Preparation method of itopride hydrochloride
A technology of itopride hydrochloride and ethyl acetate, which is applied in the field of preparation of itopride hydrochloride, can solve the problems of strict residue requirements, increased purification step loss, and high cost, and achieve improved solvent capacity, excellent stability, and The effect of increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: Take dichloromethane as solvent
[0047] Dissolve 0.738 kg of N, N-dimethylaminoethoxybenzylamine in 4.3 liters of dichloromethane, then add 8.6 liters of dichloromethane solution containing 0.969 kg of 3,4-dimethoxybenzoyl chloride, and control the temperature After reacting for 3 hours, add 3.5 liters of 1.8% dilute hydrochloric acid and stir for 30 minutes before separating the liquids;
[0048] Take the upper water phase and filter, use 20% sodium hydroxide aqueous solution to adjust the pH of the filtrate to about 8, mix slightly, stop adding lye for 1 hour, and gradually adjust to pH = 10-11 (total consumption of 20% sodium hydroxide aqueous solution is about 1L) , filtered, washed with water, and dried to obtain the prototype of itopride;
[0049] Heat and dissolve the itopride prototype in 5 liters of ethanol, add 0.62 liters of 30% isopropanol hydrochloride solution to form a salt, gradually precipitate crystals, and filter to obtain the crude ...
Embodiment 2
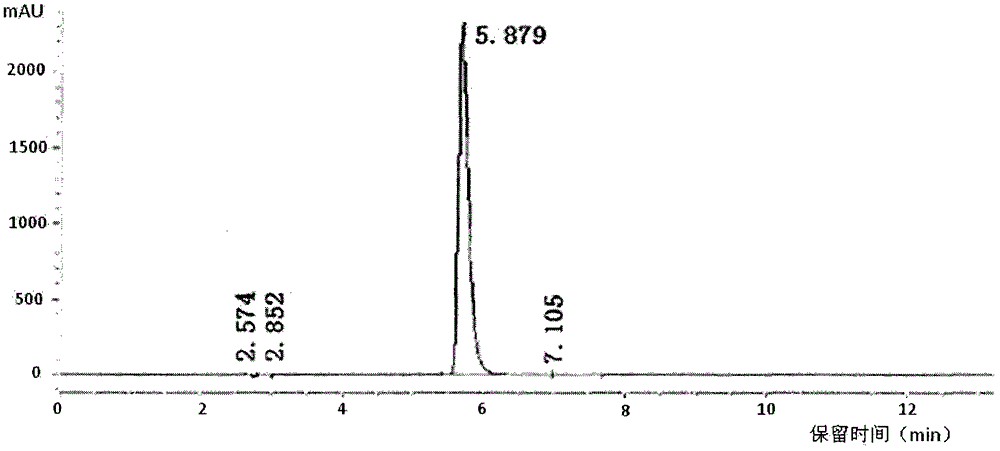
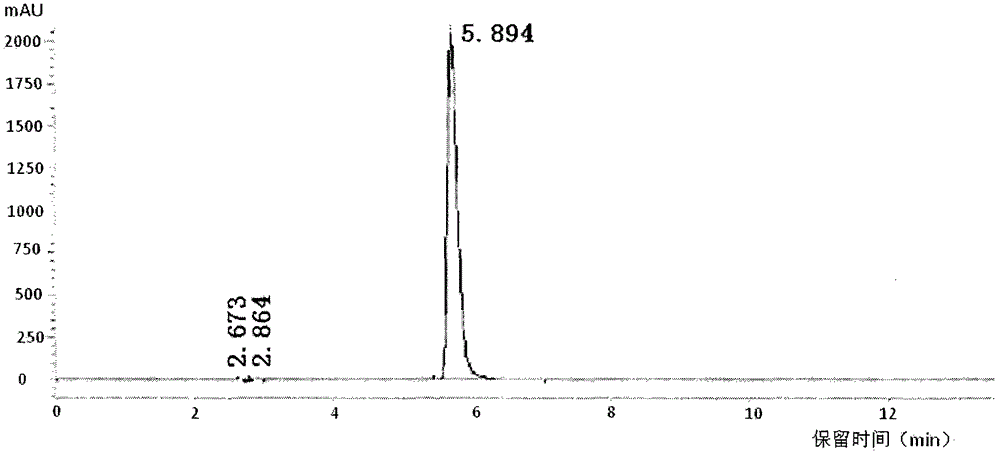
[0070] The itopride hydrochloride prepared in Example 1 was analyzed by HPLC. The same retention time and peak area of the reference substance and the test substance S100903001 are shown in Table 1 below.
[0071] Table 1
[0072]
[0073] Calculation method of relative retention time: set the peak elution time of the main peak of the product as 1, divide the peak elution time of a certain peak by the peak elution time of the main peak to obtain the relative retention time of the peak, because the use of multiple tests is different Due to instrument errors and changes in chromatographic columns and mobile phases, the eluting time of the main peak and impurity peaks cannot be the same, so the relative retention time is proposed as a unified comparison.
[0074] According to the data of the relative retention time in Table 1, it can be considered that all HPLC peaks of the S100903001 sample correspond to the control sample without new impurity production and one-to-one co...
Embodiment 3
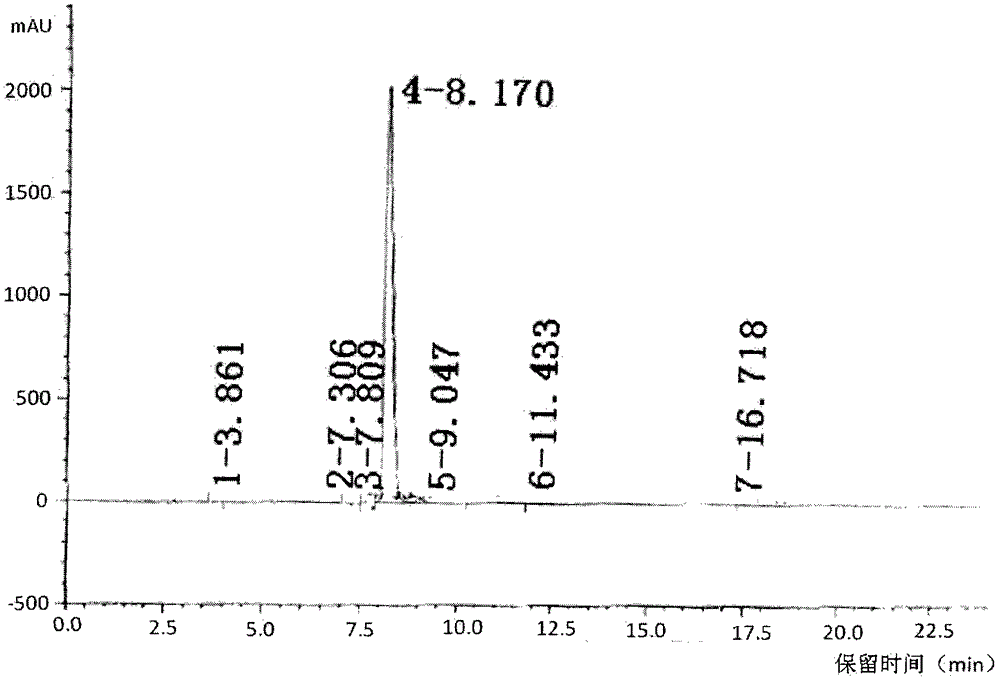
[0076] Contrastive analysis of itopride hydrochloride obtained by using ethyl acetate and itopride hydrochloride obtained by not using ethyl acetate. The results are shown in Table 2 below.
[0077] Table 2
[0078]
[0079] As can be seen from Table 2, the product main peak area of the inventive method accounts for a percentage greater than the product main peak of the reference sample, indicating that the product purity obtained is better than that of the reference sample, and that the use of ethyl acetate crystallization can effectively remove Certain impurities improve product quality.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com