Detection method for generic drug quality
A detection method and a technology for drug quality, applied in the direction of material inspection products, testing pharmaceutical preparations, etc., can solve problems such as detection result errors, and achieve the effect of improving accuracy and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
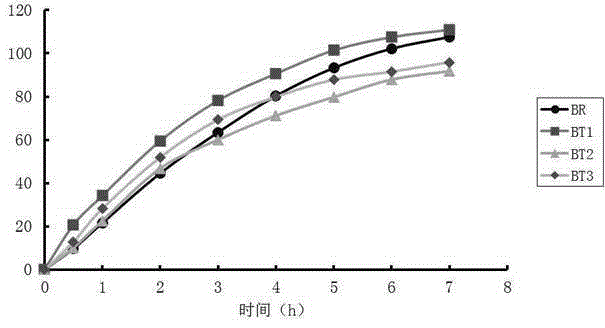
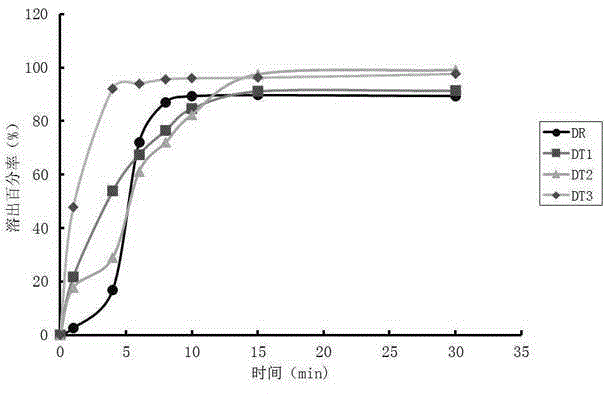
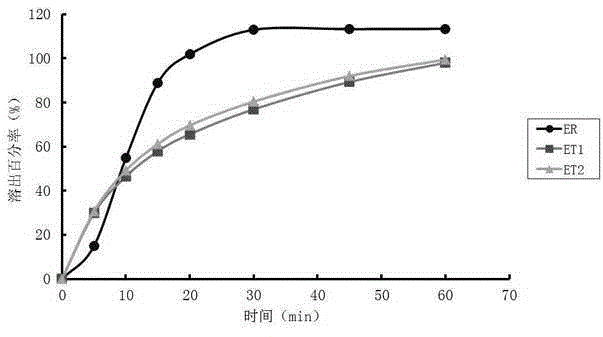
[0021] Embodiment 1: the detection method of this imitation medicine quality, carry out as follows: the first step, measure the cumulative stripping percentage of imitation medicine different time, time uses t i Indicates that the cumulative dissolution percentage of generic drugs corresponding to different times is represented by y i Indicates that i=1,2...n; the second step, using the t obtained in the first step i and y i Determine the mathematical model y=c-exp(-a (t-Ti) b ), the fitting parameters include c, α, Ti, b, and the values of c, α, Ti, and b are respectively c 0 、α 0 、Ti 0 , b 0 , put t i and y i using mathematical models After data fitting, the dissolution curve of the cumulative dissolution percentage of the generic drug is obtained; in the third step, the original drug is obtained in the mathematical model y=c-exp(-a (t-Ti) b The values of c, α, Ti, b in ) are respectively c 1 、α 1 、Ti 1 , b 1 , with the original drug in the mathematical mod...
Embodiment 2
[0022] Embodiment 2: As the optimization of the above-mentioned embodiment, the generic medicine is in the mathematical model y=c-exp(-a·(t-Ti) b The fitting parameter value in ) is obtained by the following method: the first step is to establish the .m file in matlab, and the mathematical model corresponding to the .m file is y=c-exp(-a (t-Ti) b ), call the nlinfit function in matlab, give c, α, Ti and b initial values, and the initial values of c, α, Ti and b are recorded as u=(c 2 ,α 2 , Ti 2 ,b 2 ); the second step, combining the initial values of c, α, Ti and b and t i Call the mathematical model in the .m file y=c-exp(-a (t-Ti) b ) to obtain the predicted value of the cumulative dissolution percentage, and the predicted value of the cumulative dissolution percentage is used Indicates that i=1,2...n, then, use the nonlinear least squares method to estimate the estimated value of the fitting parameter, and use the estimated value of the fitting parameter to Ind...
Embodiment 3
[0023] Example 3: As an optimization of the above example, the original research drug in y=c-exp(-a·(t-Ti) b ) in the fitting parameter value is obtained by the following method: the first step is to set up the .m file in matlab, and the mathematical model corresponding to the .m file is y=c-exp(-a (t-Ti) b ), call the nlinfit function in matlab, give c, α, Ti and b initial values, and the initial values of c, α, Ti and b are recorded as u=(c 2 ,α 2 , Ti 2 ,b 2 ); in the second step, the cumulative dissolution percentage of the original drug at different times and corresponding different times is recorded as (t i ,y i ), combining the initial values of c, α, Ti and b and t i Call the mathematical model in the .m file y=c-exp(-a (t-Ti) b ) to obtain the predicted value of the cumulative dissolution percentage, and the predicted value of the cumulative dissolution percentage is used Indicates that i=1,2...n, then, use the nonlinear least squares method to estimate th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com