Method for detecting p-hydroxybenzaldehyde in itopride hydrochloride
A technology of p-hydroxybenzaldehyde and itopride hydrochloride, which is applied in the field of medical analysis and achieves the effects of good stability, strong specificity and high resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (1) Experimental materials and instrument conditions
[0046]Experimental materials: Potassium dihydrogen phosphate, manufacturer: Guangdong Guanghua Technology Co., Ltd.; acetonitrile, manufacturer: Xilong Science Co., Ltd.; p-Hydroxybenzaldehyde, manufacturer: McLean; itopride hydrochloride, manufacturer: Zhuhai Rundu Pharmaceutical Co., Ltd.; ultrapure water, manufacturer: Zhuhai Rundu Pharmaceutical Co., Ltd.
[0047] Instruments: high performance liquid chromatography: 1260-П; electronic analytical balance: XSE205DU, GR-200; chromatographic column: Waters Nora-pak C18, 3.9×150 mm, 4.0 μm.
[0048] Inject the blank solution, the reference substance solution, and the test solution into the liquid chromatograph respectively, and record the chromatogram. The chromatographic conditions are as follows: Chromatographic column: use octadecylsilane bonded silica gel as filler; Temperature: 35°C; Injection volume: 20μl; Running time: 25min; Detection wavelength: 284nm; Mobi...
Embodiment 2
[0061] Embodiment 2 detection method system applicability test of the present invention
[0062] System suitability is achieved by measuring the RSD of the peak area of p-hydroxybenzaldehyde in the reference solution. Requirement 6 The RSD of the peak area of p-hydroxybenzaldehyde in the reference solution should meet the acceptable standard.
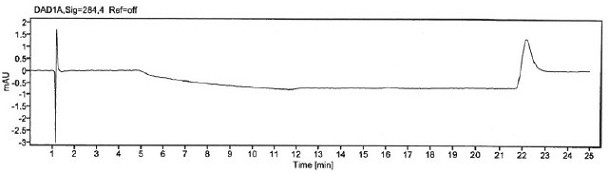
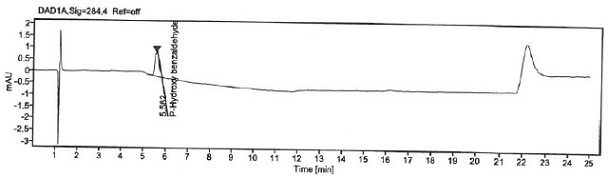
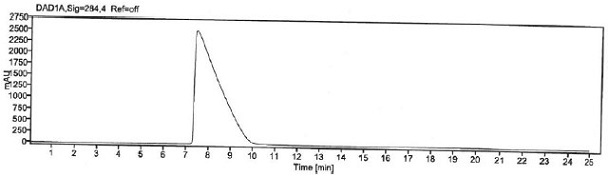
[0063] Prepare blank solution, p-Hydroxybenzaldehyde stock solution and reference substance solution as described in Example 1, under the chromatographic conditions described in Example 1, enter 1 needle of blank solution, 6 needles of reference substance solution, obtain chromatogram, as figure 1 with figure 2 , according to the formula conversion results are shown in the table below:
[0064]
Embodiment 3
[0065] Embodiment 3 Detection method specificity test of the present invention
[0066] The specificity of the method is achieved by determining that the blank solution does not interfere with the detection; the separation between p-hydroxybenzaldehyde and adjacent peaks in the selective solution and the recovery rate of p-hydroxybenzaldehyde before and after sample addition. It is required that the blank solution should not interfere with the detection; the separation between p-hydroxybenzaldehyde and adjacent peaks in the selective solution and the recovery rate of p-hydroxybenzaldehyde before and after sample addition should meet the acceptable standard.
[0067] Prepare blank solution, reference solution (p-hydroxybenzaldehyde positioning solution), test solution and selective solution as described in Example 1. After the system is balanced, inject 1 needle of blank solution, 3 needles of reference solution, and test 1 needle of product solution, 3 needles of selective sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com