PEGylated asparaginase and applications thereof
A technology of asparaginase and PEGylation, which is applied in the field of protein drugs, can solve the problems of easy shedding, reduced activity, and low yield, and achieve the effects of reducing immunogenicity, prolonging half-life, and firm binding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
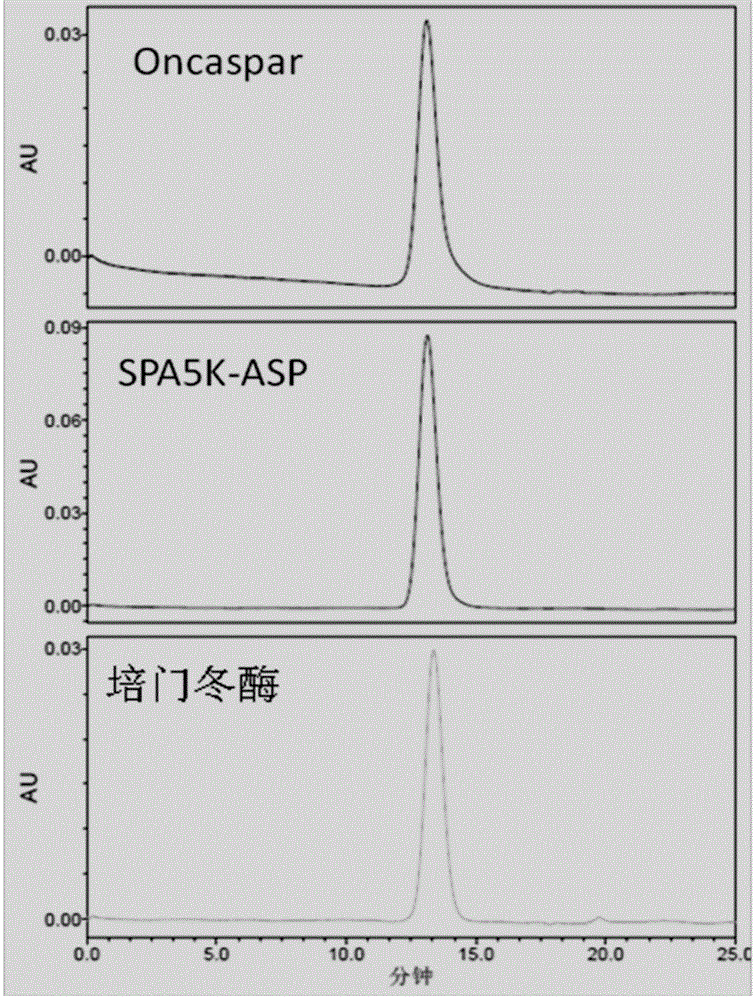
[0063] Example 1: Preparation and analysis of PEG conjugates of asparaginase
preparation example 1
[0064] Preparation example 1, pegylated asparaginase of the present invention is prepared, purified and identified by the following methods:
[0065] Step 1: Buffer Exchange
[0066] The freeze-dried powder of asparaginase was dissolved in 20 mM Tris-Hcl (pH 8.0) buffer solution to prepare a solution with a protein concentration of 5 mg / ml. Then the sample was loaded through the sample pump of the AKTA chromatography system, and the sample was sucked into the Q ion exchange column (purchased from GE Company, HiTrapQHP5mL). After loading the sample, equilibrate the chromatographic column with equilibration buffer A. After equilibrating for 5 column volumes, perform one-step elution with elution buffer B, and collect the elution peaks.
[0067] (A solution: 20mM phosphate buffer (pH8.0), B solution: 20mM phosphate buffer + 0.2M sodium chloride (pH7.5))
[0068] Step 2: Modification reaction and purification of modified products
[0069]Using M-SPA-5000 (purchased from Beijing...
Embodiment 2
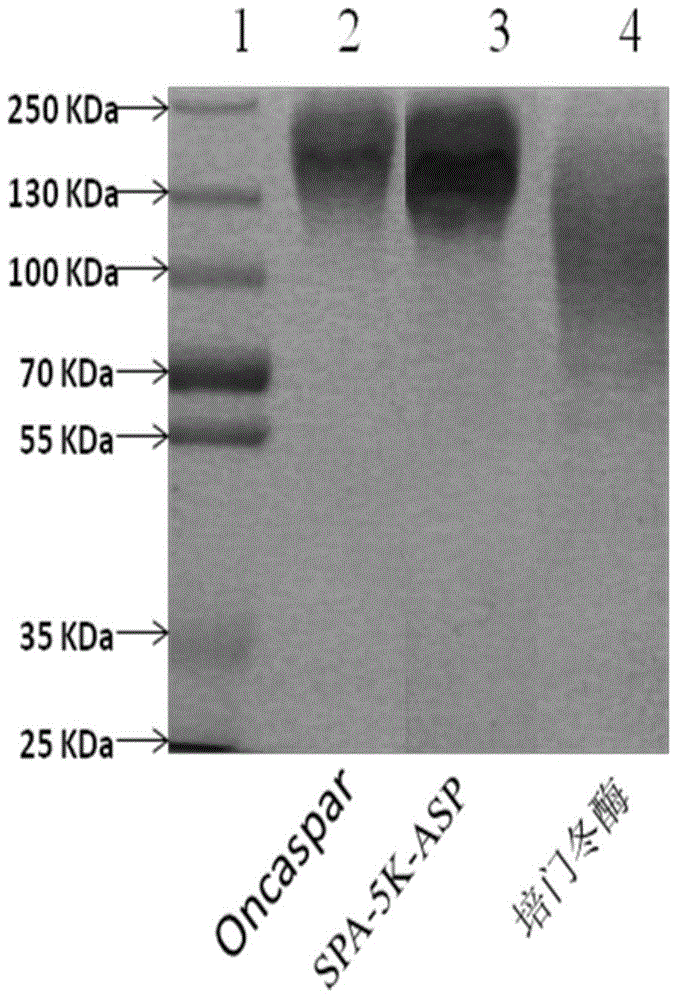
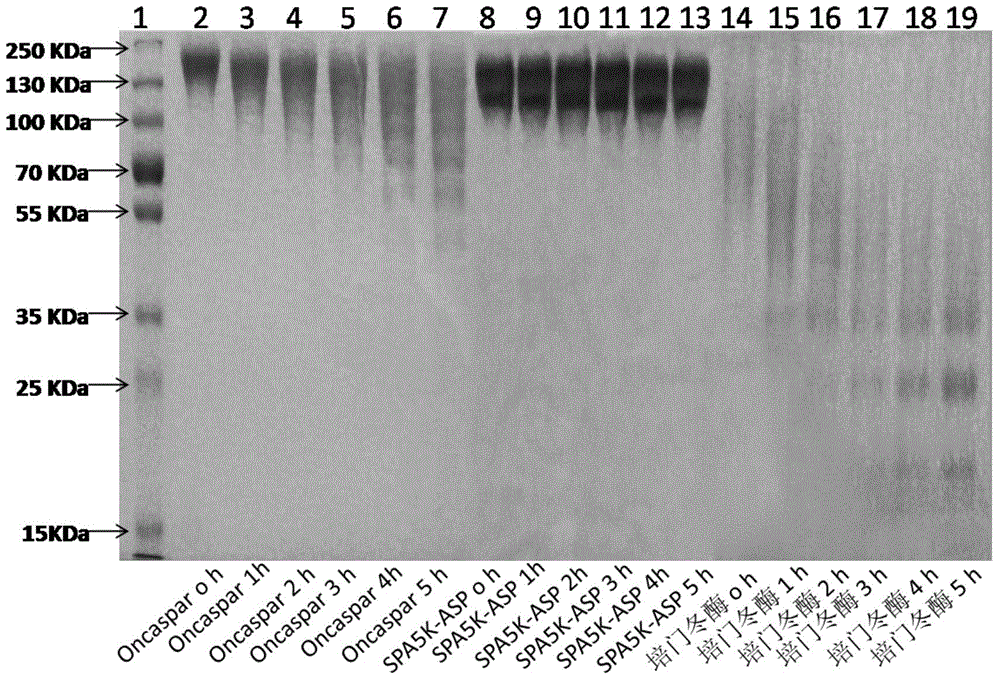
[0081] Example 2: Comparison of PEGylated ASP modification uniformity
[0082] We compared the modification uniformity of PEGylated ASP by SDS-PAGE electrophoresis. Protein stacking gel is 5%, separating gel is 8%. The stacking gel buffer is 0.5MTris-HCl buffer (pH6.8); the separating gel buffer is 1.5mol / LTris-HCl buffer (pH8.8). Take 10ug protein sample, mix it with sample buffer in equal volume, boil at 100°C for 5min, load the sample and run, and stain with Coomassie Brilliant Blue R250 (purchased from Sinopharm Group) after electrophoresis.
[0083] Depend on figure 2 It can be seen that compared with similar products on the market - Pegaspargase (purchased from Jiangsu Hengrui Medicine Co., Ltd.) and Oncaspar (purchased from SigmaTau Pharmaceutical Company), the electrophoresis of our modified product SPA5K-ASP and Oncaspar The band is relatively narrow, which shows that the modification uniformity of SPA5K-ASP and the original drug is slightly better than pegasparga...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com