Pantoprazole sodium enteric coatel tablet composition and preparation method thereof
A technology of pantoprazole sodium enteric and pantoprazole sodium is applied in the field of proton pump inhibitor pantoprazole sodium enteric-coated tablet composition and its preparation field, which can solve the problem of "peak and valley of blood drug concentration, number of administrations". Frequent problems, increased cure rate, etc., to achieve the effect of mild adverse reactions, reduced incidence of adverse reactions, and short course of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] The preparation method of pantoprazole sodium enteric-coated tablet combination preparation includes the following steps:
[0064] (1) Making pantoprazole sodium into a solid dosage unit, which has an enteric effect;
[0065] (2) The gastrointestinal motility agent Etopride Hydrochloride is made into a solid dosage unit, which has an immediate release effect;
[0066] (3) The solid dosage unit of the two active substances is mixed according to the dosage required for treatment and then compressed into tablets to achieve the therapeutic effect.
[0067] The solid dosage unit may be a pill (small pill) or granule known in the art according to the situation.
[0068] Pantoprazole sodium is unstable to acid. In the present invention, it is made into an enteric-coated solid dosage unit.
[0069] The gastrointestinal motility drug etopride hydrochloride is made into a solid dosage unit for gastric-released drugs, which can be coated with gastric dissolution or directly made into granule...
Embodiment 1
[0081] Prescription: Pantoprazole 40mg, Etopride Hydrochloride 100mg.
[0082] The preparation process is as follows:
[0083] (1) Preparation of pantoprazole sodium enteric-coated pellets:
[0084] Preparation of A pill core:
[0085]
[0086] (5% PVP liquid is made by PVP K30 And 75% ethanol aqueous solution)
[0087] Method: First, mix pantoprazole sodium with the prescription amount Na 2 CO 3 Mix evenly, mix with 1 / 2 prescription amount of starch, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, the fine powder after passing a 100-mesh sieve, mix thoroughly to obtain a mixed powder, add an appropriate amount of 5% to this mixed powder PVP or 0.5% hypromellose to prepare soft materials, spheronizing granules or pellets.
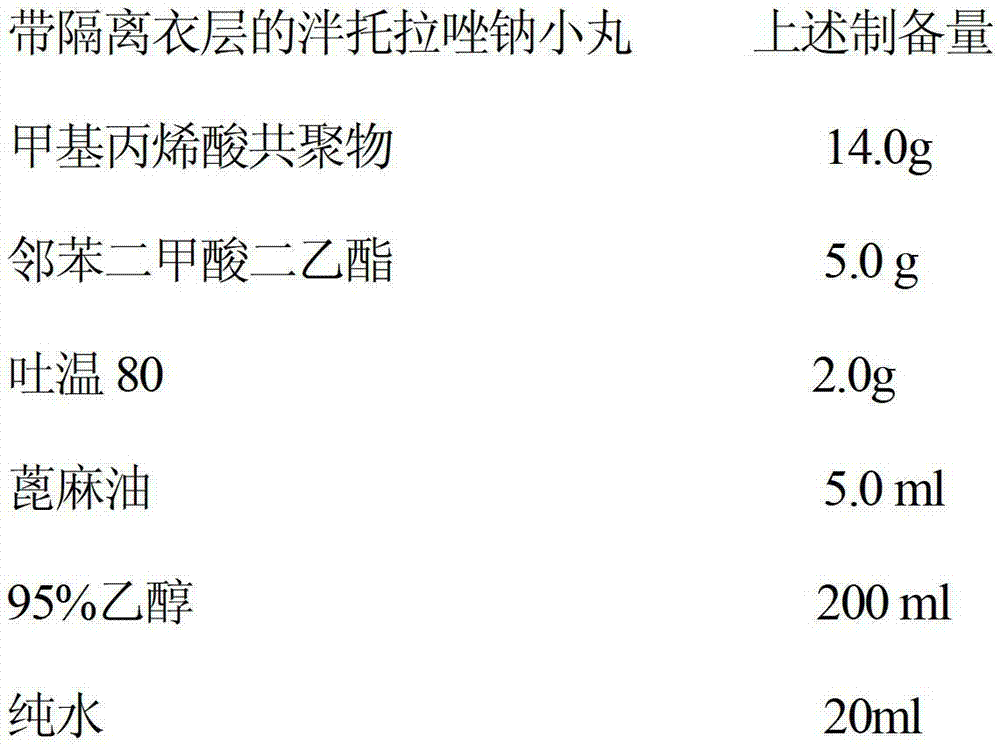
[0088] Preparation of B isolation layer:
[0089]
[0090] Soak the above-mentioned hydroxypropyl methylcellulose in 95% ethanol and pure water overnight, and add TiO 2、 Tween 80, talcum powder, and pigment are used to prepare a suspension, put th...
Embodiment 2
[0108] Prescription: Pantoprazole 20mg, Etopride hydrochloride 100mg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com