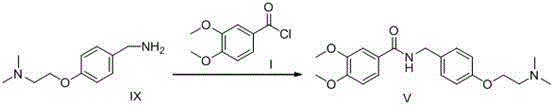
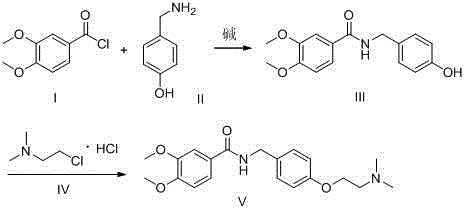
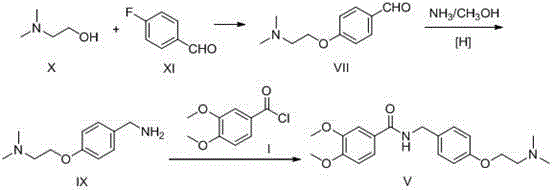
Preparation method of itopride hydrochloride
A technology of itopride hydrochloride and compound, which is applied in the field of preparation of gastrointestinal prokinetic drug itopride hydrochloride, can solve the problem of high cost, and achieve the effect of easy purchase, low price and improved safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Step 1: Add 94g (1mol) of phenol and 117.6g (2.1mol) of potassium hydroxide to 600ml of ethanol and stir to dissolve, control the temperature at 55-60°C and slowly add N,N-dimethyl chloride ethyl salt Salt 158g (1.1mol, dissolved in 300g water), after the dropwise addition, the reaction was continued for 4 hours; after the reaction was completed, ethanol was recovered, and dichloromethane was added to extract twice, combined, washed with water, and concentrated to obtain 142g oil XI, yield 86.0 %;
[0030] Step 2: Stir and dissolve 132g (0.8mol) of oil XI and 195g (1.6mol) of 30% concentrated hydrochloric acid in 400ml of water, keep the temperature below -10°C, add 24g (0.8mol) of paraformaldehyde in 4 batches, Continue the reaction for 10 hours; TLC monitors the completion of the reaction, adjust the pH to 8-9 with sodium carbonate, add dichloromethane to extract twice, combine the organic phases, concentrate to obtain an oil, then add 300ml of ethyl acetate to dissol...
Embodiment 2
[0035] Step 1: Add 94g (1mol) of phenol and 84g (2.1mol) of sodium hydroxide to 600ml of ethanol, stir and dissolve, control the temperature at 55-60°C, and slowly drop N,N-dimethylchloroethane hydrochloric acid 158g of salt (1.1mol, dissolved in 300g of water), the dropwise addition was completed and the reaction was continued for 4 hours; after the reaction was completed, ethanol was recovered, and dichloromethane was added to extract twice, combined, washed with water, and concentrated to obtain 136g of oil XI, with a yield of 82.4% ;
[0036] Step 2: Stir and dissolve 132g (0.8mol) of oil XI and 195g (1.6mol) of 30% concentrated hydrochloric acid in 400ml of water, keep the temperature below -10°C, and slowly add 40g (0.8mol) of 40% formaldehyde solution dropwise , and continued to react for 2 hours; TLC monitored the completion of the reaction, adjusted the pH to 8-9 with sodium carbonate, added dichloromethane to extract twice, combined the organic phases, concentrated t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com