Itopride hydrochloride micro-tablet and preparation method thereof
A technology of itopride hydrochloride and micro-tablets, which can be used in pharmaceutical formulas, medical preparations containing active ingredients, pill delivery, etc., can solve problems such as low Cmax and Tmax delay, and achieve the effect of improving postprandial bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 Preparation of Itopride Hydrochloride Microtablets (Specification: 0.05g)
[0065] As shown in Table 2, according to the type of material designed by the prescription, the bulk drug and auxiliary materials are vibrated through a 60-mesh sieve. The auxiliary materials include fillers, internally added disintegrants, surfactants, binders, externally added disintegrants, lubricants, and the raw materials, fillers, internally added disintegrants and The surfactant is placed in a fluidized bed one-step granulation coating machine, the fan is turned on, and mixed for 5 minutes. Weigh the binder according to the design amount of prescription 1-5, and prepare hypromellose binder solution or povidone K29 binder solution with 75% ethanol. Spray the adhesive evenly on the boiling material in the form of top spray. Set the material temperature to 50°C, dry the itopride hydrochloride granules in a fluidized dryer until the water content is not higher than 2%, and collect...
Embodiment 2
[0067] Example 2 Preparation of Itopride Hydrochloride Capsules (Specification: 0.05g)
[0068] Get the stomach-soluble film coating material, dissolve it in purified water, and prepare a solution with a solid content of about 6%. The itopride hydrochloride microchips prepared by Example 1 prescription 1 ~ 5 were collected, and prepared by 3% weight gain. weighing. Put the compressed microtablets in a non-porous coating pan for coating, control the air inlet temperature at 60-70 degrees, the material temperature at 40-50 degrees, the weight gain at 1-3%, and the moisture at 2 Within %. The coated chips prepared above were filled in No. 4 ordinary gelatin capsule shells to prepare samples A, B, C, D, and E.
Embodiment 3
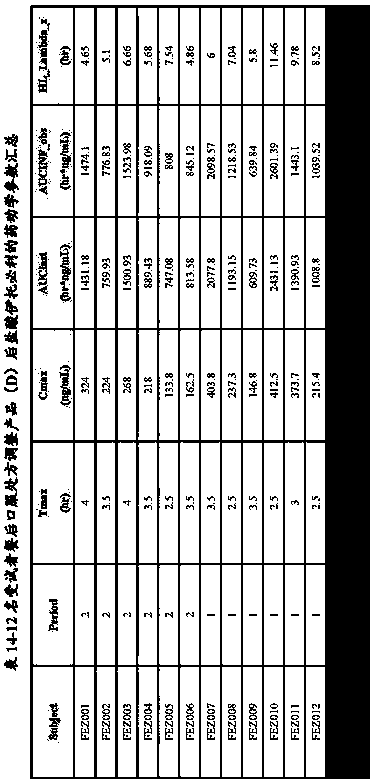
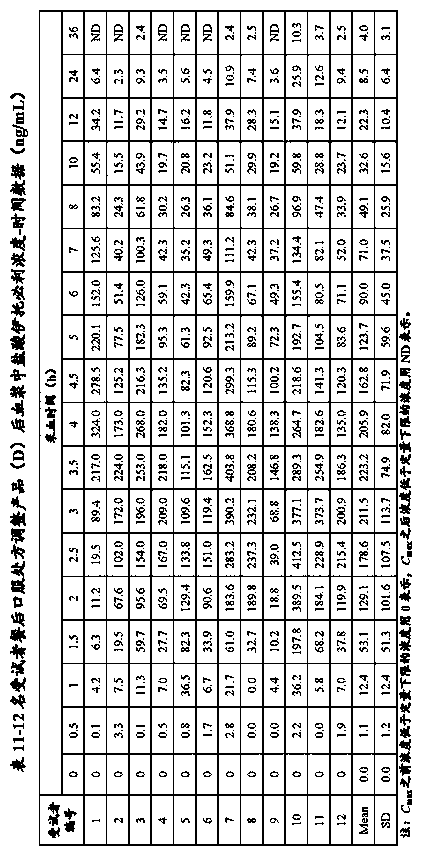
[0072] Example 3 Comparison of the in vitro dissolution curves of itopride hydrochloride capsules (A, B, C, D, E) prepared by the present invention, existing products and the original research product (R)
[0073] Take itopride hydrochloride capsules (A, B, C, D, E) prepared in Example 2, 12 capsules or tablets each of the existing product and the original research product (R), and detect its hydrochloric acid at pH 1.0 according to the following method Solution, acetate buffered saline solution at pH 4.0, phosphate buffered saline at pH 6.8 and in vitro dissolution profiles in water. See Table 3 for prescriptions.
[0074]
[0075] The measurement results are shown in Tables 4, 5, 6, and 7.
[0076] Table 4 - The average cumulative dissolution test results of itopride hydrochloride capsules (A, B, C, D, E) and original research product R in hydrochloric acid solution at pH 1.0 (n=12)
[0077]
[0078] Table 5 - The average cumulative dissolution test results of itopri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com