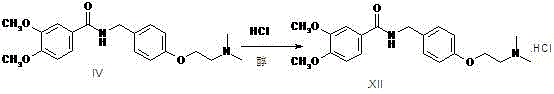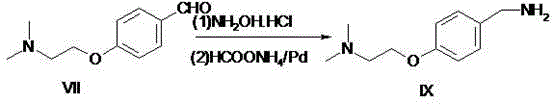Preparation method of itopride hydrochloride
A technology of itopride hydrochloride and formic acid, which is applied in the field of medicine, can solve problems such as increasing the reaction and post-treatment process, increasing the difficulty of removing impurities, and affecting product yield, so as to achieve environmental protection of the reaction, reduce solvent consumption, and save The effect of equipment costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
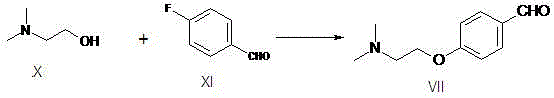
[0031] The preparation of embodiment 1, 4-(2-dimethylaminoethoxy) benzaldehyde.
[0032] N, N-dimethylethanolamine 89g (1mol), add 800mlDMF, then add 44g60%NaH (1.1mol), stir until no bubbles are generated, then add p-fluorobenzaldehyde 124g, gradually heat up to 60 ° C for 4 hours, After the reaction was detected by TLC, the solvent was recovered under reduced pressure, 1000ml of ice water was added, the pH was adjusted to about 3 with hydrochloric acid, extracted once with toluene, the aqueous layer was adjusted to about 10 with liquid caustic soda, and extracted twice with 400ml of dichloromethane. combined, washed once with saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 165 g of light yellow oil with a yield of 85.6%.
Embodiment 2
[0033] The preparation of embodiment 2, 4-(2-dimethylaminoethoxy) benzaldehyde.
[0034] Add 200g of N,N-dimethylethanolamine, add 1200ml of DMA, then add 277g of potassium tert-butoxide, stir for 1 hour, then add 293g of p-fluorobenzaldehyde, gradually raise the temperature to 80°C and react for 4 hours, TLC detects that the reaction is complete, and then depressurize Recover the solvent, add 2500ml of ice water, adjust the pH to about 3 with hydrochloric acid, extract once with toluene, adjust the pH of the water layer to about 10 with liquid caustic soda, extract twice with 800ml of chloroform, wash once with saturated saline after combining, and then It was dried over anhydrous sodium sulfate, filtered and concentrated to obtain 398 g of a light yellow oily substance with a yield of 91.7%.
Embodiment 3
[0035] Preparation of embodiment 3, 4-(2-dimethylaminoethoxy)benzylamine.
[0036] 125g of 4-(2-dimethylaminoethoxy)benzaldehyde and 45g of hydroxylamine hydrochloride were added to methanol for reflux reaction for 1 hour, and the reaction in the TLC spot plate was complete. After cooling to room temperature and under the protection of nitrogen, 122g of ammonium formate and 5g of ammonium formate were added. %Pd / C, the catalyst was recovered by filtration after 6 hours of reaction, the solvent was evaporated under reduced pressure, dichloromethane and water were added for extraction, and the organic layer was concentrated to obtain 103 g of oily 4-(2-dimethylaminoethoxy) benzylamine. The yield was 82.4%. After HPLC detection, the HPLC detection purity was 96.7%. The product was directly used in the next reaction without purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com