Metronidazole tablet composition with stable quality and preparation method thereof
A technique for metronidazole tablet and stable quality, applied in the field of metronidazole tablet composition and its preparation, capable of solving the problems of slow dissolution and large influence of dissolution rate and particle size, achieving definite curative effect, consistent quality and curative effect , the effect of stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
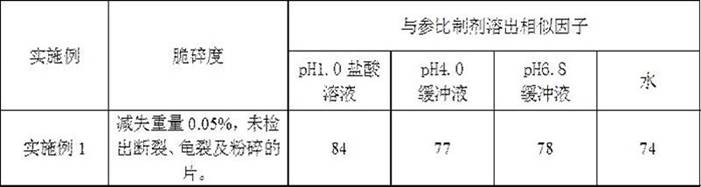
Examples
Embodiment 1
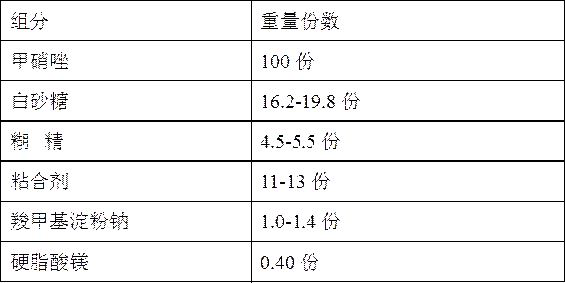
[0049] Embodiment 1: Metronidazole tablet composition
[0050] Prescription composition:
[0051]
[0052] Preparation:
[0053] (1) Preparation of raw and auxiliary materials: Metronidazole is passed through a 100-mesh sieve, white sugar is passed through a 80-mesh sieve, dextrin is passed through a 120-mesh sieve, and a 2% aqueous solution of hydroxypropyl methylcellulose is prepared. The apparent viscosity is, The viscosity is 6mPa•s;
[0054] (2) Mixing and preparation of soft materials:
[0055] ①Put metronidazole, white sugar, and dextrin in a tank mixer and dry mix for 10 minutes at a stirring rate of 200rpm and a shear rate of 800rpm;
[0056] ② The stirring rate is 200rpm, the shear rate is 800rpm, and the binder is added within 6 minutes;
[0057] ③ The stirring rate is 200rpm, the shear rate is 800rpm, stirring for 14 minutes to make a suitable soft material and then discharging;
[0058] (3) Granulation: Granulate with a 18-mesh nylon sieve swinging granula...
Embodiment 2
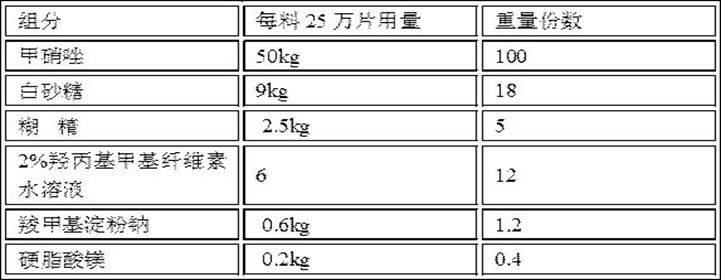
[0066] Embodiment 2: Metronidazole tablet composition
[0067] Prescription composition:
[0068]
[0069] Preparation:
[0070] (1) Preparation of raw and auxiliary materials: Metronidazole is passed through a 100-mesh sieve, white sugar is passed through a 80-mesh sieve, dextrin is passed through a 120-mesh sieve, and a 2% aqueous solution of hydroxypropyl methylcellulose is prepared. The apparent viscosity is, The viscosity is 6mPa•s;
[0071] (2) Mixing and preparation of soft materials:
[0072] ①Put metronidazole, white sugar, and dextrin in a tank mixer and dry mix for 10 minutes at a stirring rate of 200rpm and a shear rate of 800rpm;
[0073] ② The stirring rate is 200rpm, the shear rate is 800rpm, and the binder is added within 6 minutes;
[0074] ③ The stirring rate is 200rpm, the shear rate is 800rpm, stirring for 14 minutes to make a suitable soft material and then discharging;
[0075] (3) Granulation: Granulate with a 18-mesh nylon sieve swinging granula...
Embodiment 3
[0083] Embodiment 3: Metronidazole tablet composition
[0084] Prescription composition:
[0085]
[0086] Preparation:
[0087] (1) Preparation of raw and auxiliary materials: Metronidazole is passed through a 100-mesh sieve, white sugar is passed through a 80-mesh sieve, dextrin is passed through a 120-mesh sieve, and a 2% aqueous solution of hydroxypropyl methylcellulose is prepared. The apparent viscosity is, The viscosity is 6mPa•s;
[0088] (2) Mixing and preparation of soft materials:
[0089] ①Put metronidazole, white sugar, and dextrin in a tank mixer and dry mix for 10 minutes at a stirring rate of 200rpm and a shear rate of 800rpm;
[0090] ② The stirring rate is 200rpm, the shear rate is 800rpm, and the binder is added within 6 minutes;
[0091] ③ The stirring rate is 200rpm, the shear rate is 800rpm, stirring for 14 minutes to make a suitable soft material and then discharging;
[0092] (3) Granulation: Granulate with a 18-mesh nylon sieve swinging granula...
PUM
| Property | Measurement | Unit |
|---|---|---|
| shear viscosity | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| shear viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com