Method for detecting related substances in itopride hydrochloride preparation
A technology for itopride hydrochloride and related substances, which is applied to the detection of related substances in itopride hydrochloride preparations and the detection of related substances in medicines, and can solve the problems that cannot meet the needs of inspection, do not meet requirements, and do not meet the requirements. Peak and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1 detection method
[0063] (1) Instrument conditions and reagents
[0064] Instruments and conditions: high performance liquid chromatography: model: Agilent1260; chromatographic column: Thermo gold aQ C18, 4.6mm×250mm, 5μm; electronic analytical balance, pH meter, detection wavelength is 223nm, injection volume is 20μl, column temperature: 25°C; use 0.05mol / L potassium dihydrogen phosphate solution (adjust the pH value to 4.5 with dilute phosphoric acid or dilute potassium hydroxide solution) as mobile phase A, and use acetonitrile with a volume fraction of 90% as mobile phase B according to Table 1. Linear gradient elution.
[0065] Reagents and reference substances: as shown in Table 4.
[0066] (2) Test operation
[0067] ① Solution preparation:
[0068] Blank solution: 0.05mol / L potassium dihydrogen phosphate solution (adjust the pH value to 4.5 with dilute phosphoric acid or dilute potassium hydroxide solution)-90% acetonitrile (V:V=85: 15);
[00...
Embodiment 2
[0074] Embodiment 2 System suitability test
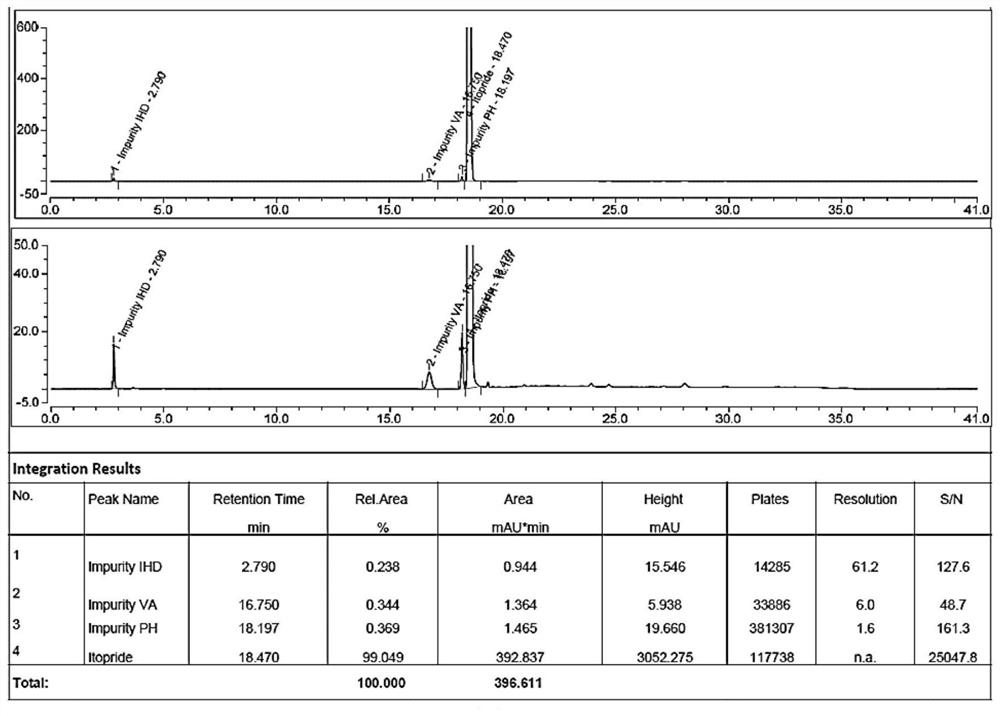
[0075] System suitability, 5 for the peak area RSD of itopride peak in the solution should not be greater than 2.0%, and the separation between the main peak and adjacent impurity peaks of the system suitability solution meets the requirements.
[0076] System suitability solution: take about 50 mg of itopride hydrochloride reference substance, accurately weigh it, put it in a 100ml measuring bottle, measure 1ml each of impurity IHD, PH, VA positioning solution, put it in the same 100ml measuring bottle, add diluent Appropriate shaking, sonication to dissolve itopride hydrochloride, add diluent to dilute to the mark, shake well, and obtain.
[0077] Need testing solution: get about 125 mg of itopride hydrochloride tablet sample powder (approximately equivalent to 50 mg of itopride hydrochloride), accurately weighed, put in a 100ml measuring bottle, add diluent and shake in an appropriate amount, and ultrasonically make itopride hyd...
Embodiment 3
[0081] Embodiment 3 sample test
[0082] Itopride hydrochloride tablets (batch number: 20190201; self-made) were tested according to the method of Example 1. The test results are as follows, which conform to the regulations of the Pharmacopoeia on the relevant substances of this product.
[0083]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com