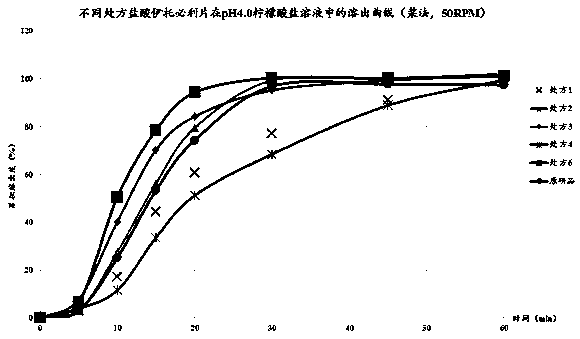
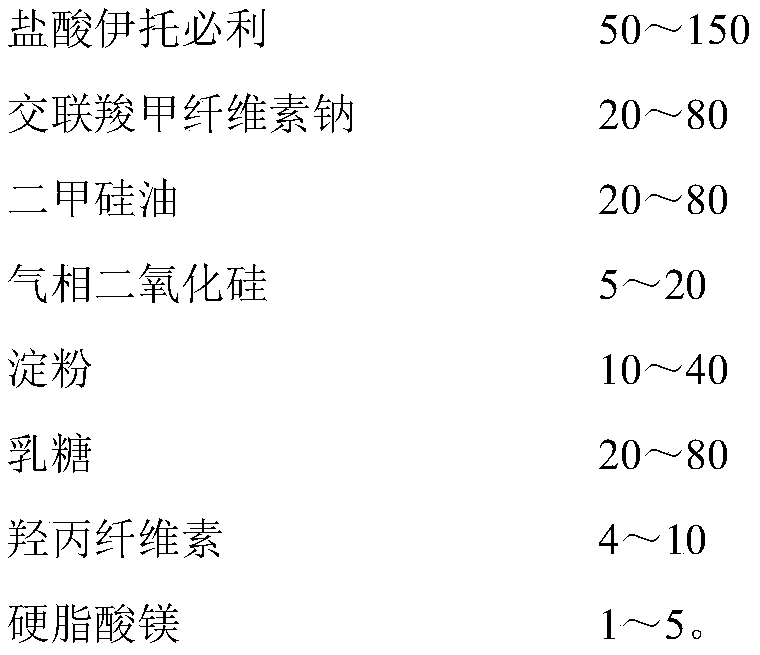
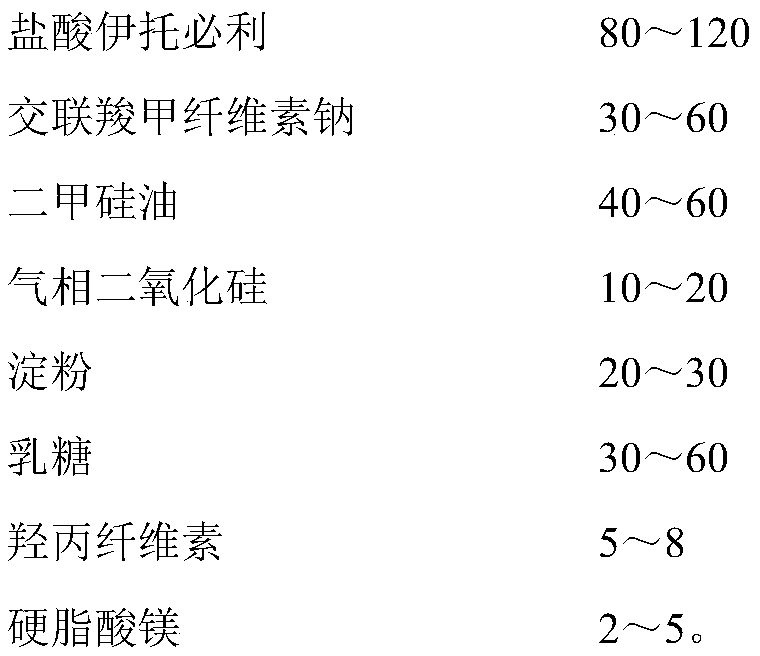
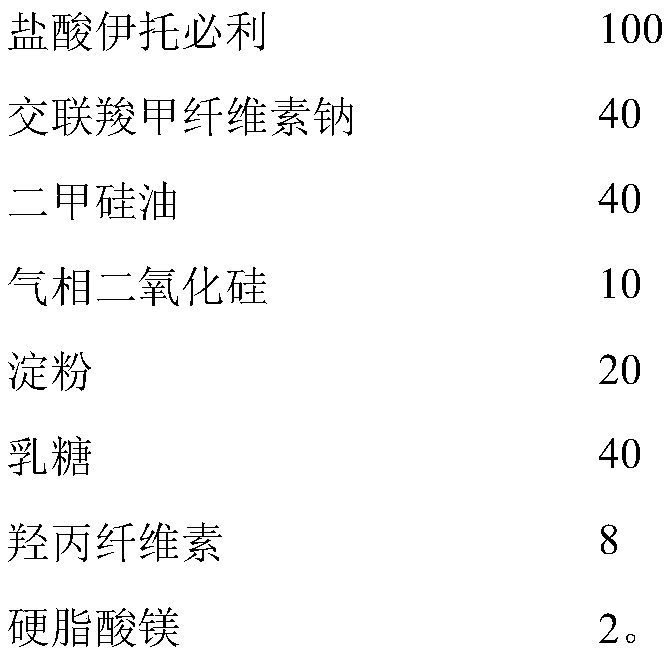
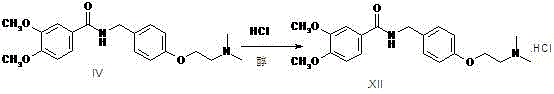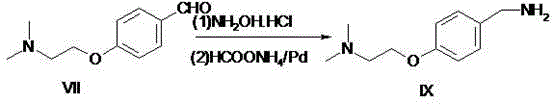Patents
Literature
30 results about "ITOPRIDE HYDROCHLORIDE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Itopride Hcl is used for the treatment, control, prevention, & improvement of the following diseases, conditions and symptoms: Functional dyspepsia Gastrointestinal conditions Itopride Hcl may also be used for purposes not listed here.
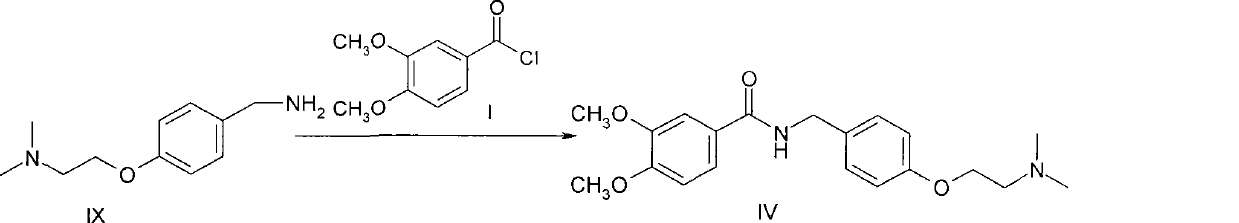
Preparation method of itopride hydrochloride
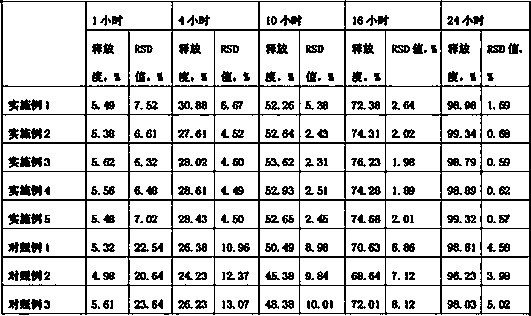
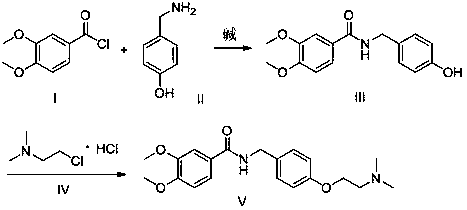
ActiveCN102993038ALow priceEasy to buyOrganic compound preparationCarboxylic acid amides preparationBenzoyl chlorideEther
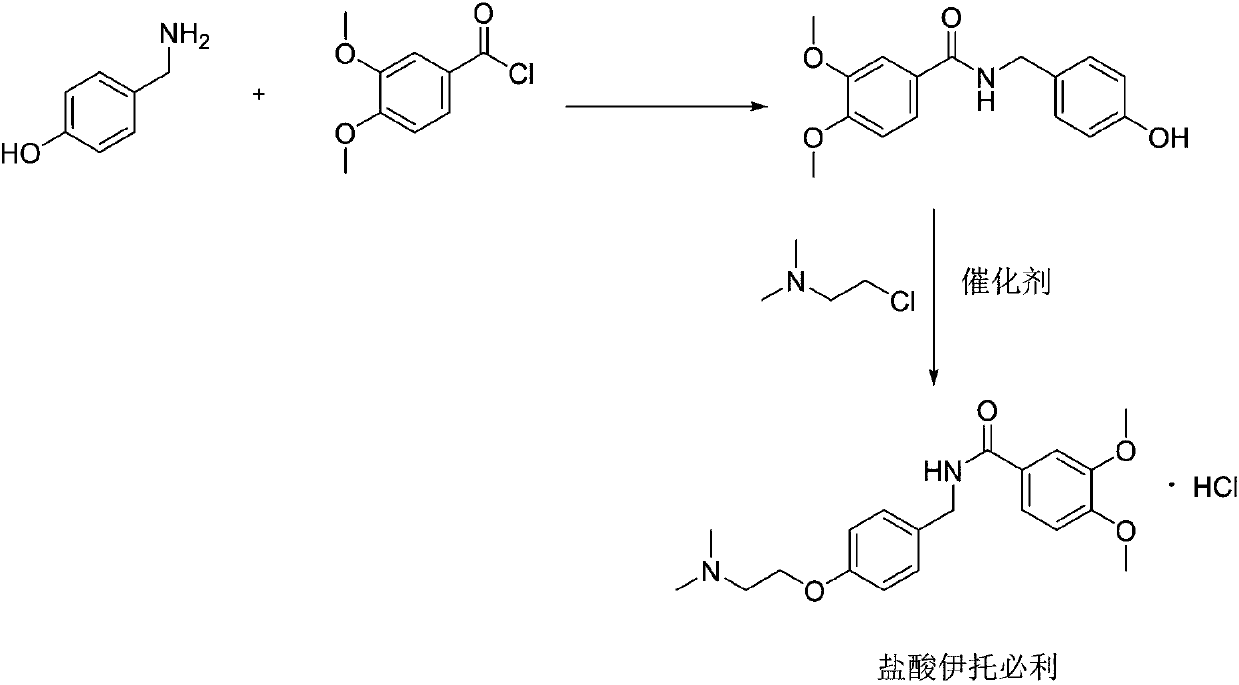
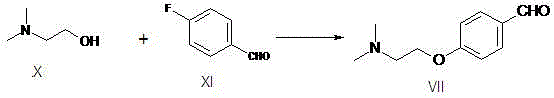
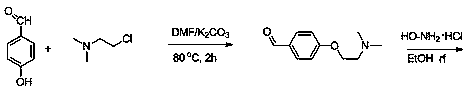
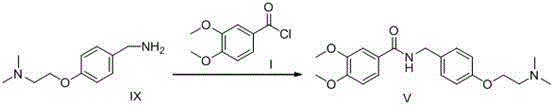
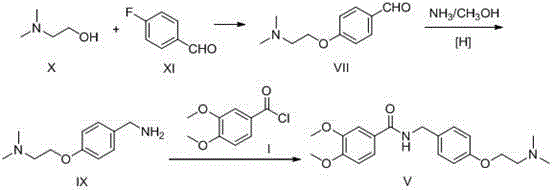
The invention relates to a preparation method of gastrointestinal crude drug itopride hydrochloride, and belongs to the field of medicines. The invention discloses a method for preparing itopride hydrochloride, which comprises the following steps of: taking cheap N,N-dimethylaminoethanol as an initial raw material; implementing an etherification reaction to obtain an intermediate product (VII) and implementing one-step reduction ammoniation to obtain a benzylamine product (IX); and reacting with 3,4-dimethoxy benzoyl chloride to generate hydrochloride. The preparation method of the itopride hydrochloride is cheap in raw material, moderate in reacting condition, and low in preparation cost of the itopride hydrochloride.
Owner:迪嘉药业集团股份有限公司
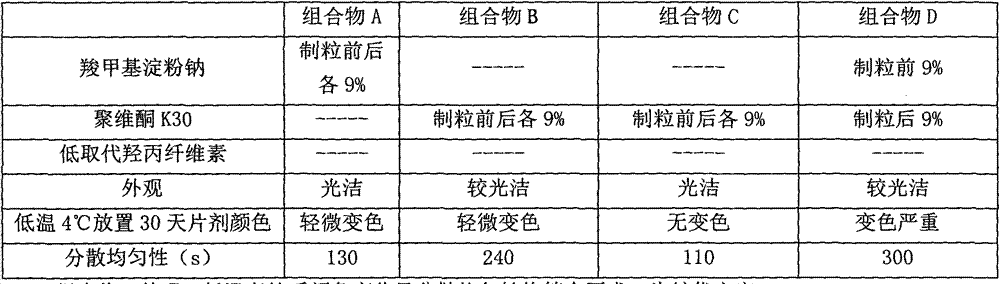
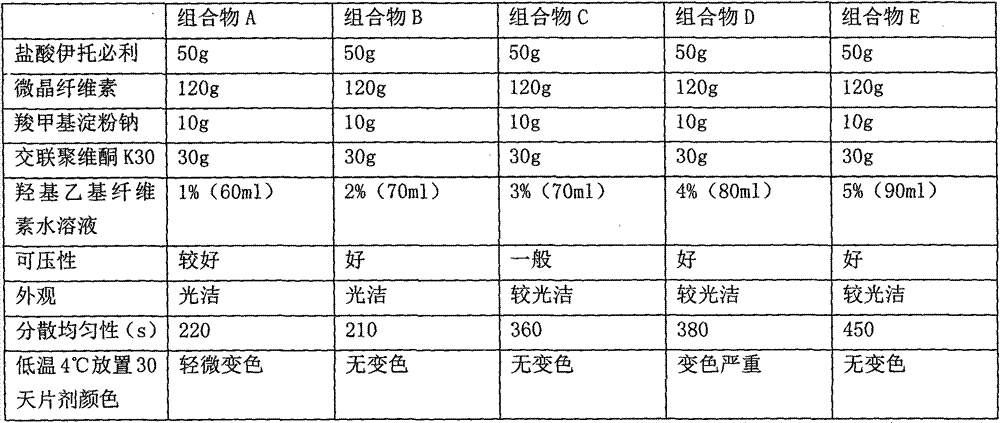
Itopride hydrochloride oral preparation
InactiveCN104116718AOvercome the problem of rough appearance and easy crackingSolve the problem of hygroscopicityOrganic active ingredientsDigestive systemMedicineDissolution
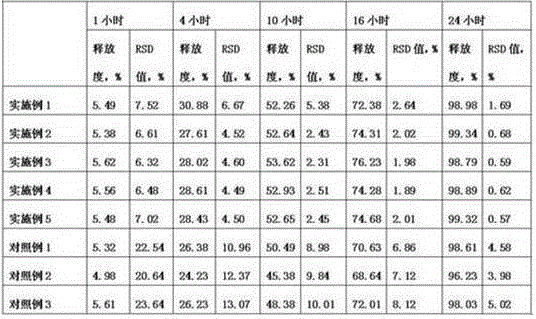
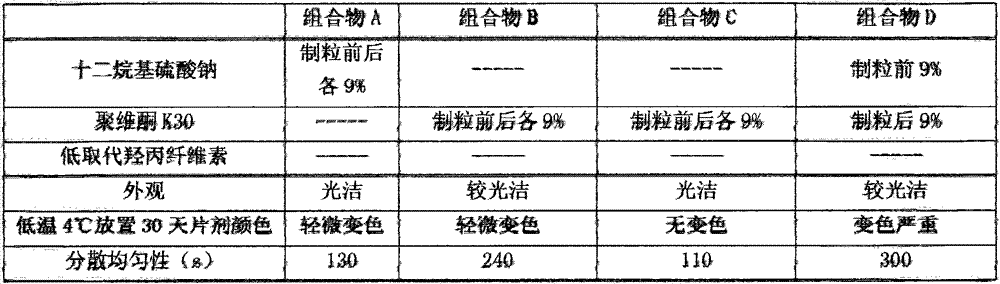
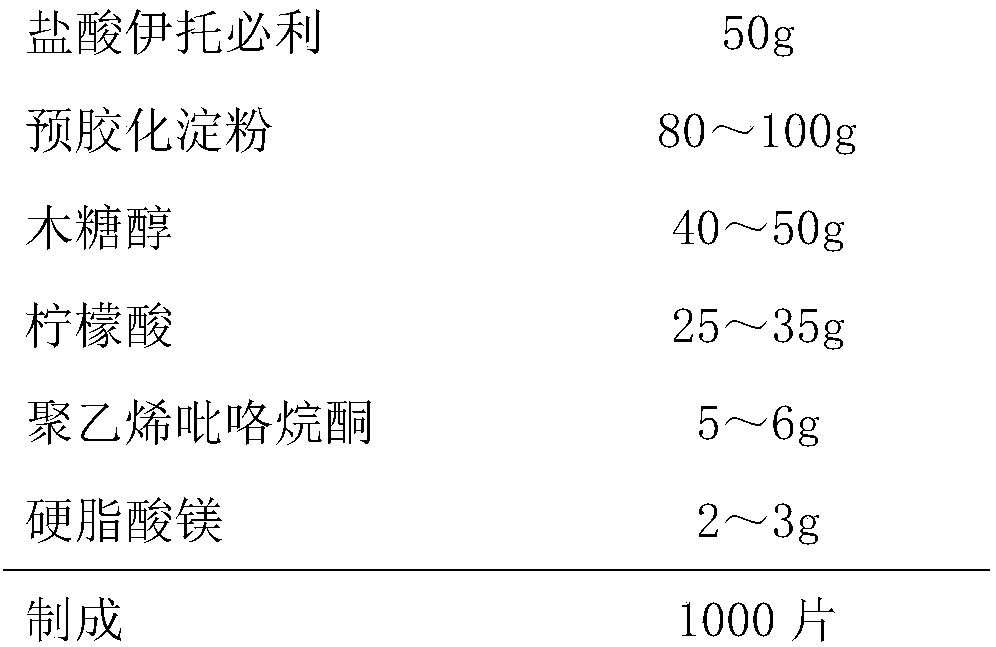
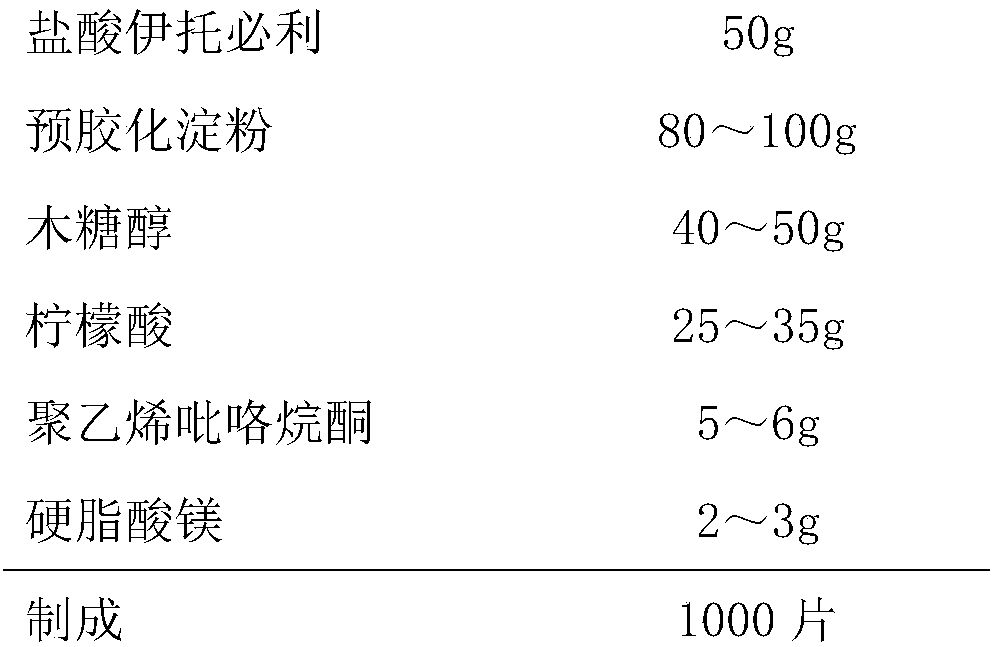
The invention discloses an itopride hydrochloride oral preparation. The oral preparation comprises 15-20 percent by weight of itopride hydrochloride and 10-30 percent by weight of a disintegrating agent, wherein the disintegrating agent is povidone. The oral preparation is used for overcoming the problems that a tablet is rough in appearance and easy to fracture, the tablet which is rough in appearance easily absorbs moisture and the color is changed in regions at low air temperature in winter and the like, is short in disintegrating time, good in dispersion uniformity, rapid in medicine dissolution, rapid in absorption, high in bioavailability, convenient and flexible to take and also good in taste and has the advantage that the compliance of patients for taking the medicine is improved.
Owner:LIVZON GROUP LIVZON PHARMA FACTORY
Preparation method of medicine of itopride hydrochloride for promoting gastrointestinal motility
InactiveCN106748862ACheap and easy to getHigh purityCarboxylic acid nitrile preparationOrganic compound preparationBenzoyl bromideMotility
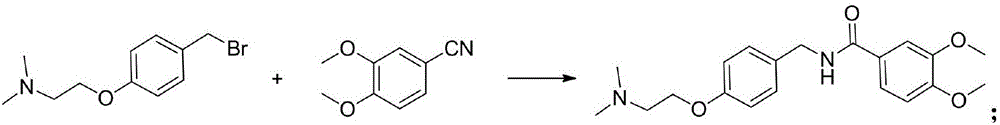
The invention discloses a preparation method of medicine of itopride hydrochloride for promoting gastrointestinal motility. The method comprises the following steps that p-hydroxy methylbenzene is used as a raw material to take a reaction with dimethylaminoethyl chloride hydrochloride; then, 4-(2-dimethylamino oxethyl) benzyl bromide is synthesized through bromination; next, the 4-(2-dimethylamino oxethyl) benzyl bromide and 3,4-dimethoxybenzonitrile take a reaction to obtain a product of itopride under the solvent-free condition through copper trifluoromethanesulfonate catalysis. The preparation method has the advantages a bran-new synthesis route is provided; the itopride is synthesized through Ritter reaction under the solvent-free condition; the advantage of green and environment-friendly effects is realized; meanwhile, the used raw material resources are wide and sufficient; the price is low; the reaction conditions are mild.
Owner:IANGSU COLLEGE OF ENG & TECH
Preparation method of itopride hydrochloride
ActiveCN103073446AIncrease lossLow yieldOrganic compound preparationCarboxylic acid amide separation/purificationBenzoyl chlorideITOPRIDE HYDROCHLORIDE
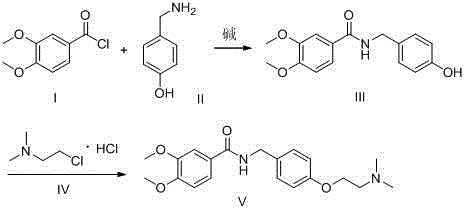
The invention provides a preparation method of itopride hydrochloride. The method comprises a step that N,N-dimethylamino ethoxy aniline and 3,4-dimethoxy benzoyl chloride are subjected to an amidation reaction in dichloromethane. The method provided by the invention also comprises a step that a hydrogen chloride isopropyl alcohol solution and an itopride prototype material are subjected to a reaction, such that a salt is produced. According to the itopride hydrochloride preparation method provided by the invention, dichloromethane is used for replacing toluene, such that the harm brought by toluene is avoided. Also, no water is introduced during the salt formation process, such that loss can be reduced, product yield can be improved, and impurities are further removed. Therefore, the quality of a finished product is improved.
Owner:珠海保税区丽珠合成制药有限公司 +1
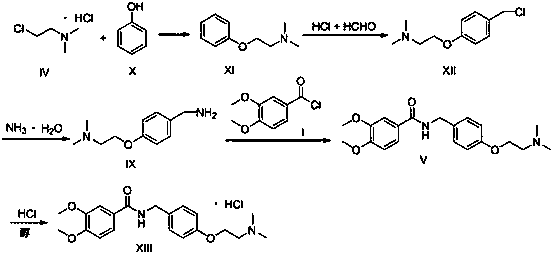
Preparation method of itopride hydrochloride
ActiveCN105985257AAvoid the restore stepLow priceOrganic compound preparationCarboxylic acid amides preparationITOPRIDE HYDROCHLORIDEPhenol
The invention relates to a preparation method of itopride hydrochloride. The preparation method includes: using 2-dimethylaminoethyl chloride hydrochloride and phenol as starting materials for reaction; subjecting the starting materials to etherification, chloromethylation, amino substitution, amidation and salifying to obtain itopride hydrochloride. Chloromethylation promoted by C / CHO is adopted, so that a step of imine reduction is omitted, solid residue generated by a reductant is eliminated, and reaction safety is improved; raw materials used in the method are low in price, sufficient in market supply and easy to purchase; reaction in each step is classic, and the preparation method is safe, easy to control and suitable for industrial production.
Owner:迪嘉药业集团股份有限公司
Method for detecting related substances in itopride hydrochloride preparation
ActiveCN113009003AEfficient detectionUnresolved peak anomaliesComponent separationPhysical chemistryMonopotassium phosphate
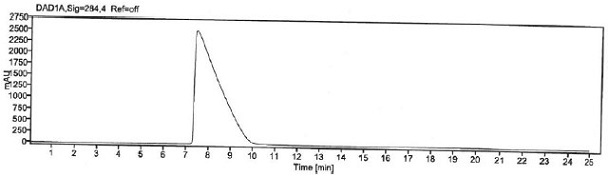
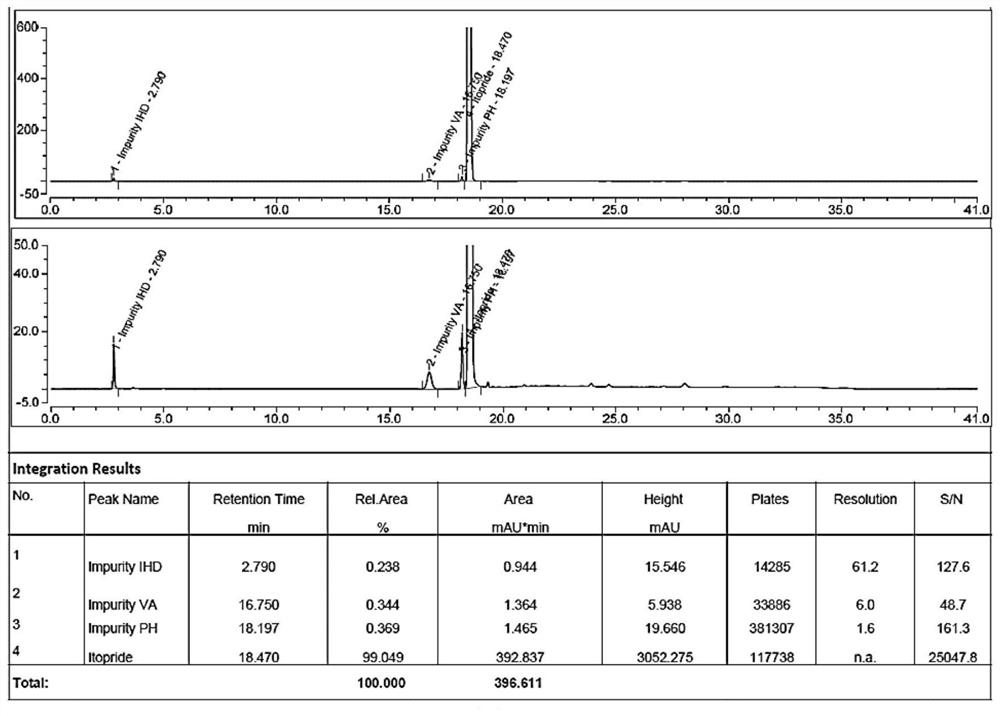
The invention provides an improved method for detecting related substances in an itopride hydrochloride preparation, which is characterized in that a monopotassium phosphate solution is used as a mobile phase A, a 90% acetonitrile solution is used as a mobile phase B to carry out gradient elution, the detection wavelength is 223 nm, the volume ratio of the monopotassium phosphate solution to the acetonitrile solution in a blank solution is 85:15, the separation degree of the itopride, the impurity VA, the impurity IHD and the impurity PH peak is more than 1.2, the abnormal phenomenon of no peak appearance during the continuous test of a plurality of samples does not exist, the method verification results show that the method meets the related requirements, the method meets the detection requirements, and the related substances in the itopride hydrochloride preparation can be effectively detected.
Owner:珠海润都制药股份有限公司
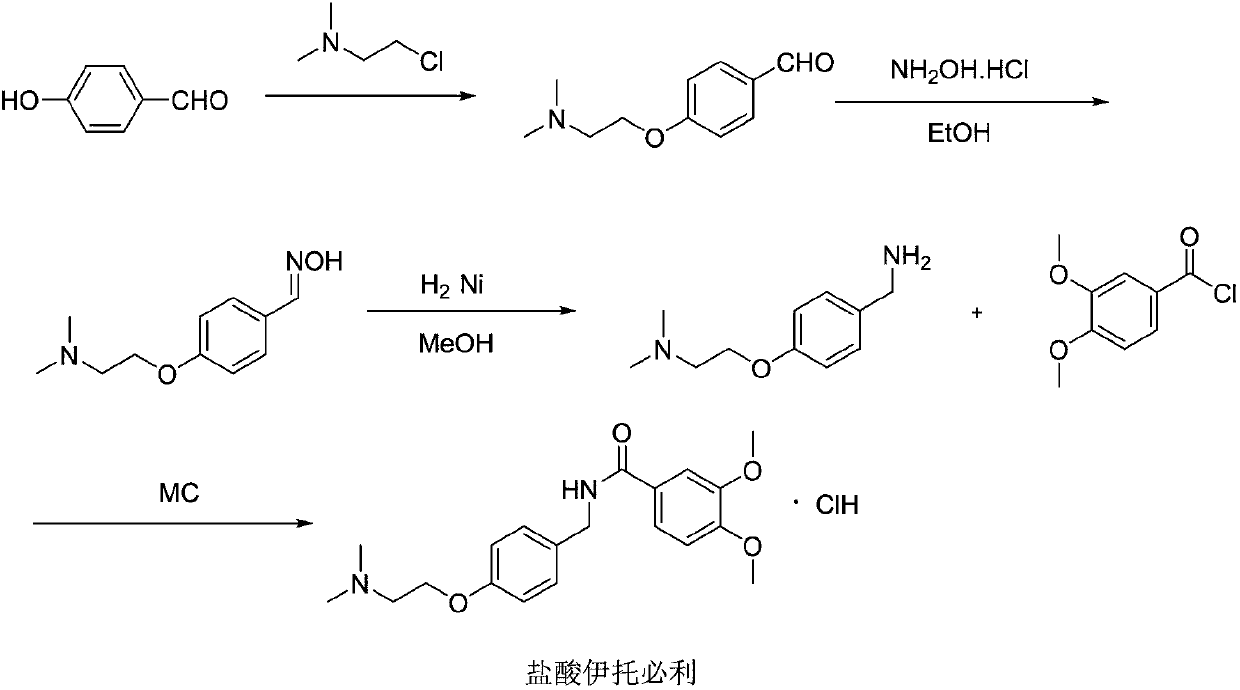
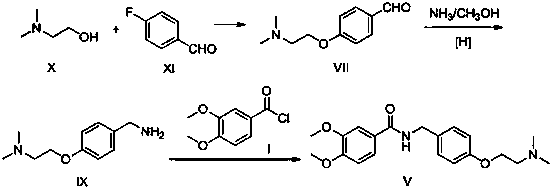
Method for preparing 4-[2-(dimethylamino)ethoxy]benzylamine as itopride-hydrocloride salt mediate
InactiveUS20090203940A1High purityHigh yieldOrganic compound preparationAmino-hyroxy compound preparationToxic gasMetal catalyst
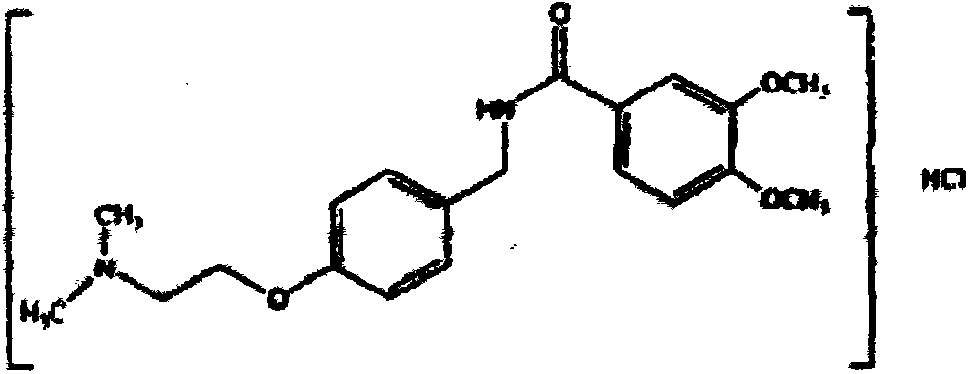
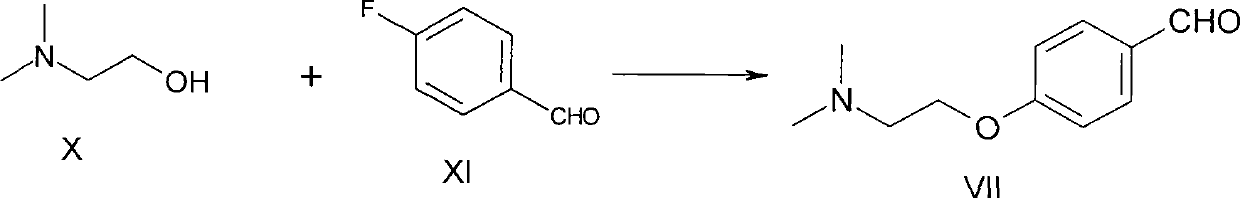
The present invention relates to a novel method for preparing an itopride-hydrochloride mediate of the formula 1, which is useful for digestive tract activator, and more particularly, the present invention provides with a method for preparing 4-[2-(dimethylamino)ethoxy]benzylamine of the formula 1, an itopride-hydrochloride mediate, whereby being capable of manufacturing in a high yield and lowering cost through a simple purification and a selective reaction, and the method is harmless and safe to human and environment due to not generating toxic gas. And specially, a super-high pressure hydrogenation and a reduction reaction using a metal catalyst are not carried out, therefore, it is very safe method, and also special manufacturing equipments are not needed.
Owner:IL YANG PHARMA CO LTD
Itopride hydrochloride sustained release preparation and preparation method thereof
InactiveCN110898023AReduce efficacyOrganic active ingredientsDigestive systemMagnesium stearateITOPRIDE HYDROCHLORIDE
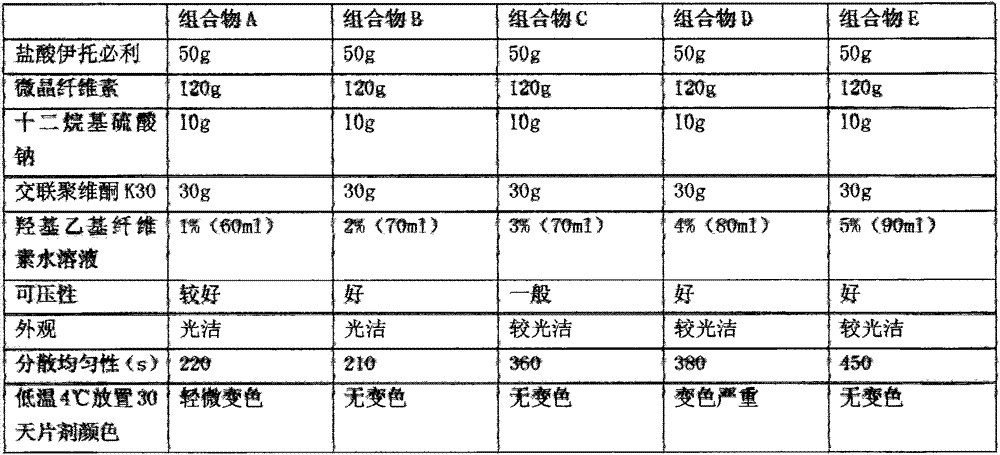
The invention discloses a itopride hydrochloride sustained release preparation which is prepared from the following components in parts by weight: 50 to 100 parts of itopride hydrochloride, 40 to 50 parts of hydroxypropyl methylcellulose K100, 20 to 40 parts of hydroxypropyl methylcellulose E5, 20 to 30 parts of microcrystalline cellulose, 20 to 30 parts of lactose and 1 to 2 parts of magnesium stearate. The invention further discloses a preparation method thereof. The preparation method has the beneficial effects that the preparation is convenient, and the effective components of prepared tablets are slowly released.
Owner:CHENGDU HENGRUI PHARMA
Itopride hydrochloride micro-tablet and preparation method thereof
ActiveCN109125277AIncrease postprandial bioavailabilitySolve the problem of inconsistent dissolution profiles in vitroOrganic active ingredientsDigestive systemGeneric drugAdditive ingredient
The invention discloses an itopride hydrochloride micro-tablet, a preparation method thereof and application thereof in consistency evaluation of generic drugs. According to the itopride hydrochloridemicro-tablet, the preparation method thereof and the application thereof in the consistency evaluation of the generic drugs, itopride hydrochloride and various ingredients are mixed, pelletized and dried, and then evenly mixed with an extra disintegrant and a lubricant, and the mixture is pressed into the micro-tablet of which the diameter is no more than 3MM. A capsule can be filled with the micro-tablet, multiple dissolution curves of a prepared itopride hydrochloride capsule in vitro are consistent with an original product (product name: itopride hydrochloride tablet; trade name: Weilisu;specification: 0.05g; certificate holding merchant: ABBOTT LABORATORIES (M) SDN. BHD), in the process of accelerated test and long-term test stability investigation, related substances have no significant change, and the micro-tablet has a bioequivalence trend with the original product after the meal.
Owner:珠海润都制药股份有限公司
Synthesis method of itopride hydrochloride
InactiveCN107892657AHigh purityLow costOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsOrganic synthesis
The invention discloses a synthesis method of itopride hydrochloride, and relates to the technical field of organic synthesis. Hydroxybenzylamine is taken as starting material, in non-polar solvent, in the presence of an acid-binding agent, the Hydroxybenzylamine and 3,4-dimethoxybenzoyl chloride are subjected to amidation to obtain an intermediate N-(4-hydroxyl) benzyl-3,4-dimenthoxybenzamide, and in polar solvent, in the presence of a catalyst, the intermediate N-(4-hydroxyl) benzyl-3,4-dimenthoxybenzamide and 2-chlorine-N,N-dimethylethylamine are subjected to etherification to obtain the itopride hydrochloride. According to the method, the raw materials are easy to obtain, the route is short, only two steps of synthetic reactions are needed, the high yield of 85-97% can be achieved in each step, by means of amidation and etherification with high product purity, a dangerous process of high-pressure hydrogenation is avoided successfully, and meanwhile, the production and raw materialcosts are greatly reduced.
Owner:安徽修一制药有限公司
Itopride hydrochloride composition
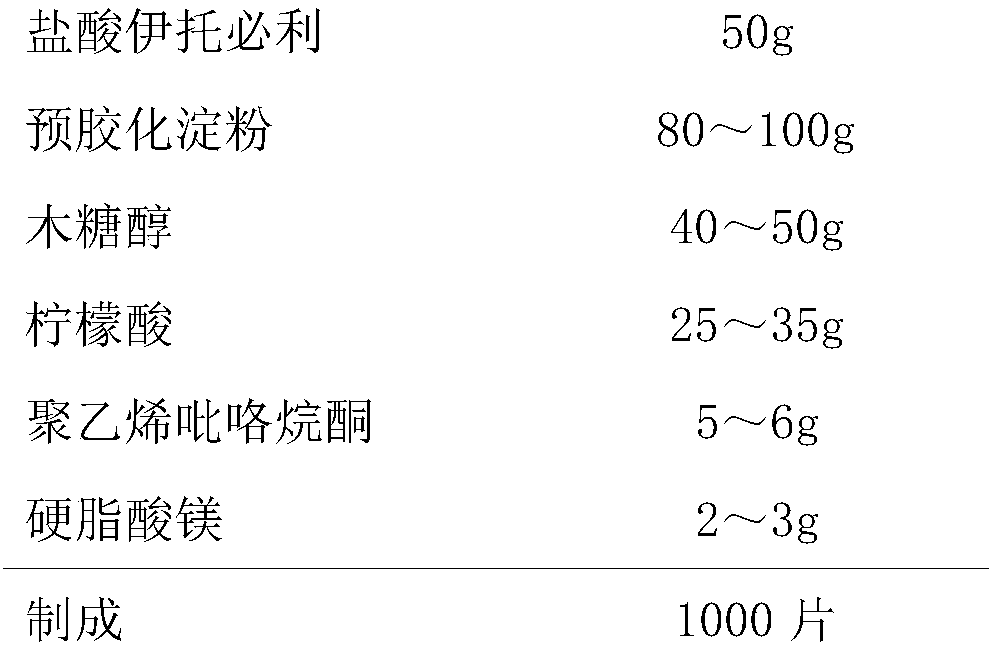
ActiveCN105267171AMeet clinical drug needsSolve the problem of large differences in releaseOrganic active ingredientsDigestive systemExtended release tabletsMethyl cellulose
The invention relates to an itopride hydrochloride sustained-release tablet composition. The technical scheme comprises that the cores of per 1000 tablets of itopride hydrochloride composition comprises 75 g of itopride hydrochloride, 45 g of Hydroxypropyl methyl cellulose, 15 g of sodium alginate, 40 g of microcrystalline cellulose, 8 g of aerosi, 7 g of polyvinylpyrrolidone K30, and 3 g of magnesium stearate. The beneficial effects comprise that the itopride hydrochloride sustained-release tablet according with clinic requirements is obtained through reasonable prescription adjusting, and the problem that the table release degree difference of the itopride hydrochloride sustained-release tablets is large is solved.
Owner:DISHA PHARMA GRP
Itopride hydrochloride orally disintegrating tablets and preparation method thereof
InactiveCN107789331AStrong water swelling effectHigh hardnessOrganic active ingredientsDigestive systemIrritationOrally disintegrating tablet
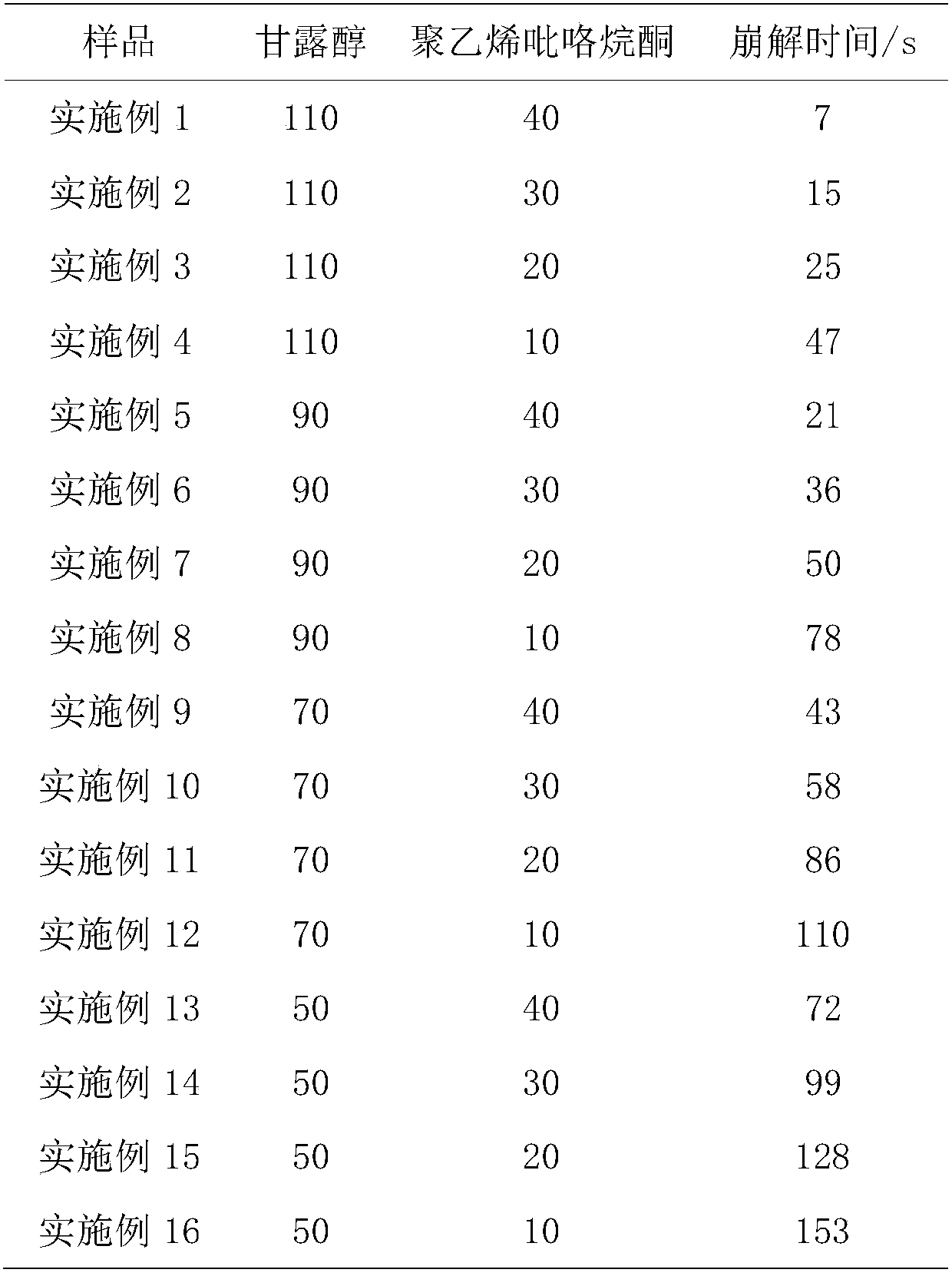
The invention relates to the technical field of pharmaceutical preparations, in particular to an orally disintegrating itopride hydrochloride tablet and a preparation method thereof: 50 parts by weight of itopride hydrochloride, 90-110 parts by weight of mannitol, and 10-30 parts by weight of lemon Acid, 30-40 parts by weight of polyvinylpyrrolidone and an appropriate amount of 50% ethanol solution are mixed evenly and added to the above-mentioned mixed material powder, 10-30 parts by weight of citric acid and stevioside are mixed in an appropriate amount to make a uniform soft material, and passed through 30 mesh Wet granules are prepared by nylon sieve, dried at 50-60°C for about 30 minutes, sieved with 30 meshes for granulation, the granules and 2-3 parts by weight of magnesium stearate and 1-2 parts by weight of micro-powdered silica gel are mixed evenly, and pressed into tablets. It can disintegrate quickly in the oral cavity under anhydrous conditions (or only a small amount of water exists), enter the digestive tract with swallowing action, and have no mucosal absorption in the oral cavity. The absorption and metabolism process in the body are consistent with ordinary tablets. Compared with common preparations, it has the advantages of convenient administration, fast absorption, high bioavailability, and less irritation to the mucous membrane of the digestive tract.
Owner:HUAYI PHARMA ANHUI CO LTD
Itopride hydrochloride oral preparation
InactiveCN104257617AOvercome the problem of rough appearance and easy crackingSolve the problem of hygroscopicityOrganic active ingredientsDigestive systemDissolutionITOPRIDE HYDROCHLORIDE
The invention discloses an itopride hydrochloride oral preparation. The oral preparation comprises 15-20 percent by weight of itopride hydrochloride and 10-30 percent by weight of disintegrating agent, wherein the disintegrating agent is povidone. According to the oral preparation, the problems that the appearance of tablets is rough, the tablets are easily broken, the tablets with rough appearance are easily moisturized and the colors of the tablets are easily changed in areas where the temperature is relatively low in winter are overcome. The oral preparation is short in disintegration time, high in dispersion uniformity, high in medicine dissolution speed, high in absorption and high in bioavailability, is convenient and flexible to orally take and has good taste; moreover, the medication compliance of patients is improved.
Owner:GUANGZHOU WEIXI PHARMA
Itopride hydrochloride tablet and preparation method of tablet
PendingCN111374958AAvoid difficultiesSimple production processOrganic active ingredientsDigestive systemGeneric drugITOPRIDE HYDROCHLORIDE
The invention discloses an itopride hydrochloride tablet, a preparation method of the tablet, and application in consistency evaluation of generic drugs. The raw material medicine of itopride hydrochloride has the characteristics of easily absorbing moisture, becoming sticky in water and crystallization, so that the materials become sticky and has poor granulation. Therefore, wet granulation is not suitable for preparing itopride hydrochloride tablets. In the present invention, itopride hydrochloride and various auxiliary materials are mixed uniformly, and then tablets with a diameter of about7 mm, a thickness of about 3 mm and a weight of about 130 mg, are obtained by directly pressing the above mixture. According to the itopride tablets produced by directly pressing powder, not only thedifficulty of granulation can be solved, but also the production process is simple, and the manufacturing cost is low. The multiple dissolution curves of the tablets in vitro are consistent with thedissolution curves of the original research product, related substances have no obvious change in the process of an accelerated test and long-term test stability investigation, and the tablets are bioequivalent with the original research product after meal.
Owner:珠海润都制药股份有限公司
Itopride hydrochloride medicine composition and preparation method thereof
ActiveCN110787155AEliminate flatulenceReduce resistanceDigestive systemPharmaceutical non-active ingredientsCellulosePharmacologic action
Owner:CHENGDU HENGRUI PHARMA
Itopride hydrochloride fast-release preparation and preparation method thereof
PendingCN110755394AFast releasePromote digestionOrganic active ingredientsDigestive systemITOPRIDE HYDROCHLORIDEStearic acid
The invention relates to an itopride hydrochloride fast-release preparation and a preparation method thereof. The itopride hydrochloride fast-release preparation comprises the following components inparts by weight: 50-100 parts of itopride hydrochloride, 10-50 parts of croscarmellose sodium, 20-150 parts of starch and 1-2 parts of magnesium stearate. The invention achieves the following beneficial effects: in order to release an effective drug component, namely the itopride hydrochloride, as soon as possible, the types of other auxiliary materials are few, a small amount of croscarmellose sodium is used as a swelling agent, a small amount of thickening agent is used, and the only auxiliary material with a higher content is the starch, which is beneficial to human digestion, so that the release speed of the whole tablet is very high.
Owner:CHENGDU HENGRUI PHARMA
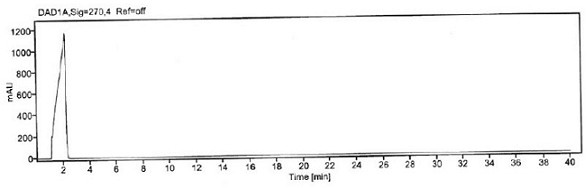
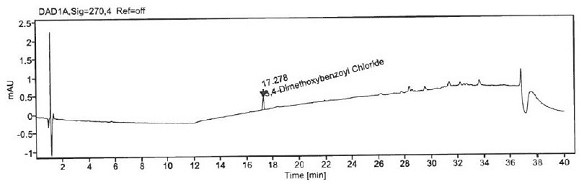
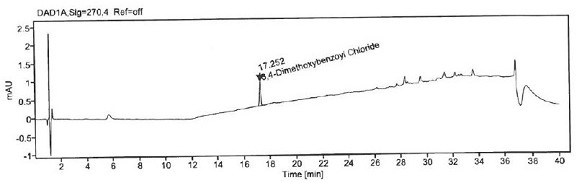
Method for detecting 3, 4-dimethoxybenzoyl chloride in itopride hydrochloride
ActiveCN111693633AStrong specificityHigh precisionComponent separationStationary phasePhysical chemistry
The invention aims to provide a method for detecting 3, 4-dimethoxybenzoyl chloride in itopride hydrochloride. The method comprises the following steps: adding aniline into itopride hydrochloride to carrt out pre-column derivatization on 3, 4-dimethoxybenzoyl chloride; then, using octadecylsilane chemically bonded silica as a stationary phase to separate 3, 4-dimethoxybenzoyl chloride from itopride hydrochloride so as to confirm the residual amount of 3, 4-dimethoxybenzoyl chloride in itopride hydrochloride. The system applicability, specificity, precision, detection limit, quantification limit, linearity and range, accuracy, durability and the like are verified to confirm the feasibility and effectiveness of the method. The method for detecting 3, 4-dimethoxybenzoyl chloride in itopride hydrochloride is provided for the first time, has the characteristics of high accuracy, high precision, good reproducibility, good stability, strong specificity and the like, and has the advantages ofshort time consumption, simple operation, low cost and the like.
Owner:珠海润都制药股份有限公司
Itopride hydrochloride chewable tablets and preparation method thereof
InactiveCN107854442AGreat tasteFast drug releaseOrganic active ingredientsDigestive systemFiller ExcipientMixed materials
The invention relates to the technical field of pharmaceutical preparations, in particular to an itopride hydrochloride chewable tablet and a preparation method thereof: 50 parts by weight of itopride hydrochloride, 100-170 parts by weight of a filler, 20-40 parts by weight of citric acid, 3-8 parts by weight of polyvinylpyrrolidone and an appropriate amount of 50% ethanol solution are mixed and added to the above-mentioned mixed material powder, mixed to make a uniform soft material, passed through a 30-mesh nylon sieve to make wet granules, and dried at 50-60 ° C for about 30 minutes. The 30-mesh sieve is sized, and the granules are mixed with 2 to 3 parts by weight of magnesium stearate evenly and pressed into tablets. The filler is one or more of pregelatinized starch, lactose, dextrin, microcrystalline cellulose, mannitol, sorbitol and xylitol. It has the characteristics of simple preparation, convenient administration, fast absorption and high bioavailability.
Owner:HUAYI PHARMA ANHUI CO LTD
Preparation method of itopride hydrochloride
ActiveCN102993038BLow priceEasy to buyOrganic compound preparationCarboxylic acid amides preparationBenzoyl chlorideEther
The invention relates to a preparation method of gastrointestinal crude drug itopride hydrochloride, and belongs to the field of medicines. The invention discloses a method for preparing itopride hydrochloride, which comprises the following steps of: taking cheap N,N-dimethylaminoethanol as an initial raw material; implementing an etherification reaction to obtain an intermediate product (VII) and implementing one-step reduction ammoniation to obtain a benzylamine product (IX); and reacting with 3,4-dimethoxy benzoyl chloride to generate hydrochloride. The preparation method of the itopride hydrochloride is cheap in raw material, moderate in reacting condition, and low in preparation cost of the itopride hydrochloride.
Owner:迪嘉药业集团股份有限公司
Detection method of demethylated itopride nitrosamine
The invention provides a detection method of an impurity demethylated itopride nitrosamine in itopride hydrochloride, wherein the method comprises the steps: selecting octadecylsilane chemically bonded silica as a filler, and an ammonium acetate aqueous solution and acetonitrile as mobile phases to carry out liquid chromatography separation, and detecting target molecules by using combination of mass spectrometry. According to the method, the system applicability, specificity, precision, quantitation limit, detection limit, linearity and range all meet the requirements, the separation effect is good, operation is easy and convenient, and the method sensitivity is high.
Owner:珠海润都制药股份有限公司
A kind of itopride hydrochloride microtablet and preparation method thereof
ActiveCN109125277BIncrease postprandial bioavailabilitySolve the problem of inconsistent dissolution profiles in vitroOrganic active ingredientsDigestive systemGeneric drugAbbott Laboratories
The invention discloses an itopride hydrochloride micro-tablet, a preparation method thereof and application thereof in consistency evaluation of generic drugs. According to the itopride hydrochloridemicro-tablet, the preparation method thereof and the application thereof in the consistency evaluation of the generic drugs, itopride hydrochloride and various ingredients are mixed, pelletized and dried, and then evenly mixed with an extra disintegrant and a lubricant, and the mixture is pressed into the micro-tablet of which the diameter is no more than 3MM. A capsule can be filled with the micro-tablet, multiple dissolution curves of a prepared itopride hydrochloride capsule in vitro are consistent with an original product (product name: itopride hydrochloride tablet; trade name: Weilisu;specification: 0.05g; certificate holding merchant: ABBOTT LABORATORIES (M) SDN. BHD), in the process of accelerated test and long-term test stability investigation, related substances have no significant change, and the micro-tablet has a bioequivalence trend with the original product after the meal.
Owner:珠海润都制药股份有限公司
Itopride hydrochloride combination
ActiveCN105106167ASolve the problem that the release rate can only reach about 70%Increase profitOrganic active ingredientsDigestive systemCellulosePolyethylene glycol
The invention relates to an itopride hydrochloride controlled-release tablet combination. The technical scheme includes that an itopride hydrochloride controlled-release tablet comprises a tablet core and a coating layer; the itopride hydrochloride controlled-release tablet is characterized in that the tablet core contains itopride hydrochloride, polyethylene glycol 1000, lactose, mannitol, aerosil and povidone K30, and the coating layer contains ethyl cellulose and hydroxypropyl methyl cellulose. By the technical scheme, the problem that release rate of single-chamber itopride controlled-release tablets can reach about 70% only is solved, and a pharmaceutical which is safe, effective and high in utilization rate is provided for clinical medication.
Owner:DISHA PHARMA GRP
Itopride hydrochloride composition
ActiveCN105267171BEvenly mixedSolve the problem of large differences in releaseOrganic active ingredientsDigestive systemSustained Release TabletMethyl cellulose
The invention relates to an itopride hydrochloride sustained-release tablet composition. The technical scheme comprises that the cores of per 1000 tablets of itopride hydrochloride composition comprises 75 g of itopride hydrochloride, 45 g of Hydroxypropyl methyl cellulose, 15 g of sodium alginate, 40 g of microcrystalline cellulose, 8 g of aerosi, 7 g of polyvinylpyrrolidone K30, and 3 g of magnesium stearate. The beneficial effects comprise that the itopride hydrochloride sustained-release tablet according with clinic requirements is obtained through reasonable prescription adjusting, and the problem that the table release degree difference of the itopride hydrochloride sustained-release tablets is large is solved.
Owner:DISHA PHARMA GRP
A kind of preparation method of itopride hydrochloride
ActiveCN105985257BAvoid the restore stepLow priceOrganic compound preparationCarboxylic acid amides preparationITOPRIDE HYDROCHLORIDEDimethylaminoethyl chloride
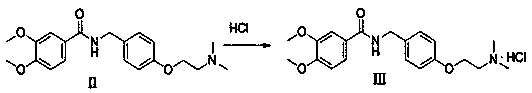
A kind of preparation method of itopride hydrochloride The present invention relates to the preparation method of itopride hydrochloride, the reaction takes 2-dimethylaminochloroethane hydrochloride and phenol as starting materials, through etherification, chloromethylation , amino substitution, amidation, and salt-forming 5-step reaction to obtain itopride hydrochloride. In the present invention, the chloromethylation promoted by HCl / CHO avoids the imine reduction step, eliminates the solid waste generated by the reducing agent, and improves the safety of the reaction; the raw materials used are cheap, the market supply is sufficient, and they are easy to purchase; The one-step reaction is more classic, safe and easy to control, and suitable for industrial production.
Owner:迪嘉药业集团股份有限公司
Preparation method of itopride hydrochloride
ActiveCN103073446BIncrease lossLow yieldOrganic compound preparationCarboxylic acid amide separation/purificationBenzoyl chlorideITOPRIDE HYDROCHLORIDE
The invention provides a preparation method of itopride hydrochloride. The method comprises a step that N,N-dimethylamino ethoxy aniline and 3,4-dimethoxy benzoyl chloride are subjected to an amidation reaction in dichloromethane. The method provided by the invention also comprises a step that a hydrogen chloride isopropyl alcohol solution and an itopride prototype material are subjected to a reaction, such that a salt is produced. According to the itopride hydrochloride preparation method provided by the invention, dichloromethane is used for replacing toluene, such that the harm brought by toluene is avoided. Also, no water is introduced during the salt formation process, such that loss can be reduced, product yield can be improved, and impurities are further removed. Therefore, the quality of a finished product is improved.
Owner:珠海保税区丽珠合成制药有限公司 +1
A kind of itopride hydrochloride composition
ActiveCN105106167BSolve the problem that the release rate can only reach about 70%Increase profitOrganic active ingredientsDigestive systemMANNITOL/SORBITOLPolyethylene glycol
The invention relates to an itopride hydrochloride controlled-release tablet combination. The technical scheme includes that an itopride hydrochloride controlled-release tablet comprises a tablet core and a coating layer; the itopride hydrochloride controlled-release tablet is characterized in that the tablet core contains itopride hydrochloride, polyethylene glycol 1000, lactose, mannitol, aerosil and povidone K30, and the coating layer contains ethyl cellulose and hydroxypropyl methyl cellulose. By the technical scheme, the problem that release rate of single-chamber itopride controlled-release tablets can reach about 70% only is solved, and a pharmaceutical which is safe, effective and high in utilization rate is provided for clinical medication.
Owner:DISHA PHARMA GRP
Itopride preparation method
InactiveCN106518706AWide variety of sourcesLow priceCarboxylic acid nitrile preparationOrganic compound preparationBenzaldehydeHydroxylamine Hydrochloride
The invention discloses an itopride preparation method, the method comprises the steps of 1, 2-(dimethylamino) chloroethane hydrochloride and hydroxybenzaldehyde synthesizing into 4-(2- dimethylamino ethoxy) benzaldehyde, then synthesizing into 4-(2- dimethylamino ethoxy) benzyl alcohol in alcohol solvent by revivification; 2, in alcohol solvent 3, 4-dimethoxy benzaldehyde and hydroxylamine hydrochloride creating reaction, then in nonpolar solvent synthesizing into 3, 4- dimethoxybenzonitrile by dehydration using the dehydrant; 3, the 4-(2-dimethylamino ethoxy) benzyl alcohol and the 3, 4- dimethoxybenzonitrile synthesizing into itopride in one step; 4, obtaining hydrochloric acid itopride by dissolving itopride in hydrogen chloride alcohol solution and salifying. The adopted raw material in the method is wide in sourcing scope, simple in preparation processing, and is suitable for large scale industrialization production; the preparation process involves no danger process, the production equipment is simple, the synthesized circuit is shorter than the existed circuits, the preparation time is short and the use effect is good.
Owner:安徽省诚联医药科技有限公司
Pantoprazole sodium enteric coatel tablet composition and preparation method thereof
InactiveCN103156846AImprove toleranceHigh synergistic effectOrganic active ingredientsDigestive systemOral medicationITOPRIDE HYDROCHLORIDE
The invention relates to a pantoprazole sodium enteric coatel tablet composition and a preparation method thereof. The composition is characterized by consisting of a proton pump inhibitor pantoprazole sodium and a gastrointestinal excitomotor itopride hydrochloride. The composition is used for treating gastric ulcer, gastroesophageal reflux diseases and functional dyspepsia. In the combined preparation, the weight ratio of pantoprazole sodium and itopride hydrochloride is (2:5)(-2:10). Compared with a single component preparation, the cure rate is relatively increased remarkably, the occurrence rate of untoward effect is greatly reduced, and the symptom recurrence rate is remarkably reduced. The composition has important effect of treating gastric ulcer, gastroesophageal reflux diseases and functional dyspepsia, and is simple and convenient in preparation method, low in cost, suitable for oral administration of patients and good in compliance.
Owner:LIAONING NIRVANA PHARMA
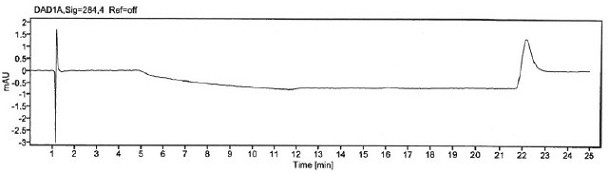
Method for detecting p-hydroxybenzaldehyde in itopride hydrochloride
ActiveCN111679029AHigh separation of chromatographic peaksImprove system applicabilityComponent separationAgainst vector-borne diseasesEthoxidineFluid phase
The invention provides a method for detecting p-hydroxybenzaldehyde in itopride hydrochloride. 4-[(2-dimethylamino) ethoxy] benzylamine is a key intermediate for synthesizing itopride hydrochloride; p-hydroxybenzaldehyde is a starting material for synthesizing 4-[(2-dimethylamino) ethoxy] benzylamine; therefore, p-hydroxybenzaldehyde may not react completely in the process of synthesizing 4-[(2-dimethylamino) ethoxy] benzylamine and remains in 4-[(2-dimethylamino) ethoxy] benzylamine; further, the p-hydroxybenzaldehyde is possibly introduced into an itopride hydrochloride finished product; inorder to detect the content of p-hydroxybenzaldehyde in itopride hydrochloride, the invention provides a liquid chromatography detection method of p-hydroxybenzaldehyde in itopride hydrochloride for the first time, and the liquid chromatography detection method has the characteristics of high accuracy, high precision, good reproducibility, good stability, strong specificity and the like, and has the advantages of short time consumption, simple operation, low cost and the like.
Owner:珠海润都制药股份有限公司
Preparation method of itopride hydrochloride
InactiveCN108610266ASave materialReduce stepsOrganic compound preparationCarboxylic acid amides preparationITOPRIDE HYDROCHLORIDEPhenol
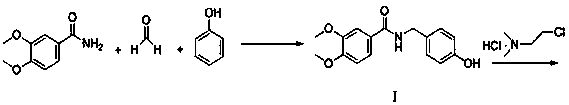
The invention relates to a preparation method of itopride hydrochloride and belongs to the technical field of raw material preparation. The technical scheme is as follows: the preparation method of itopride hydrochloride comprises the steps as follows: an intermediate I is prepared from initial materials including 3,4-dimethoxybenzamide, formaldehyde and phenol with a one-pot method; the intermediate I and N,N-dimethyl chloroethane hydrochloride are subjected to a substitution reaction to produce an intermediate II; finally, itopride hydrochloride is prepared through salification. The itopridepreparation process comprises a short and convenient route and is economical and environmentally friendly.
Owner:迪嘉药业集团股份有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
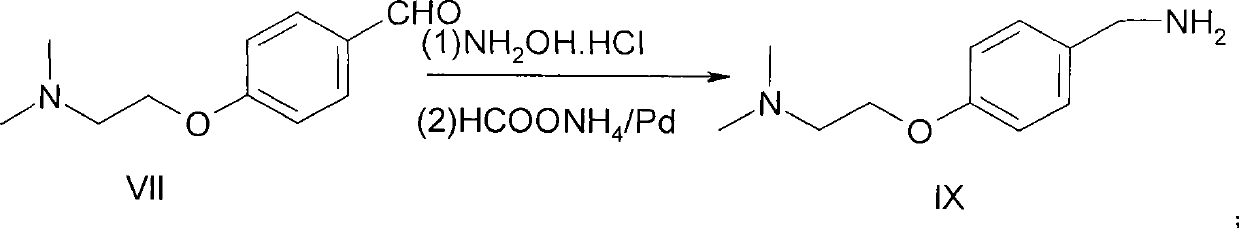



![Method for preparing 4-[2-(dimethylamino)ethoxy]benzylamine as itopride-hydrocloride salt mediate Method for preparing 4-[2-(dimethylamino)ethoxy]benzylamine as itopride-hydrocloride salt mediate](https://images-eureka.patsnap.com/patent_img/611b3c77-cf7e-40e2-83c1-88f54b6a5d07/US20090203940A1-20090813-C00001.png)
![Method for preparing 4-[2-(dimethylamino)ethoxy]benzylamine as itopride-hydrocloride salt mediate Method for preparing 4-[2-(dimethylamino)ethoxy]benzylamine as itopride-hydrocloride salt mediate](https://images-eureka.patsnap.com/patent_img/611b3c77-cf7e-40e2-83c1-88f54b6a5d07/US20090203940A1-20090813-C00002.png)
![Method for preparing 4-[2-(dimethylamino)ethoxy]benzylamine as itopride-hydrocloride salt mediate Method for preparing 4-[2-(dimethylamino)ethoxy]benzylamine as itopride-hydrocloride salt mediate](https://images-eureka.patsnap.com/patent_img/611b3c77-cf7e-40e2-83c1-88f54b6a5d07/US20090203940A1-20090813-C00003.png)