Inspection method of isosorbide mononitrate sustained-release capsule related substances
A technology of isosorbide mononitrate and related substances, which is applied in the field of testing related substances of isosorbide mononitrate sustained-release capsules, can solve the problems of no detection method for isosorbide mononitrate sustained-release capsules, prolong the action time, etc., and achieve a systematic The effect of good applicability, good accuracy and good linear relationship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Experimental materials and instrument conditions
[0035] Instrument: High performance liquid chromatography: Agilent 1260; Chromatographic column: BDS HYPERSIL C18, 250mm×4.6mm, 5μm; Flow rate: 1.0ml / min; Column temperature: 30±2℃; Injection volume: 20μl; Detection wavelength: 210nm , using methanol-water (30:70) as mobile phase A, methanol as mobile phase B, and eluted according to the following gradient:
[0036] .
[0037] (2) Experimental steps
[0038] (1) Prepare solutions, respectively prepare blank solution, blank excipient solution, system suitability solution, impurity reference solution, test solution and control solution;
[0039] Blank solution: mobile phase A.
[0040] Blank excipient solution: Weigh about 135mg of the blank excipient (approximately equivalent to 50mg of isosorbide mononitrate) in the prescribed amount, put it in a 50ml measuring bottle, add an appropriate amount of mobile phase A, dissolve it by ultrasonication, dilute to the ma...
Embodiment 2
[0086] Embodiment 2 Detection method system applicability test of the present invention
[0087] System suitability is achieved by measuring the repeatability of the reference solution. Requirement 5 RSD for the peak area of isosorbide mononitrate in the reference solution is ≤5.0%. -5 pins, record chromatograms.
Embodiment 3
[0088] Embodiment 3 Detection method specificity of the present invention
[0089] (1) Specificity test
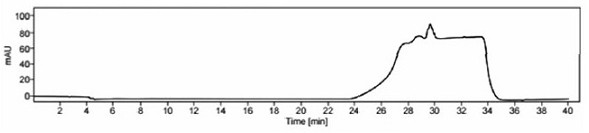
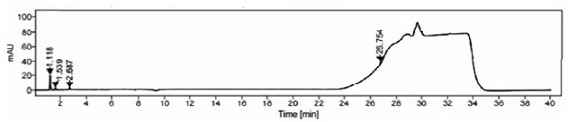
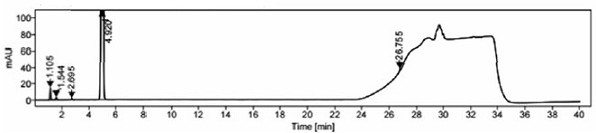
[0090] The specificity of the method is mainly to examine the peak identification and selectivity, and it is required that the blank solution and blank excipients should not interfere with the detection; in the system suitability solution, the separation between the isosorbide mononitrate peak and the isosorbide 2-mononitrate peak is >2.0 , the number of theoretical plates of the main peak ≥ 3000; the separation of each peak in the selective solution ≥ 1.5, the number of theoretical plates of the main peak ≥ 3000, the purity of the main peak ≥ 980; the separation of the main peak and adjacent impurity peaks in the test solution ≥ 1.5, the main peak Theoretical plate number ≥ 3000, main peak purity ≥ 980, after the system is stable, blank solution, blank excipient solution, 2-isosorbide mononitrate localization solution, isosorbide dinitrate localization solution, system su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com