curve of a reference preparation by using the similarity factor f2. The method is scientific, durable and reproducible, can be usedfor evaluating the quality consistency evaluation of a generic drug and an original drug, and also can provide a guarantee for the consistency of the inter-batch quality of the drug.
Owner:四川省食品药品检验检测院
System and method for automatically switching prescriptions in a retail pharmacy to a new generic drug manufacturer
ActiveUS7895056B2Easy to useReduce in quantityFinanceDrug and medicationsGeneric drugTiered approach
An automatic manufacturer switchover function to switch a set of future new, transfer, refill, and / or copy prescriptions to a new manufacturer product for a pharmacy. Furthermore, the claimed method and system may allow for a tiered approach to a manufacturer switch by allowing a corporate entity or owner of a pharmacy network to designate a pharmacy wide preferred manufacturer (or generic product) while giving a local pharmacy the power to decide when to implement a switchover at a local level. In one embodiment, the claimed switching system and process may also provide indications to pharmacists and customers to guide a transition from one manufacturer to another, thereby preserving customer perception of quality and pharmacy reputation.
Owner:WALGREEN CO
Method for determining dissolution curve of lanthanum carbonate chewable tablets
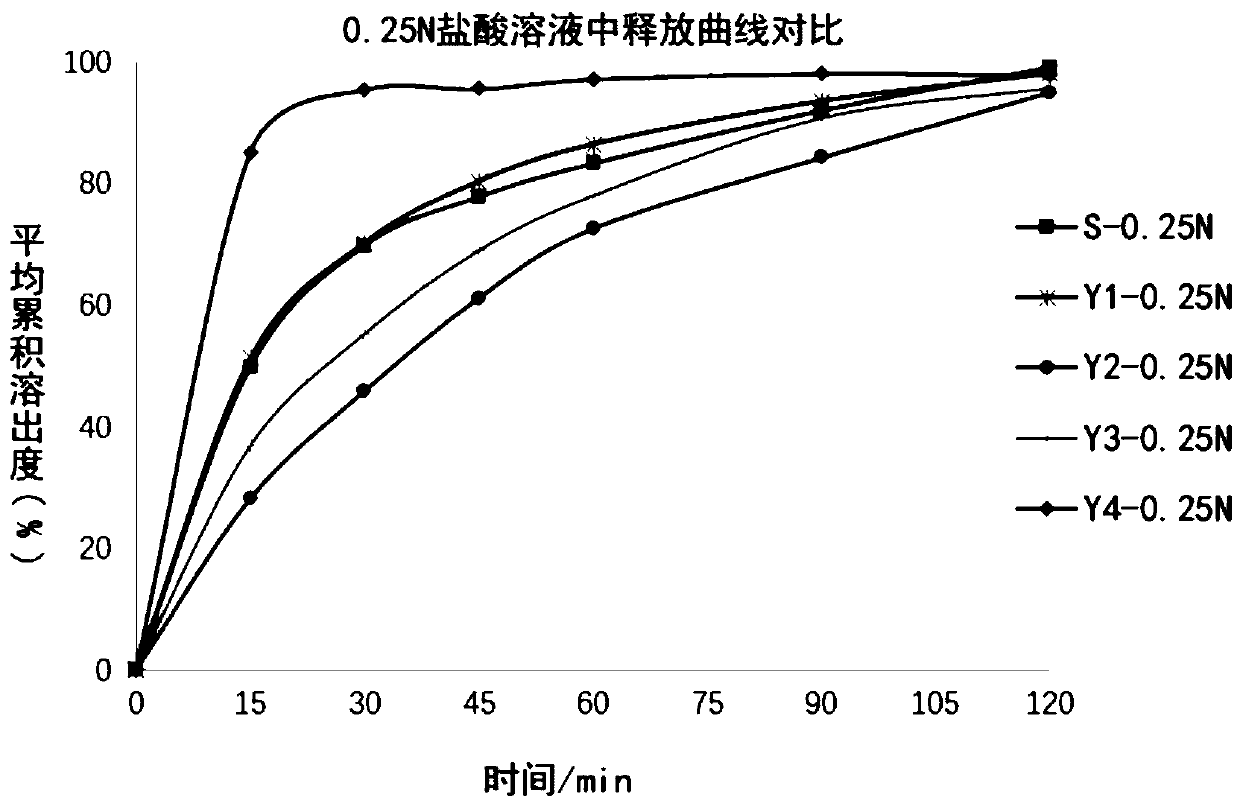
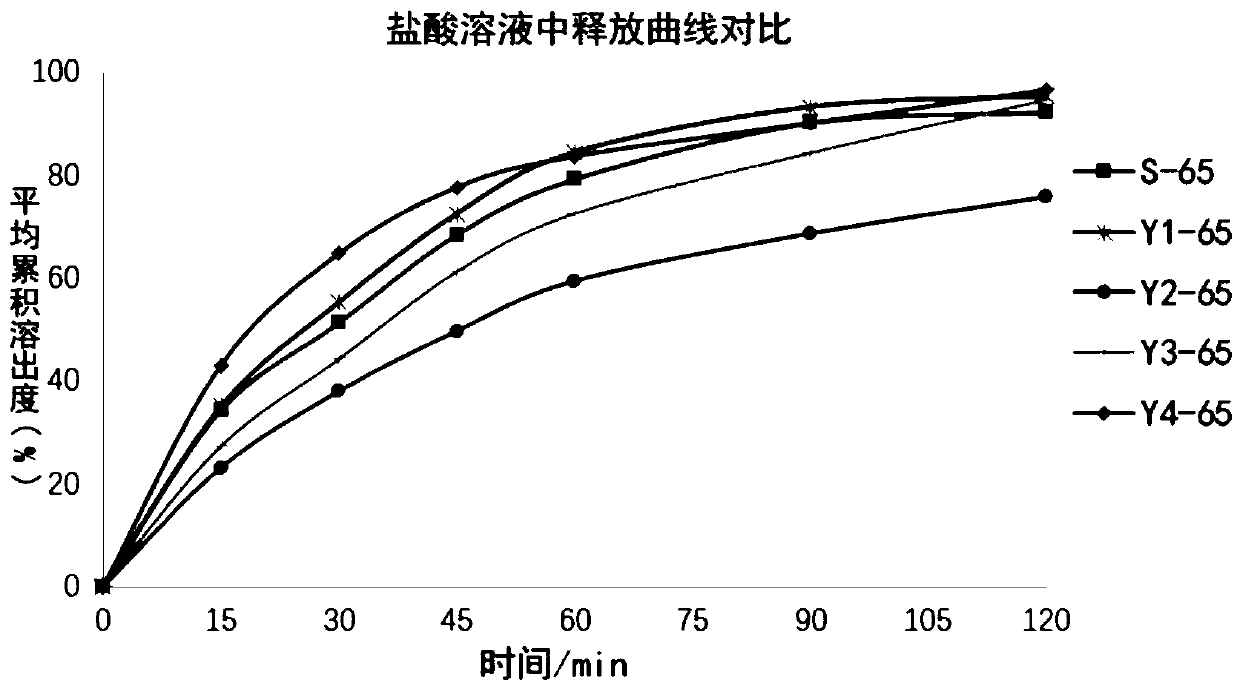
InactiveCN110108832AEfficiently distinguish the effects of in vitro releaseQuality assuranceComponent separationLanthanum carbonate Chewable TabletGeneric drug
The invention relates to the field of analytical chemistry, in particular to a method for determining a dissolution curve of lanthanum carbonate chewable tablets. An oar method is adopted, wherein a dissolution medium is 0.25 N hydrochloric acid solution, and the rotating speed is 50-75 revolutions per minute. The method for determining the dissolution curve of the lanthanum carbonate chewable tablets has the characteristics of science, durability, reproducibility and the like, the influence of different prescriptions and process variables on the in vitro release of the lanthanum carbonate chewable tablets can be effectively distinguished, the method for determining the dissolution curve of the lanthanum carbonate chewable tablets can be used for quality consistency evaluation of generic drugs and original research drugs, guarantee can be provided for consistency of quality between batches of drugs, the quality of the drugs is further ensured, and consistency of quality and curative effect is achieved.
Owner:BEIJING YUEKANGKECHUANG PHARM TECH CO LTD
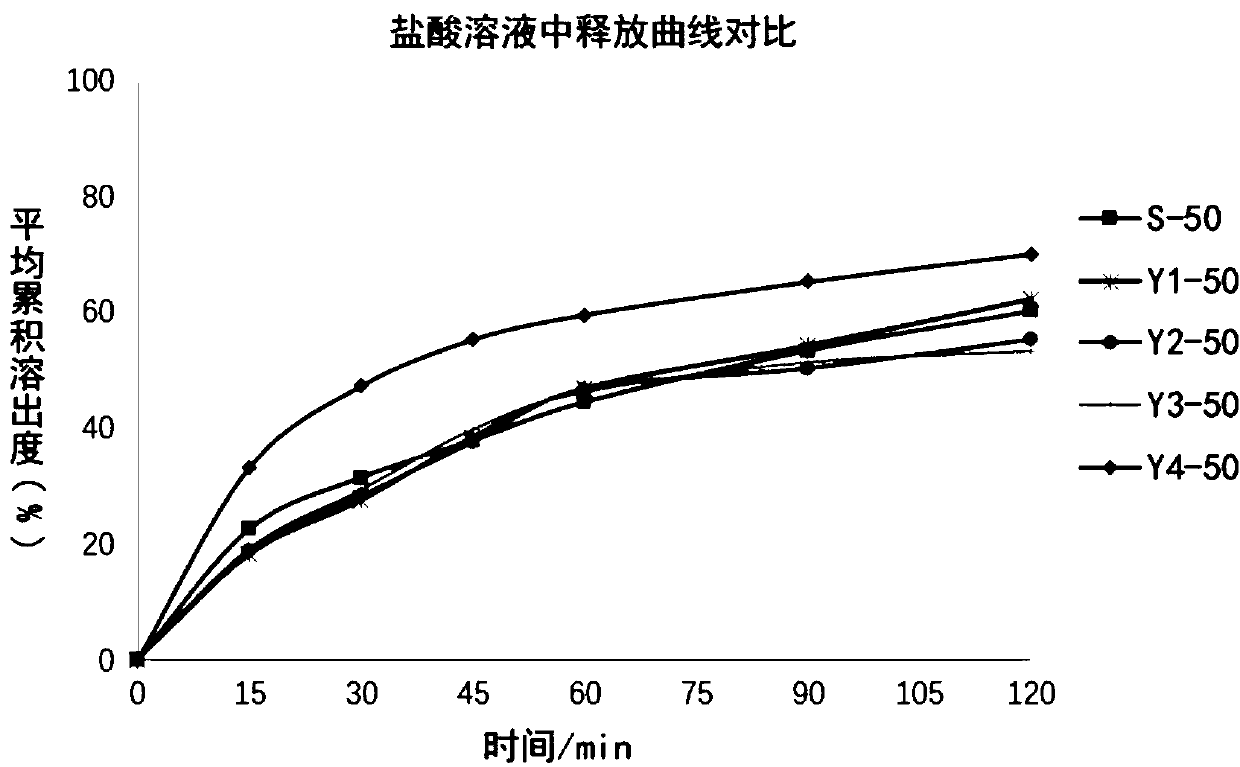
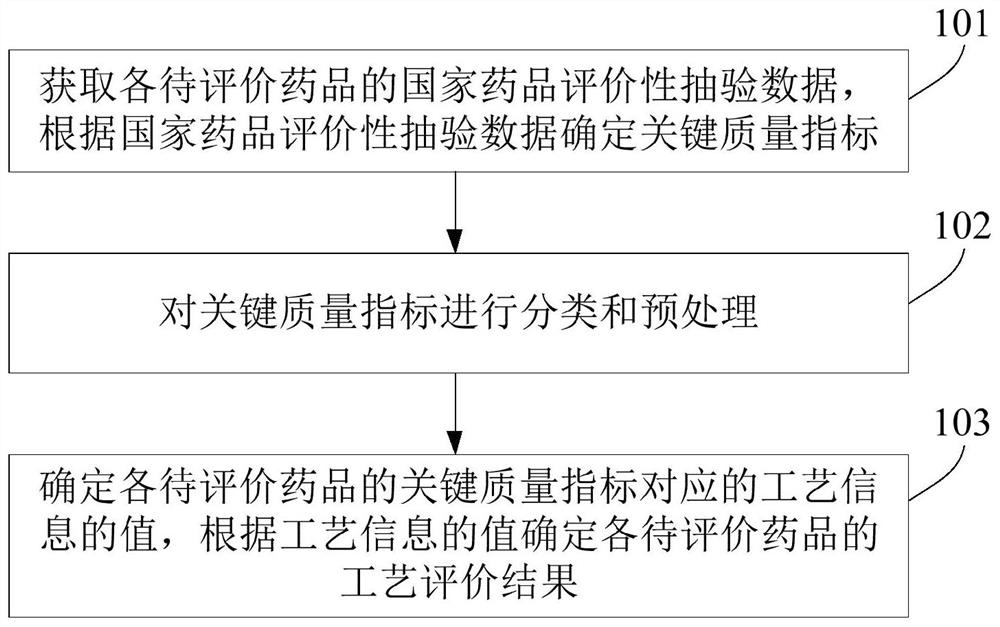
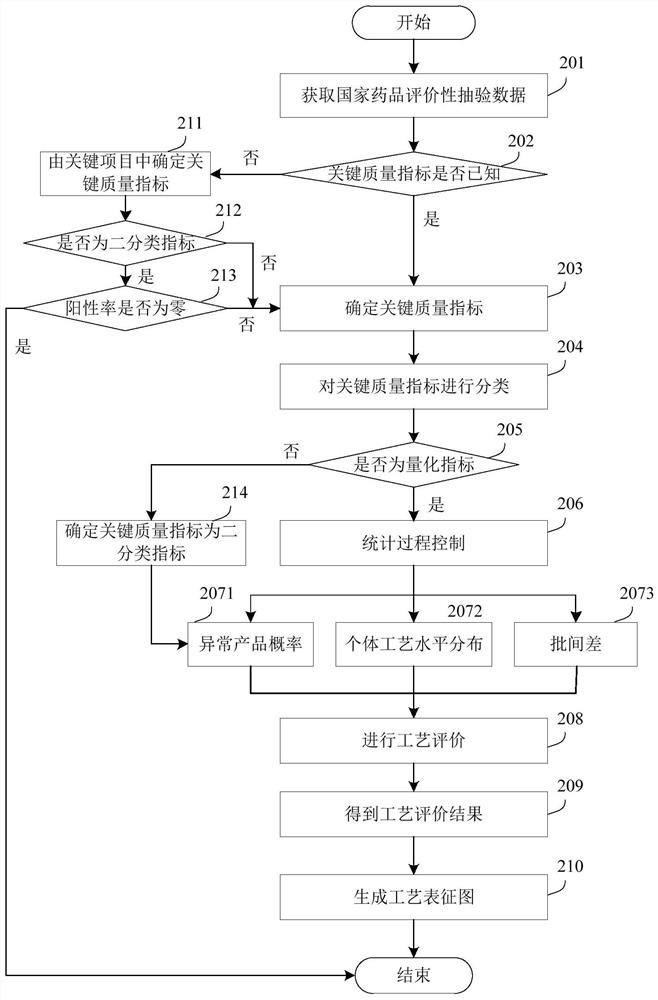
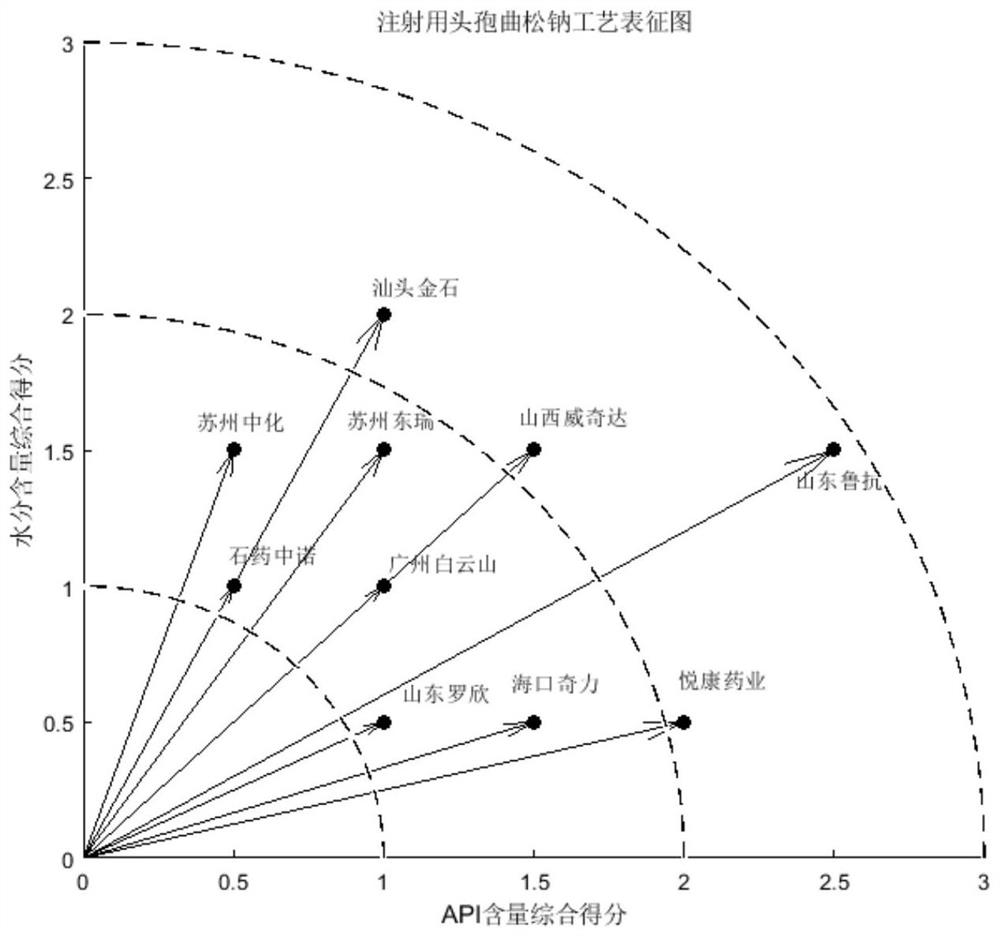
A process evaluation method and system for generic drugs
The embodiment of the present invention relates to a process evaluation method and system for generic drugs, so as to realize a more comprehensive process evaluation of drugs; further, it can better characterize the process information of key quality indicators. The method includes: obtaining national drug evaluation random test data of each drug to be evaluated, determining key quality indicators according to the national drug evaluation random test data; classifying and preprocessing the key quality indicators; determining each drug to be evaluated According to the value of the process information corresponding to the key quality index, the process evaluation result of each drug to be evaluated is determined according to the value of the process information; the process information includes individual process level distribution, inter-batch difference and abnormal product probability.
Owner:NAT INST FOR FOOD & DRUG CONTROL
Methods and systems for pharmacy modeling
Methods and systems for pharmacy modeling are described. The risk adjusted pharmacy predictive model is created from member data, claims data, and population data. This model can be used to compare the actual pharmacy performance to an expected actual pharmacy performance value, which can be used to identify pharmacies at risk or not performing to an acceptable level. The model can be used for adherence and generic drug utilization ratings of pharmacies. The pharmacy can be judged on a therapy class by therapy class basis with factors that reflect the demographic, socio-economic, location, benefits attributes, etc. that actually affect the performance of the pharmacy and may assist in determining the quality of care by a pharmacy.
Owner:EXPRESS SCRIPTS STRATEGIC DEV INC
Itopride hydrochloride tablet and preparation method of tablet
PendingCN111374958AAvoid difficultiesSimple production processOrganic active ingredientsDigestive systemGeneric drugITOPRIDE HYDROCHLORIDE
The invention discloses an itopride hydrochloride tablet, a preparation method of the tablet, and application in consistency evaluation of generic drugs. The raw material medicine of itopride hydrochloride has the characteristics of easily absorbing moisture, becoming sticky in water and crystallization, so that the materials become sticky and has poor granulation. Therefore, wet granulation is not suitable for preparing itopride hydrochloride tablets. In the present invention, itopride hydrochloride and various auxiliary materials are mixed uniformly, and then tablets with a diameter of about7 mm, a thickness of about 3 mm and a weight of about 130 mg, are obtained by directly pressing the above mixture. According to the itopride tablets produced by directly pressing powder, not only thedifficulty of granulation can be solved, but also the production process is simple, and the manufacturing cost is low. The multiple dissolution curves of the tablets in vitro are consistent with thedissolution curves of the original research product, related substances have no obvious change in the process of an accelerated test and long-term test stability investigation, and the tablets are bioequivalent with the original research product after meal.
Owner:珠海润都制药股份有限公司
Bacteriostat-free water-soluble vitamin freeze-dried preparation for injection
The invention relates to a freeze-dried preparation of water-soluble vitamin composition for injection without bacteriostatic agent. According to the technical scheme provided by the present invention, the components of this preparation are that each bottle contains: 2.79-3.41 mg of thiamine nitrate, 4.41-5.39 mg of riboflavin sodium phosphate, 36-44 mg of nicotinamide, 4.41-5.39 mg of pyridoxine hydrochloride mg, sodium pantothenate 14.85-18.15mg, sodium vitamin C 101.7-124.3mg, biotin 54-66μg, folic acid 0.36-0.44mg, vitamin B124.5-5.5μg, glycine 270-330mg, edetate disodium 0.45 -0.55 mg. The invention can fundamentally eliminate clinical adverse reactions such as allergy induced by antibacterial agents to users, and improve product safety.
Owner:费森尤斯卡比华瑞制药有限公司
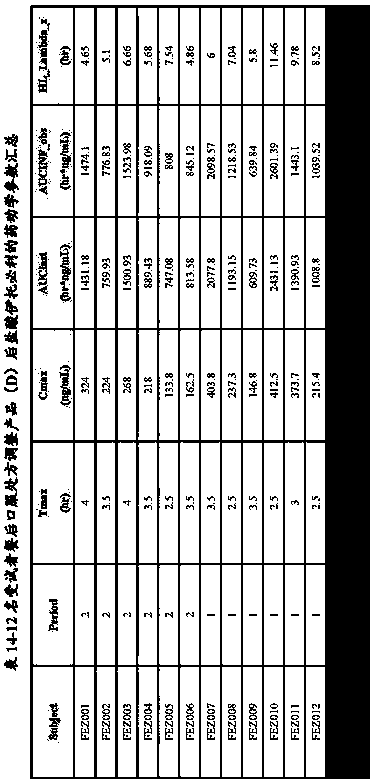
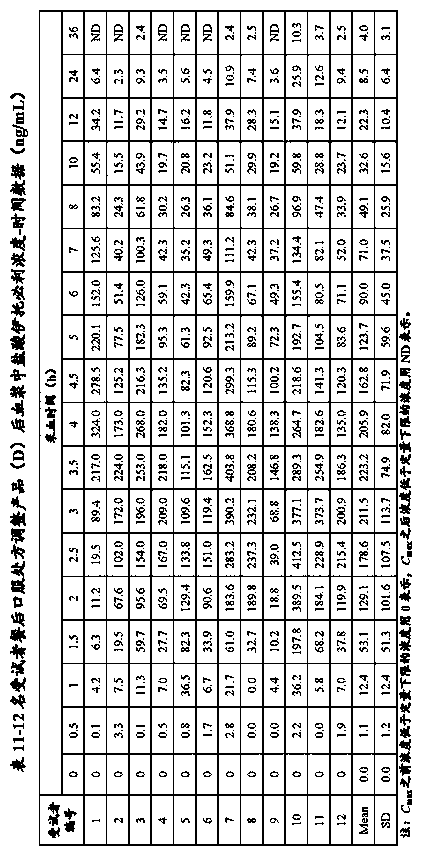
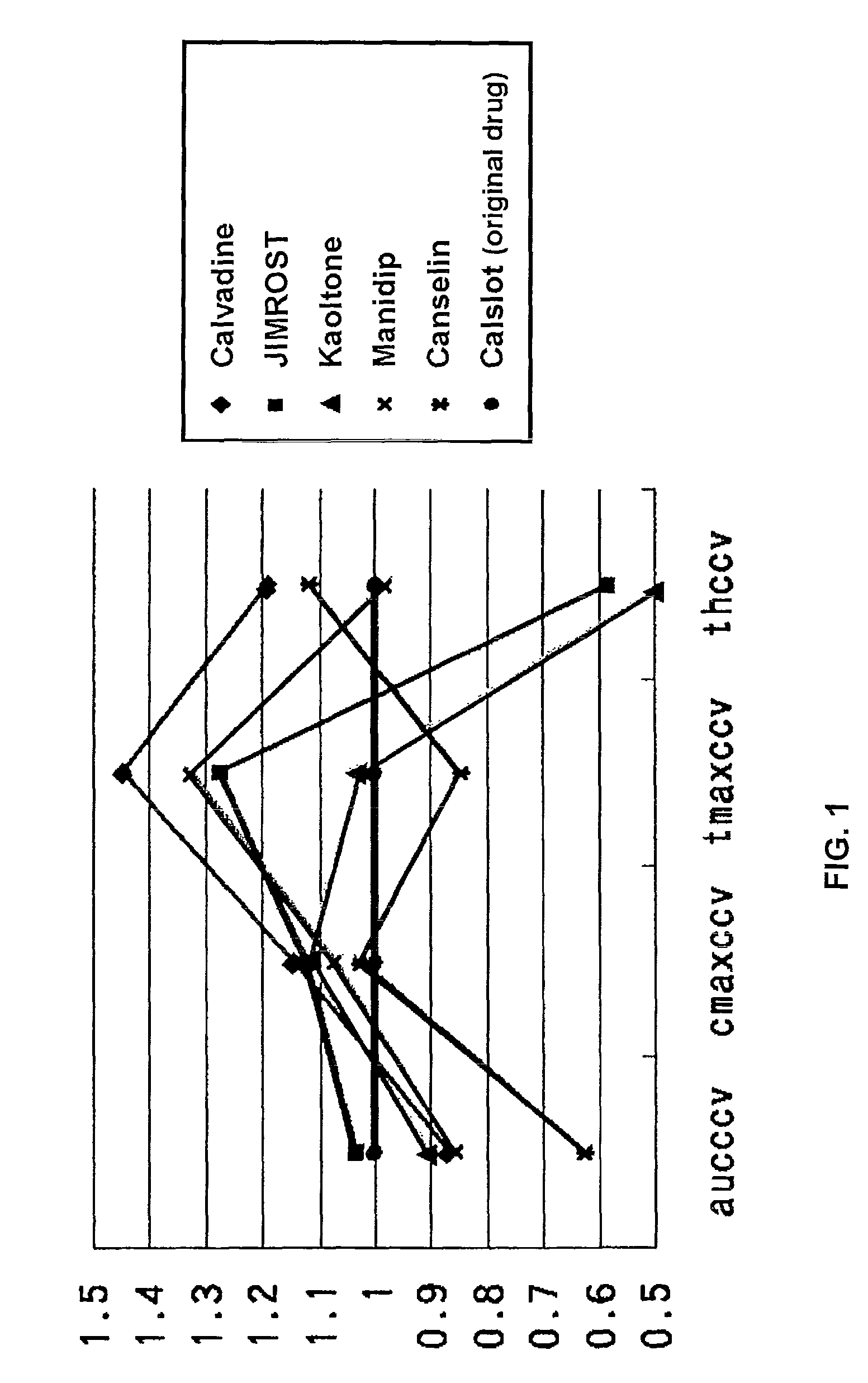
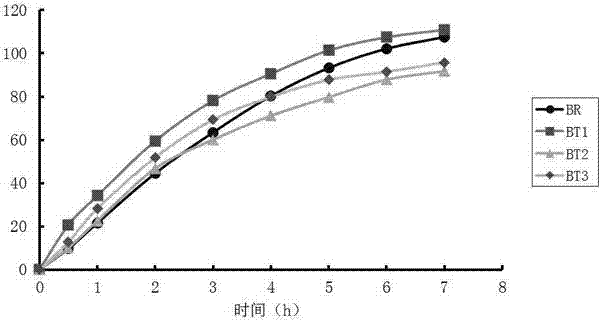
Bioequivalence evaluation method of evaluating bioequivalence of a generic drug to the corresponding original drug
[Problem] There is provided a bioequivalence evaluation method not only of evaluating bioequivalence between an original drug and a corresponding generic drug but also of enabling to compare bioequivalence between generic drugs.[Means of Solving the Problem] The bioequivalence between generic drugs is evaluated according to the Expression (1) wherein CCVCmax g stands for the calibration coefficient of variation of Cmax (the maximum blood concentration), CCVTmax g stands for the calibration coefficient of variation of Tmax (the time to maximum blood concentration), CCVT1 / 2 g stands for the calibration coefficient of variation of the half-life (T1 / 2), and CCVAUC g stands for the calibration coefficient of variation of AUC (the blood concentration-area under the curve).
Owner:TERASHITA MASATO
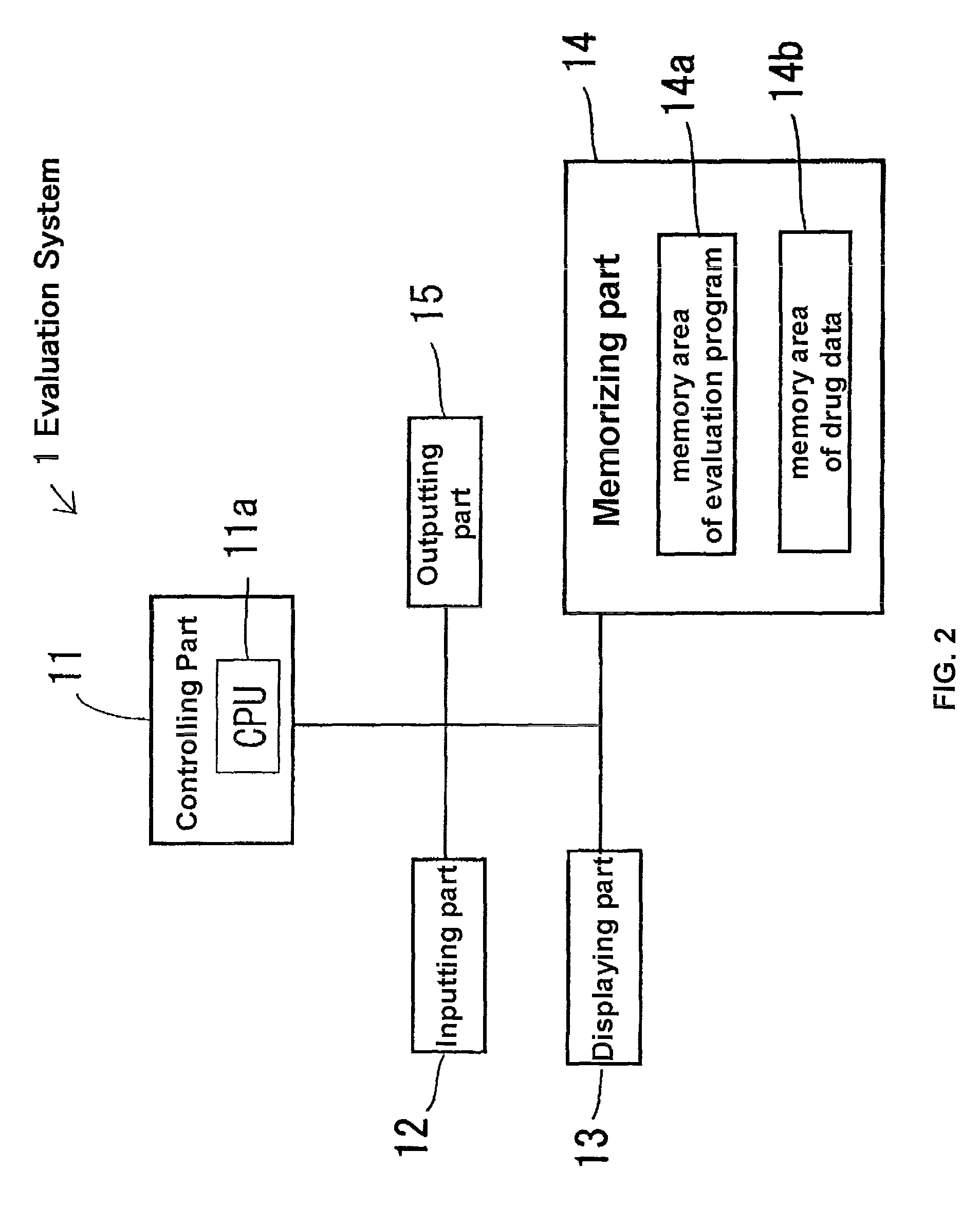
Early-stage information monitoring method and device for generic drug, electronic equipment and storage medium
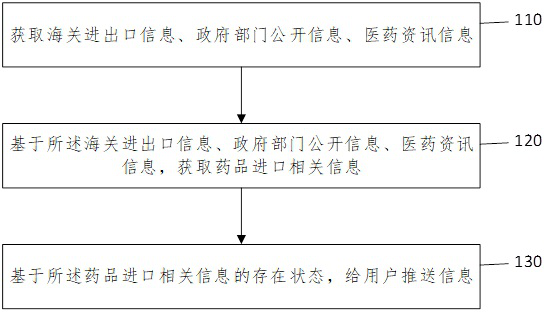
The invention provides an early-stage information monitoring method and device for generic drugs, electronic equipment and a storage medium. The method comprises the following steps: acquiring customs import and export information, government department public information and medicine information; based on the customs import and export information, the government department public information and the medicine information, obtaining medicine import related information; and pushing information to a user based on the existence state of the drug import related information. According to the method, the device, the electronic equipment and the storage medium provided by the invention, a user can be helped to obtain early-stage enterprise generic drug project approval information, high manpower, capital and time investment is saved, and the enterprises are guided to reasonably approve projects.
Owner:PHARMCUBE (BEIJING) CO LTD
Determination method of povidone k30 content by hplc
The invention discloses an HPLC determination method for povidone K30 content. In the determination method, a mixed solution of potassium nitrate-acetonitrile-water is used as a mobile phase, and molecular exclusion chromatography is used to determine the content of povidone K30 in a product to be tested. The method of the present application is simple, fast, accurate and sensitive, can accurately determine the content of povidone K30, can be applied to the reverse engineering research of povidone K30 in oral solid preparations, and is useful for generic drug development and consistency evaluation And bioequivalence studies provide supporting data to speed up the research and development process.
Owner:东莞西典医药科技有限公司
Quality testing methods for generic drugs
ActiveCN105372401BImprove accuracyHigh sensitivityTesting medicinal preparationsGeneric drugMedicine
The invention relates to the technical field of detection methods for drug quality, in particular to a detection method for the generic drug quality. The method comprises the steps that when two or more fitting parameters of a generic drug are out of a confidence interval of a brand-name drug, the quality of the generic drug is not similar to the quality of the brand-name drug; when three or more fitting parameters of the generic drug are within the confidence interval of the fitting parameters of the brand-name drug, the quality of the generic drug is similar to the quality of the brand-name drug. According to the detection method for the generic drug quality, the accuracy and sensitivity of a quality detection result can be improved, and therefore the quality of the generic drug can be detected; in addition, the detection method for the generic drug quality is applicable to quality detection of different drugs and drugs of different dosage forms, and therefore the application range of the detection method for the generic drug quality is enlarged.
Owner:中国人民解放军新疆军区联勤部药品仪器检验所
A method for measuring the dissolution profile of simvastatin tablets
ActiveCN108414656BImprove discriminationImprove stabilityComponent separationFluid phaseGeneric drug
Owner:四川省食品药品检验检测院
A kind of itopride hydrochloride microtablet and preparation method thereof
ActiveCN109125277BIncrease postprandial bioavailabilitySolve the problem of inconsistent dissolution profiles in vitroOrganic active ingredientsDigestive systemGeneric drugAbbott Laboratories
The invention discloses an itopride hydrochloride micro-tablet, a preparation method thereof and application thereof in consistency evaluation of generic drugs. According to the itopride hydrochloridemicro-tablet, the preparation method thereof and the application thereof in the consistency evaluation of the generic drugs, itopride hydrochloride and various ingredients are mixed, pelletized and dried, and then evenly mixed with an extra disintegrant and a lubricant, and the mixture is pressed into the micro-tablet of which the diameter is no more than 3MM. A capsule can be filled with the micro-tablet, multiple dissolution curves of a prepared itopride hydrochloride capsule in vitro are consistent with an original product (product name: itopride hydrochloride tablet; trade name: Weilisu;specification: 0.05g; certificate holding merchant: ABBOTT LABORATORIES (M) SDN. BHD), in the process of accelerated test and long-term test stability investigation, related substances have no significant change, and the micro-tablet has a bioequivalence trend with the original product after the meal.
Owner:珠海润都制药股份有限公司
A kind of irbesartan capsule and preparation method thereof
ActiveCN109157527BHigh Cmax valueQuick releaseOrganic active ingredientsPharmaceutical non-active ingredientsGeneric drugActive agent
Owner:珠海润都制药股份有限公司
Determination method for dissolution curve of ulipristal acetate solid preparation
The invention relates to the technical field of medicine quality control, in particular to a determination method for a dissolution curve of an ulipristal acetate solid preparation. The method comprises the following steps: by taking a solvent containing Tween 80 as a dissolution medium, detecting the accumulated dissolution rates of an ulipristal acetate solid preparation in the dissolution medium at different dissolution time points by adopting a paddle method in a stirring state, and then drawing a dissolution curve, wherein the volume percentage concentration of the Tween 80 in the dissolution medium is 0.1-2%. According to the method, the surfactant Tween 80 is added into the dissolution medium to evaluate the dissolution curve of the ulipristal acetate tablet, so that the quality of the ulipristal acetate tablet is scientifically and objectively evaluated, the method can be used for evaluating the quality consistency (similarity) of a generic drug and an original drug, and the quality consistency between batches of drugs can be guaranteed.
Owner:HENAN TAIFENG BIOTECH CO LTD
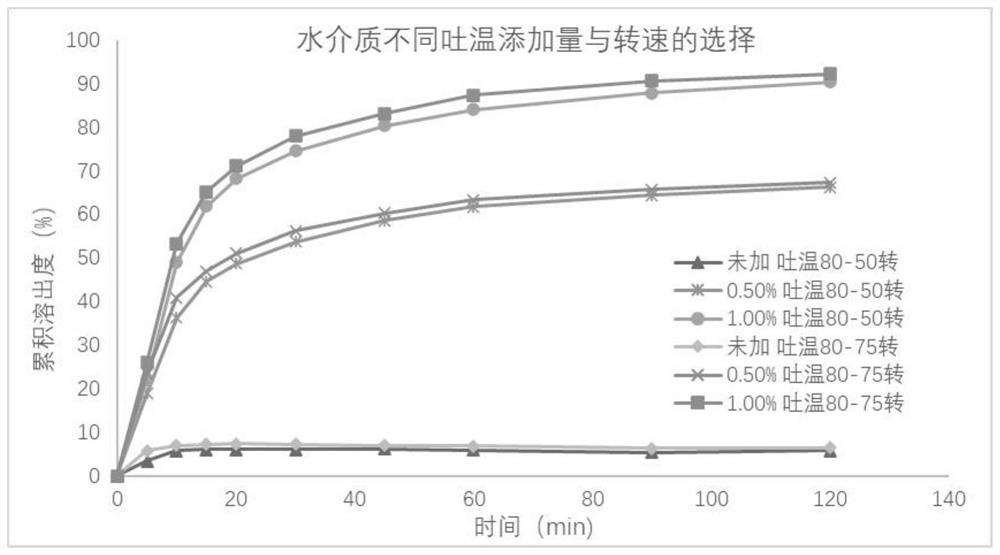
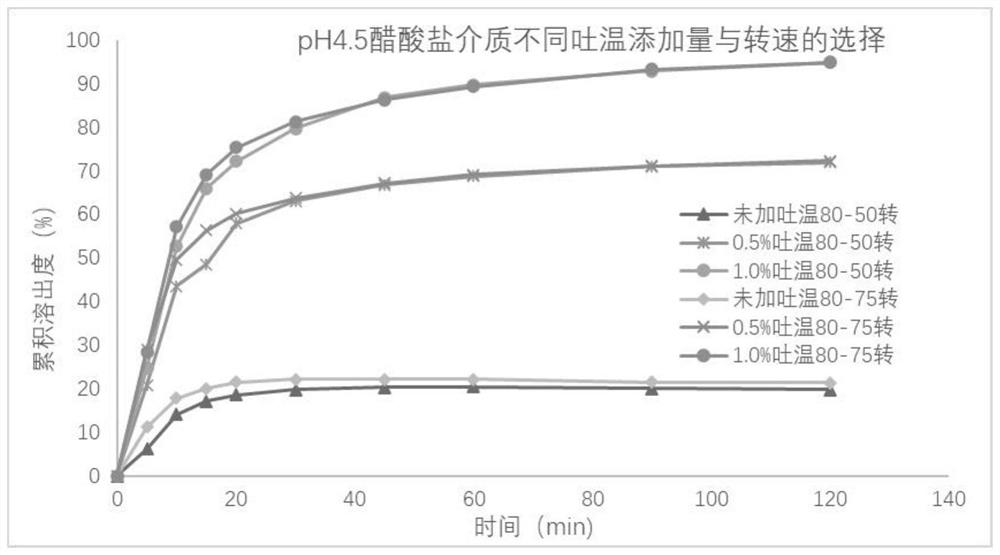
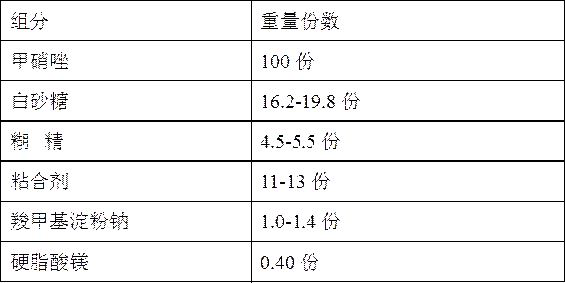
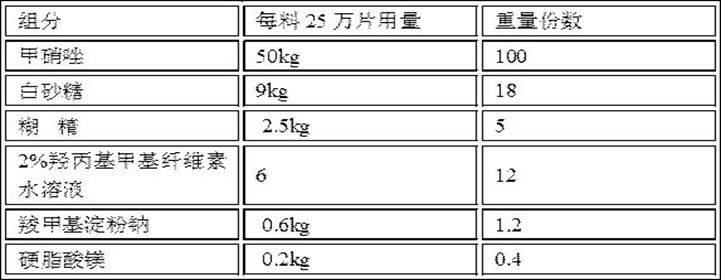
Metronidazole tablet composition with stable quality and preparation method thereof
ActiveCN112007002AGood curative effectDetermination of curative effectAntibacterial agentsOrganic active ingredientsGeneric drugCurative effect
The invention provides a metronidazole tablet composition with stable quality. A preparation method comprises the following steps: (1) preparation of raw materials and auxiliary materials; (2) mixingto prepare a soft material; (3) granulating; (4) drying; (5) size stabilization; (6) total mixing; and (7) tableting. The metronidazole tablet composition disclosed by the invention is definite in curative effect and stable in quality, and is consistent with reference listed drugs in quality and curative effects, and generic drugs can well replace the reference listed drugs; and the production process is simple, and the process tolerance is good.
Owner:河北君临药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com