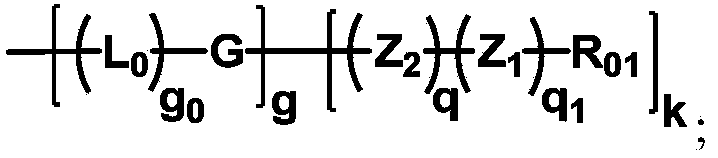Biologically related substances modified by multifunctional H-type polyethylene glycol derivative
A bio-related substance, polyethylene glycol technology, applied in the field of polymer synthesis, can solve problems such as failure to meet biological safety requirements, decreased drug activity, limited application range, etc., to improve pharmacokinetics or tissue distribution, Weaken the effect, reduce the effect of immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
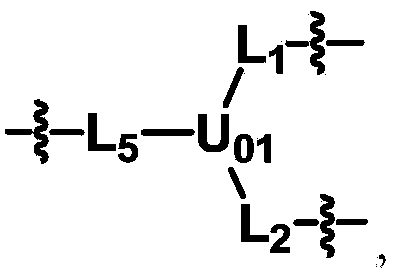
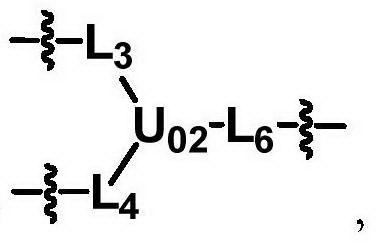
[0118] In the preparation method part of the present invention, dotted lines are used in the structural formulas of some skeleton groups to indicate that the skeletons will be directly connected to the groups shown in the structural formulas in the specified compounds.
[0119] In the present invention, the ring structure is represented by a circle, and different labels are given according to the difference of the ring structure. For example,
[0120] represents any ring structure;
[0121] Represents an aliphatic ring structure without any aromatic or heteroaromatic rings, also known as aliphatic rings;
[0122] Represents an aromatic ring structure, containing at least one aromatic ring or heteroaromatic ring, also known as an aromatic ring;
[0123] Indicates the skeleton of sugars or sugar derivatives containing cyclic monosaccharide skeletons, also known as sugar rings;
[0124] Indicates a ring containing amide bonds, ester bonds, imides, acid anhydrides and...
Embodiment 1
[1148] Example 1: Preparation of interferon α-2a modified by polyfunctional H-type polyethylene glycol succinimide active ester derivative (amide bond connection)
[1149] Add 645 mg (about 2 times the molar ratio) of polyfunctional H-type polyethylene glycol succinimide active ester derivative (A1-1, molecular weight about 26 kDa) into a dry and clean 50 mL round bottom flask, nitrogen protection, add PBS The pH value of the buffer was adjusted to pH=8.0, and 7.5 mL of interferon α-2a (NH 2 - IFN) in PBS buffered saline solution, shake and react at 25°C for 4 hours, shake and react at 4°C for 12 hours, add 1.5ml of glycine solution to terminate the reaction. The concentration of interferon α-2a was diluted to 0.5 mg / mL with PBS buffered saline solution with pH=8.0, and then purified by agarose gel exchange resin, and the single-modified and double-modified components were collected respectively, and concentrated by ultrafiltration. MALDI-TOF-MS test showed that the molecular...
Embodiment 2
[1156] Example 2: Preparation of recombinant human granulocyte colony-stimulating factor (rhG-CSF) modified by multifunctional H-type polyethylene glycol aldehyde derivatives (secondary amine linkage)
[1157] Add 600mg (about 1.5 times the molar ratio) of multifunctional H-type polyethylene glycol aldehyde derivatives (D5-1, molecular weight about 20kDa) into a dry and clean 50mL round bottom flask, nitrogen protection, add PBS buffer solution to adjust the pH Adjust pH value to 5.0, add 7.6mL rhG-CSF PBS buffered saline solution, react at room temperature for 4 hours, add sodium cyanoborohydride, react at room temperature for about 24 hours, add saturated ammonium chloride solution to quench, dilute with water to protein The concentration is about 0.1mg / mL. Use dilute hydrochloric acid to adjust the pH value to about 5.0, and use Resource S ion-exchange column chromatography to elute with a gradient of 0-0.5mol / L NaCl solution (containing 20mmol / L NaAc, pH5.0) to collect sin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com