Itopride hydrochloride orally disintegrating tablets and preparation method thereof
A technology of itopride hydrochloride and orally disintegrating tablets, which can be applied in pharmaceutical formulations, medical preparations of non-active ingredients, and drug delivery, and can solve problems such as high energy consumption, inapplicability, and excessive use of excipients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
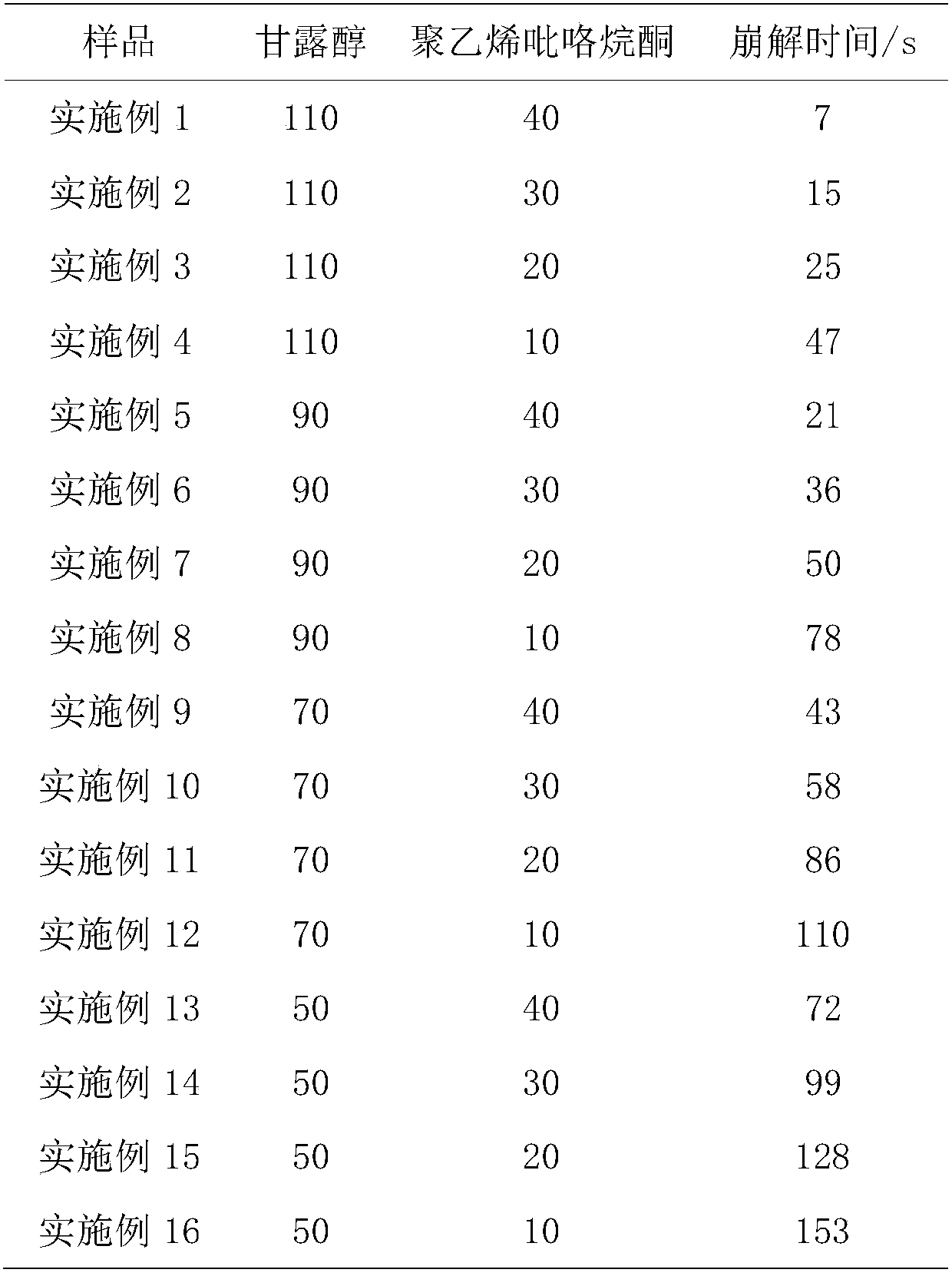
[0015] Mix 50mg of itopride hydrochloride, 110mg of mannitol, 20mg of citric acid in parts by weight, 40mg of polyvinylpyrrolidone and an appropriate amount of 50% ethanol solution and evenly add them to the above mixed material powder, mix 20mg of citric acid and stevioside in an appropriate amount to prepare Uniform soft material, passed through a 30-mesh nylon sieve to make wet granules, dried at 50-60°C for about 30 minutes, granulated through a 30-mesh sieve, mixed with 2 mg of magnesium stearate and 1 mg of micro-powdered silica gel, and pressed into tablets.
Embodiment 2
[0017] Mix 50mg of itopride hydrochloride, 110mg of mannitol, 20mg of citric acid by weight, 30mg of polyvinylpyrrolidone and an appropriate amount of 50% ethanol solution and add them into the above mixed material powder, mix 20mg of citric acid and stevioside in an appropriate amount to prepare Uniform soft material, passed through a 30-mesh nylon sieve to make wet granules, dried at 50-60°C for about 30 minutes, granulated through a 30-mesh sieve, mixed with 2 mg of magnesium stearate and 1 mg of micro-powdered silica gel, and pressed into tablets.
Embodiment 3
[0019] Mix 50mg of itopride hydrochloride, 110mg of mannitol, 20mg of citric acid in parts by weight, 20mg of polyvinylpyrrolidone and an appropriate amount of 50% ethanol solution and evenly add them to the above mixed material powder, mix 20mg of citric acid and stevioside in an appropriate amount to prepare Uniform soft material, passed through a 30-mesh nylon sieve to make wet granules, dried at 50-60°C for about 30 minutes, granulated through a 30-mesh sieve, mixed with 2 mg of magnesium stearate and 1 mg of micro-powdered silica gel, and pressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com