A kind of detection method of isomer in valsartan hydrocarbon compound
A valsartan hydrocarbon compound and detection method technology, applied to measuring devices, instruments, scientific instruments, etc., to achieve high separation, high system applicability, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1) Experimental materials and instrument conditions
[0022] High performance liquid chromatography: Shimadzu LC-15C; Chromatographic column: 5μm, 4.6×250 mm, OD column; Chromatographic conditions: Flow rate: 0.6mL / min; Column temperature: 30℃; Injection volume: 10μl; Running time: 30min; detection wavelength: 222nm; mobile phase: n-hexane: isopropanol: trifluoroacetic acid: dimethylamine, the volume ratio is 70:30:0.1:0.1.
[0023] 2) Experimental steps
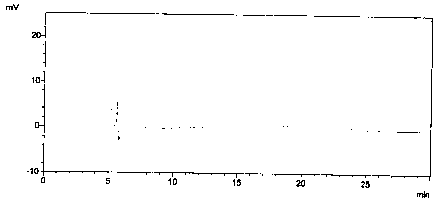
[0024] Prepare blank solution: mobile phase.
[0025] Preparation of enantiomer localization solution: Accurately weigh 5.29 mg of valsartan alkylate isomer reference substance, put it in a 10ml volumetric flask, add mobile phase to dissolve and dilute to the mark, and shake well.
[0026] Preparation of control solution: Accurately weigh 12.51mg of valsartan alkylate reference substance, put it in a 25ml volumetric flask, add mobile phase to dissolve and dilute to the mark, and shake well.
[0027] Preparation of ...
Embodiment 2
[0029] Embodiment 2 detection method system applicability test of the present invention
[0030] Prepare blank solution, enantiomer localization solution, contrast solution and need testing solution as described in Example 1, then dilute enantiomer localization solution 100 times, then accurately measure 2mL, dilute 10mL, as the system Suitability solution.
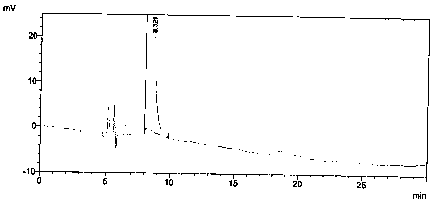
[0031] After the system is balanced, add the above-mentioned solutions respectively, record the chromatogram, and obtain the system suitability spectrum as follows: Figure 5 As shown, the obtained system adaptability results are shown in Table 1.
[0032] Table 1 - System Suitability Test Results
[0033]
Embodiment 3
[0034] Embodiment 3 detection method limit test of the present invention
[0035] Prepare quantitative limit solution: take 1 mL of the enantiomeric localization solution prepared in Example 1, dilute 100 times with mobile phase, then accurately measure 3 mL and add mobile phase to dilute to a 25 mL measuring bottle, then accurately measure 1 mL and add The mobile phase was diluted to 10mL and prepared.
[0036] Preparation of detection limit solution: Accurately measure 3mL of quantification limit solution, and then dilute to 10mL with mobile phase to prepare.
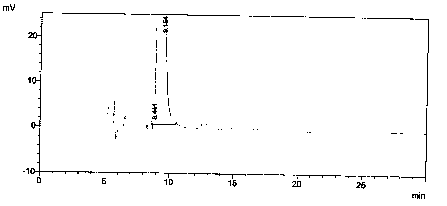
[0037] Assay method and result, as described in embodiment 1, chromatographic conditions are measured, and the result obtained is as shown in table 2.
[0038] Table 2 - Limit test results
[0039]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com