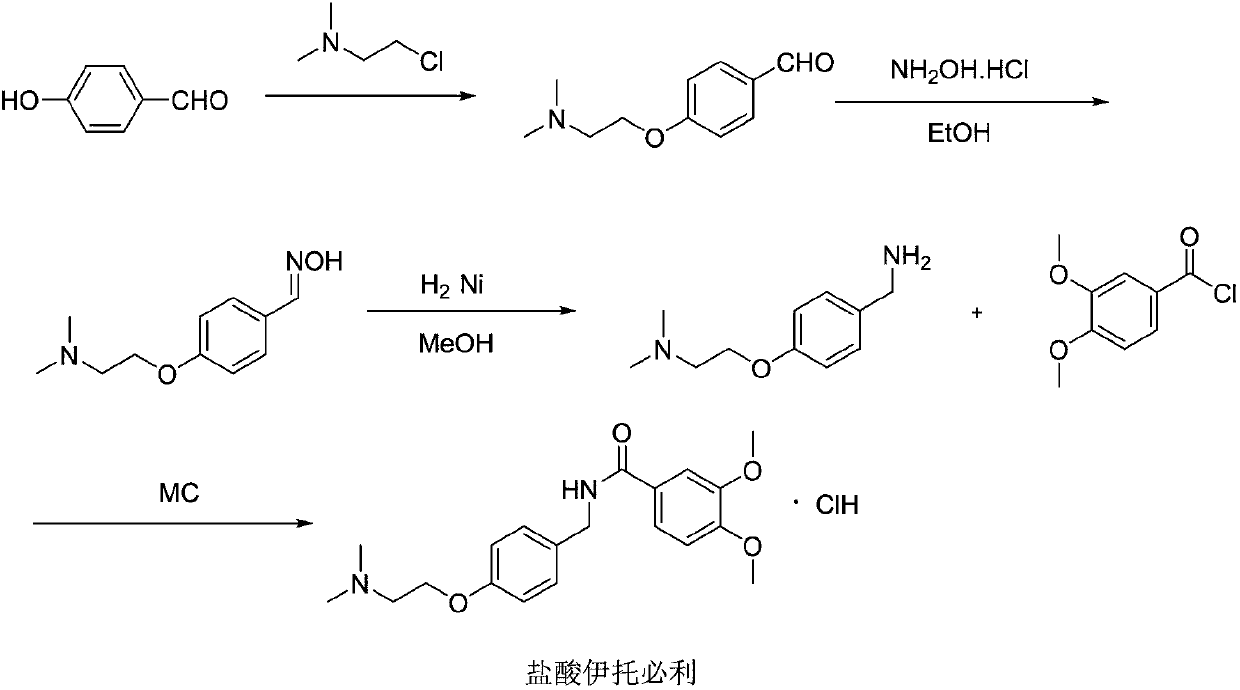
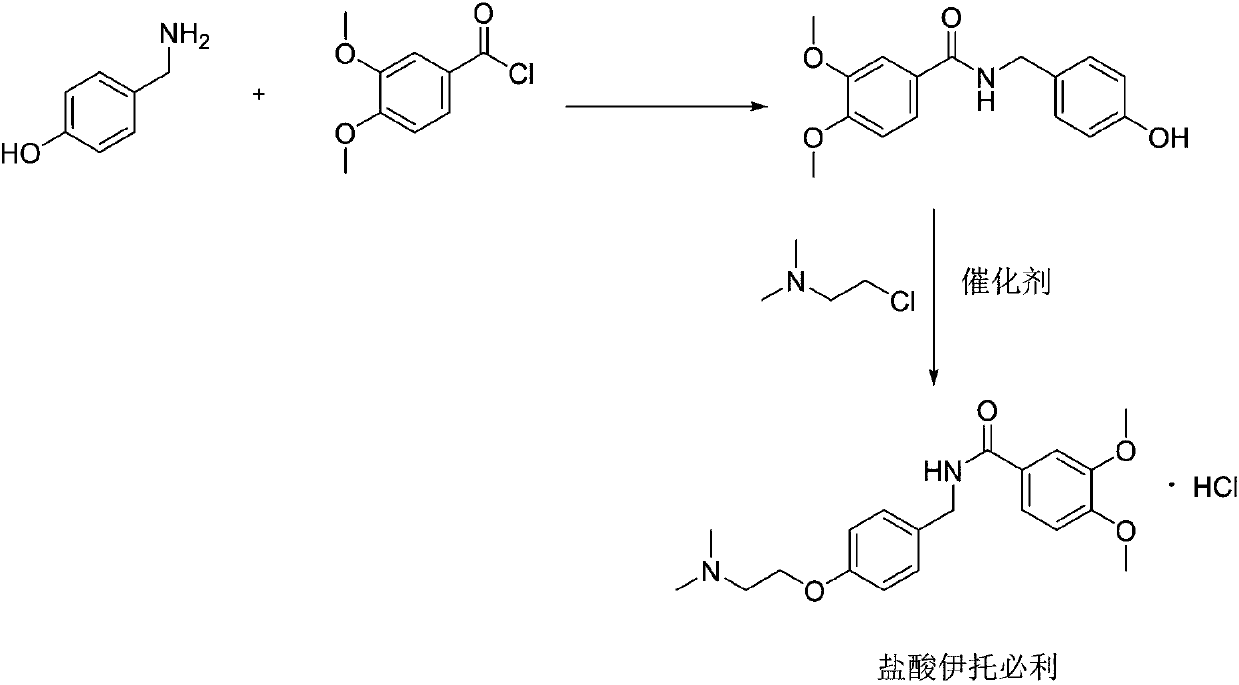
Synthesis method of itopride hydrochloride
A technology of itopride hydrochloride and a synthesis method is applied in the synthesis field of itopride hydrochloride, and can solve the problem of not conforming to the concept of green and safe pharmaceutical production, tedious preparation of ethoxybenzylamine, and low total yield of the synthesis process and other problems to achieve the effect of reducing production risks, reducing production and raw material costs, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Preparation of N-(4-hydroxy)benzyl-3,4-dimethoxybenzamide:
[0021] 200 g of p-hydroxybenzylamine and 326 g of 3,4-dimethoxybenzoyl chloride were put into a dry reaction flask containing 1000 ml of dichloromethane. 129g of pyridine was carefully added under stirring at room temperature, heated to reflux for 2h, the reaction was completed, after the solvent was evaporated, the residue was washed with water and dried to obtain a yellow solid product, weight 448g, purity 98%. The yield is 95%.
[0022] (2) Preparation of Itopride Hydrochloride:
[0023] Put 448g of the obtained amide product and 260g of 2-chloro-N,N-dimethylethylamine into a dry reaction flask containing 1300ml DMF, then put in 2.6g potassium iodide, stir and raise the temperature to 130-135°C, and react for 8-10h. After the reaction was completed, after the solvent was evaporated under reduced pressure, 1200ml of absolute ethanol was put into the bottle, and after stirring to dissolve, 8ml of 30% hydrochlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com