Itopride hydrochloride composition
A technology of itopride hydrochloride and magnesium stearate, applied in the directions of drug combination, drug delivery, organic active ingredients, etc., can solve the problem of large difference in release degree, etc., and achieve the effect of solving large difference in release degree between tablets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
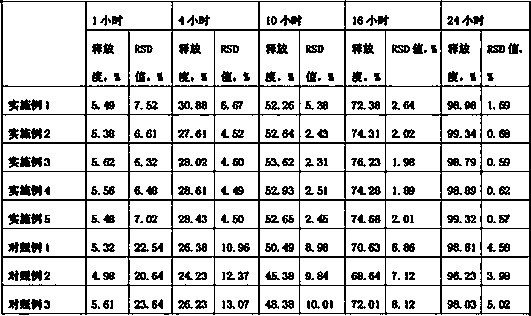
Embodiment 1
[0024] Embodiment 1, itopride hydrochloride 75g, hydroxypropyl methylcellulose K4M25g, hydroxypropylmethylcellulose K10015g, sodium alginate 25g, microcrystalline cellulose 30g, micropowder silica gel 8g, povidone K30 6g, Magnesium stearate 3g. Prepare 1000 tablets according to the preparation method described in the technical solution section.
Embodiment 2
[0025] Example 2, 75g of itopride hydrochloride, 45g of hydroxypropyl methylcellulose K4M, 15g of sodium alginate, 40g of microcrystalline cellulose, 8g of micronized silica gel, 7g of povidone K30, and 3g of magnesium stearate. Prepare 1000 tablets according to the preparation method described in the technical solution section.
Embodiment 3
[0026] Example 3, 75g of itopride hydrochloride, 50g of hydroxypropyl methylcellulose K100, 18g of sodium alginate, 50g of microcrystalline cellulose, 13g of micronized silica gel, 6g of povidone K30, and 4g of magnesium stearate. Prepare 1000 tablets according to the preparation method described in the technical solution section.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com