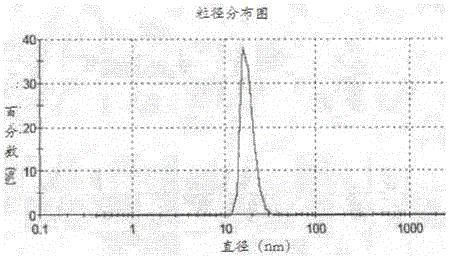
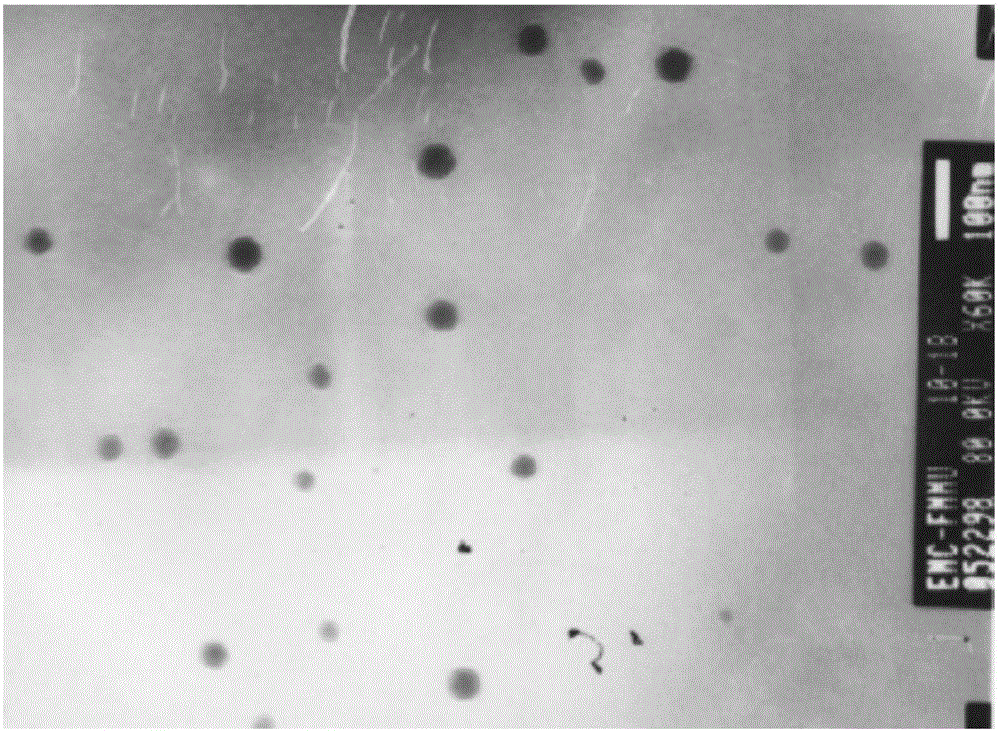
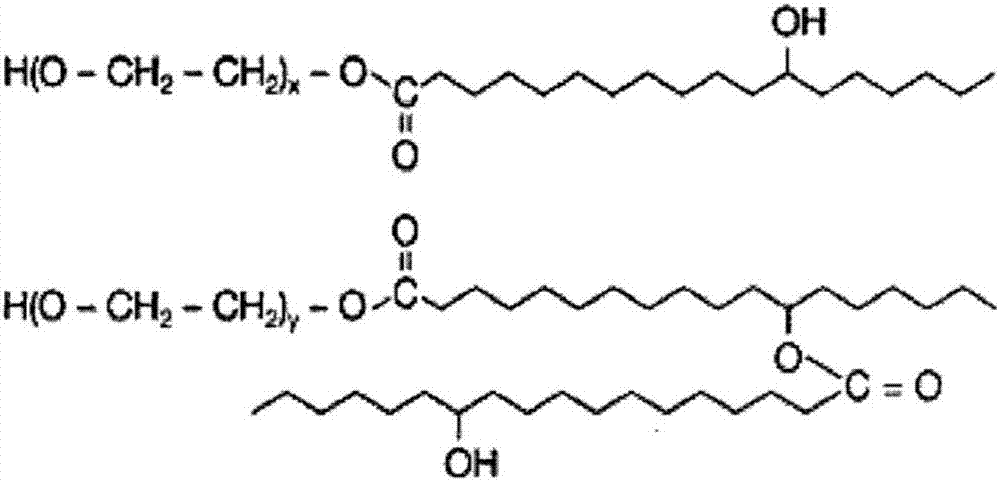
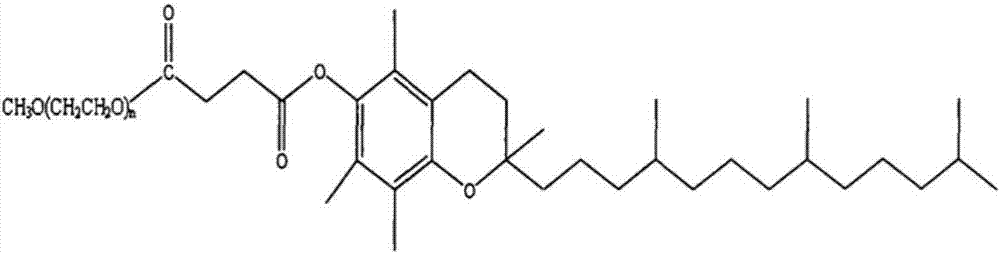
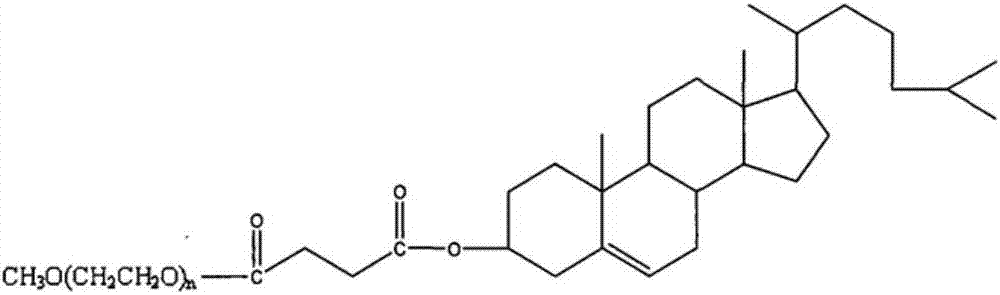
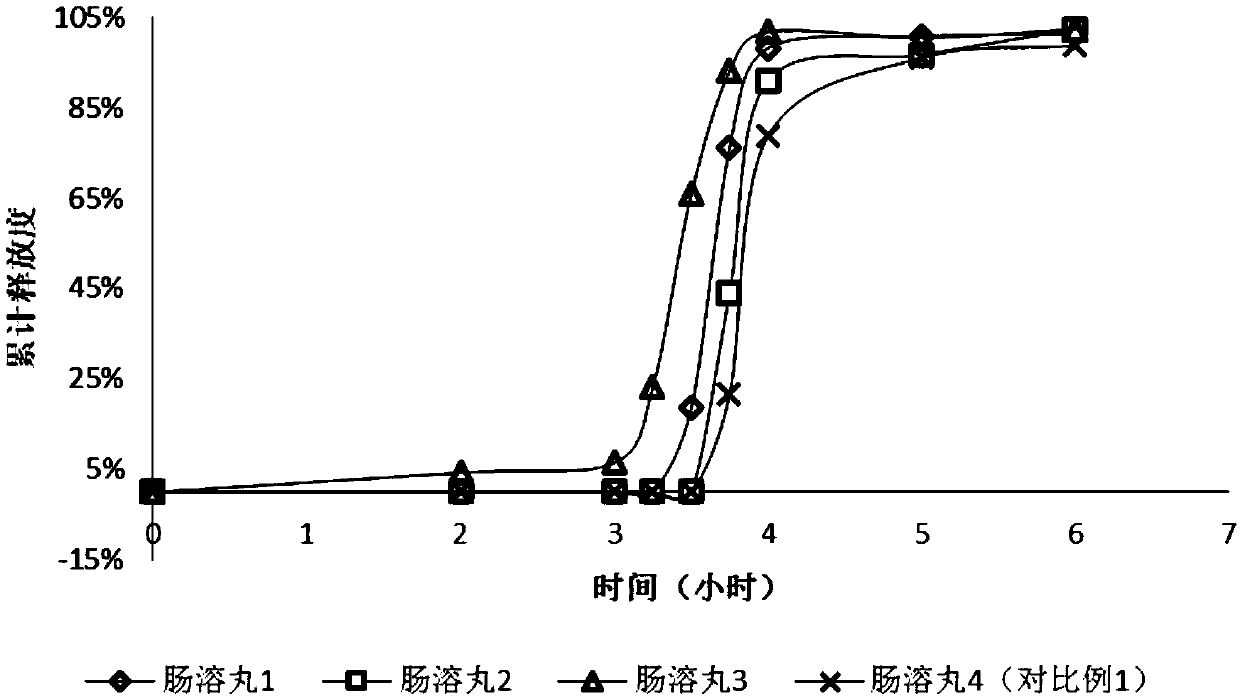
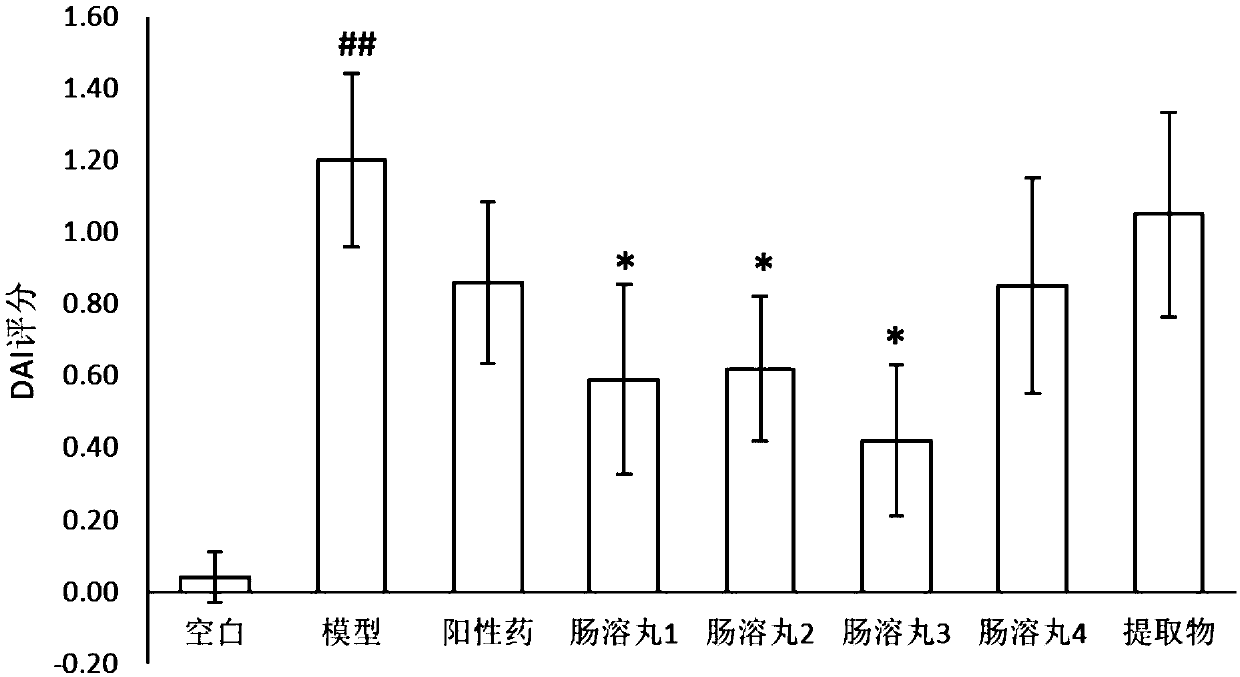
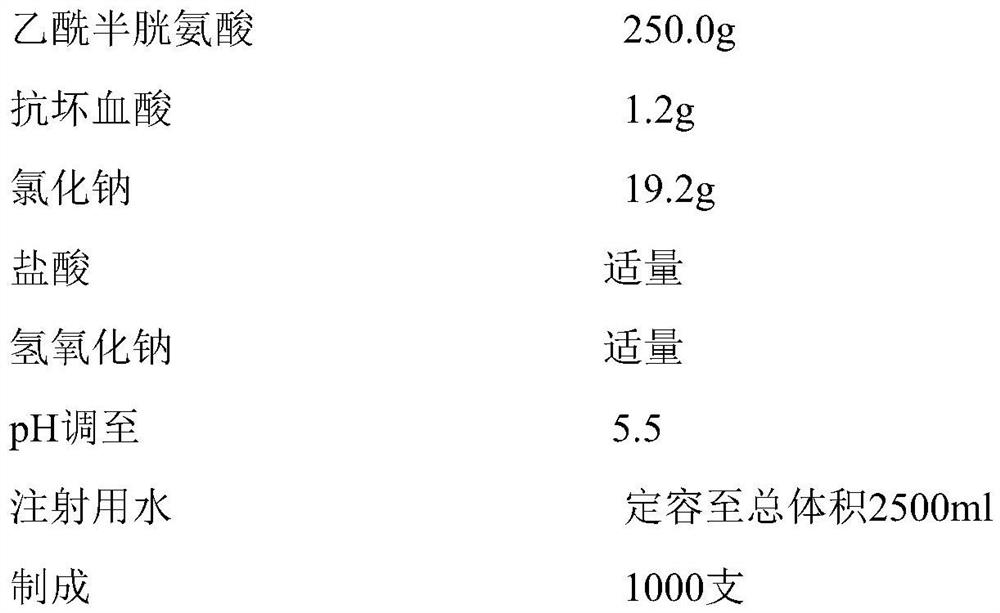
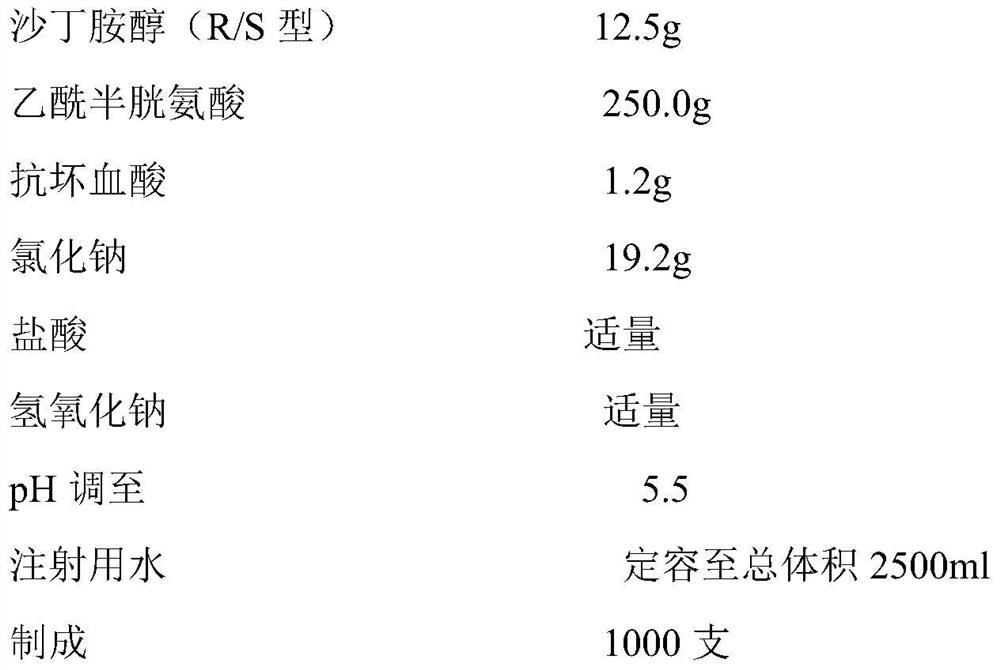
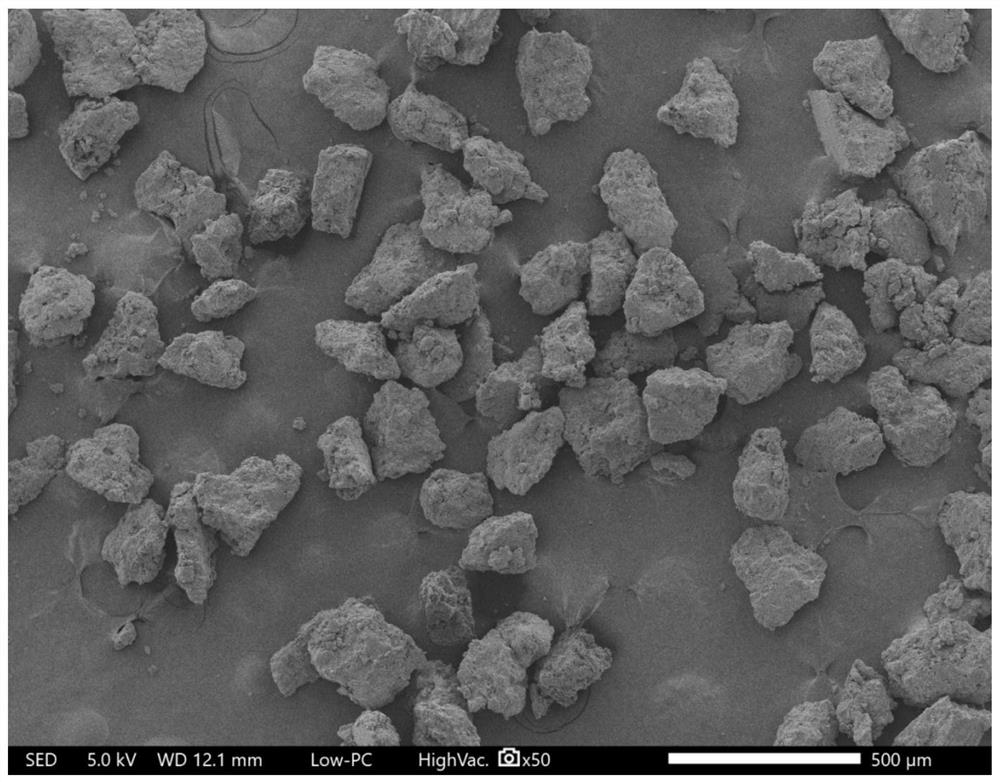
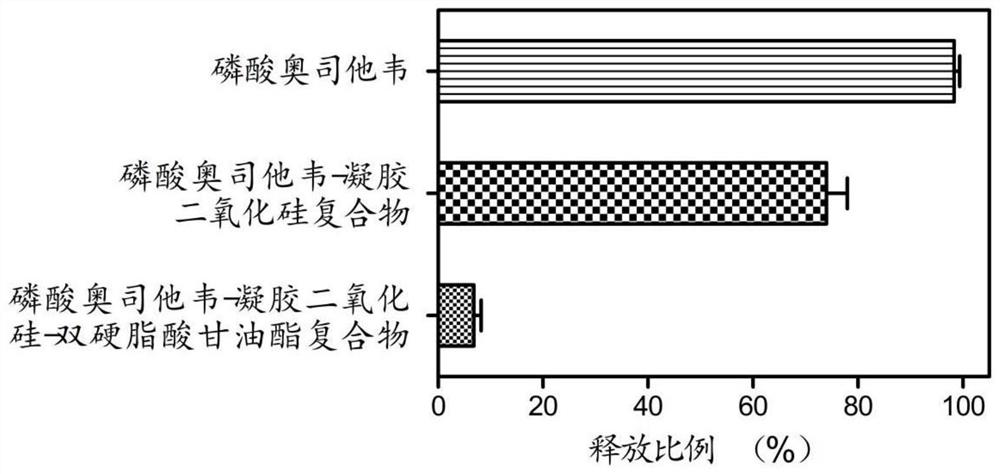
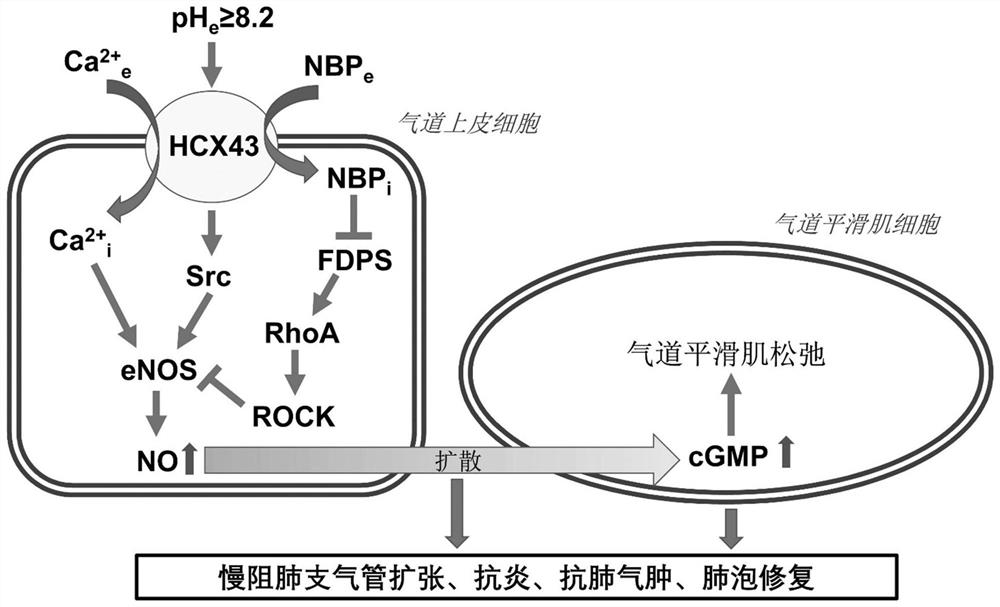
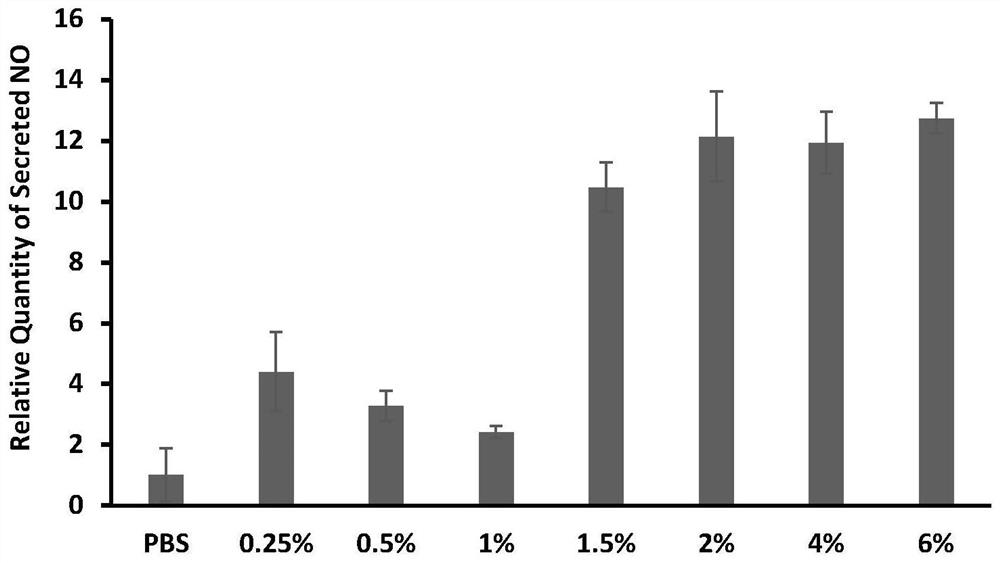
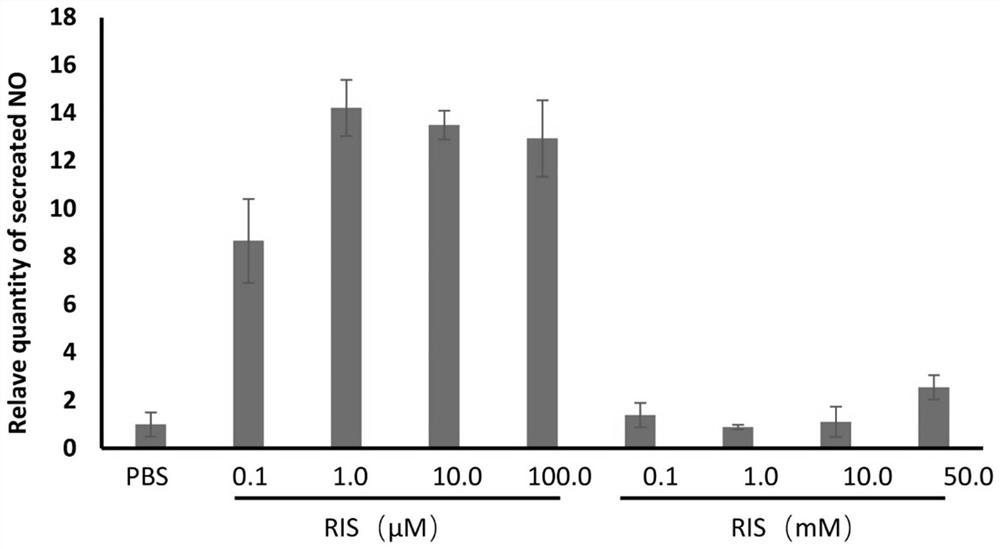
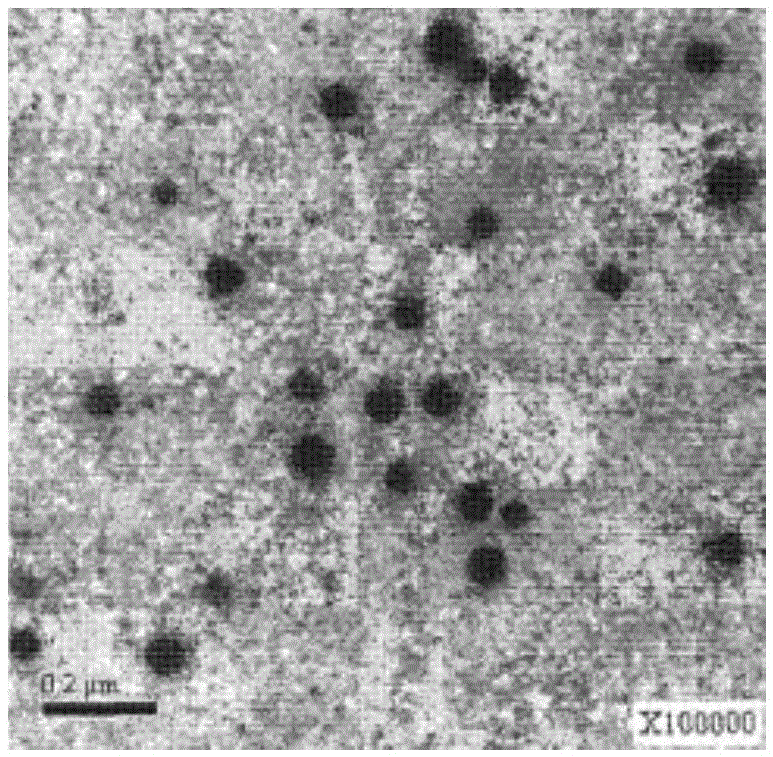
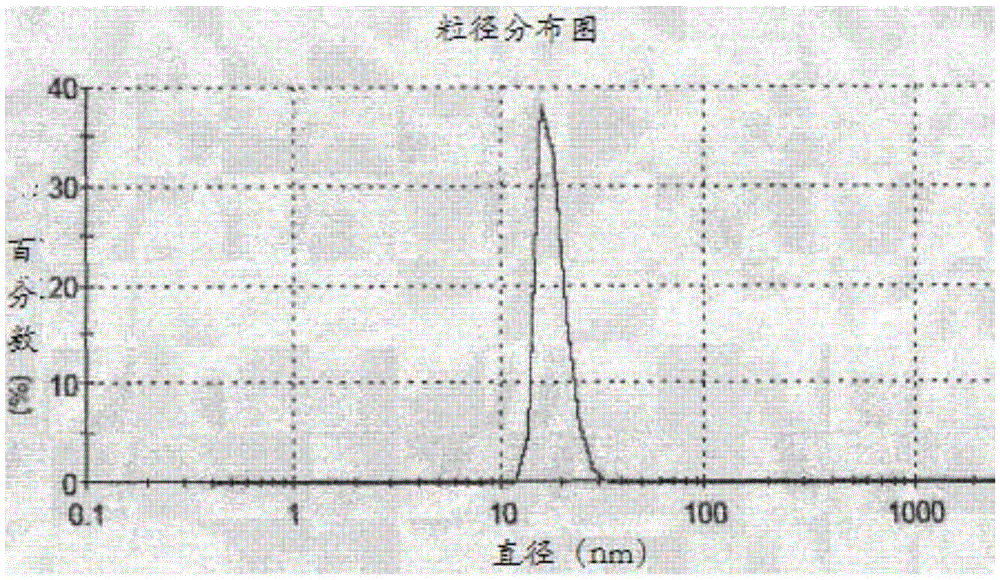
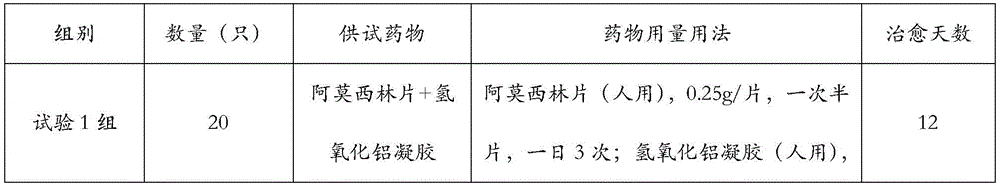
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30results about How to "Meet clinical drug needs" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oral proliposome containing atorvastatin calcium and preparation method of oral proliposome
InactiveCN103690485AHighlight substantive featuresSignificant progressMetabolism disorderPharmaceutical non-active ingredientsOral medicationPhospholipid
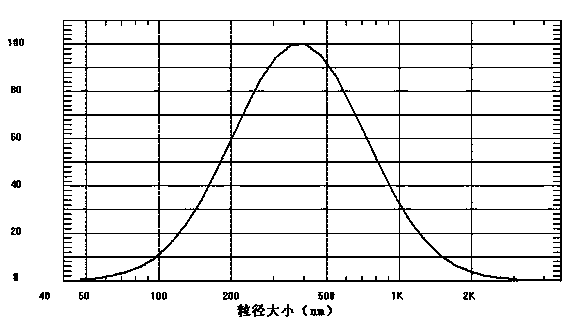
The invention relates to an oral proliposome containing atorvastatin calcium and a preparation method of the oral proliposome. The oral proliposome comprises the following components: 0.5%-10% of the atorvastatin calcium, 10%-30% of phospholipid, 2%-10% of poloxamer, 50%-87.3% of absolute ethanol and 0.2%-3% of a stabilizer. The preparation method comprises the following steps: adding the atorvastatin calcium, the phospholipid, the poloxamer and the stabilizer into the absolute ethanol solvent, mixing in the absence of water, and dissolving the mixture into a clear anhydrous solution so as to obtain an atorvastatin calcium proliposome preparation. The proliposome can be quickly self-assembled into the medicated proliposome preparation with a proper particle size and high encapsulation efficiency after being diluted by a proper hydration medium before use and is used for oral administration, the operation is easy and convenient, and the oral administration mode can be easily accepted by patients.
Owner:RUNZE PHARMACEUTICAL (SUZHOU) CO LTD
3-n-butylphthalide injection and preparation method thereof
ActiveCN103505409AEffectively mask special fragranceMask special fragranceOrganic active ingredientsPowder deliveryVinyl etherActive agent
The invention relates to a 3-n-butylphthalide injection and a preparation method thereof. The 3-n-butylphthalide injection disclosed by the invention comprises 3-n-butylphthalide or a derivant thereof, a surfactant and water for injection, wherein the surfactant is selected from one or more of phospholipid, polyethylene glycol-12-hydroxy stearate, polyethylene glycol-VE (Vinyl Ether) carbonic ester, polyethylene glycol-VE succinate, polyethylene glycol-DSPE (distearoyl phosphoethanolamine), polyethylene glycol-cholesteryl hemisuccinate, polyethylene glycol-cholesterol methyl ester, polyethylene glycol-cholesterol sulfate, polyoxyethylene dehydration sorbitol fatty acid ester and poloxamer. By adopting the 3-n-butylphthalide injection disclosed by the invention, a special fragrance of the 3-n-butylphthalide can be effectively concealed; the 3-n-butylphthalide injection is stable in quality, high in 3-n-butylphthalide content, simple in preparation technology, strong in operability and beneficial to industrialization, can be separately subpackaged into small volume of preparation, can be applied to intravenous injection, and also can be applied to intramuscular injection; the clinical drug delivery requirements are met.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Tibetan medicine of Zuotai and processing method thereof
InactiveCN107375321AStrong medicineSolve the problem of drug safetyAntibacterial agentsHeavy metal active ingredientsProcessing costRhizome
The invention provides Tibetan medicine of Zuotai and a processing method thereof, and belongs to the field of Tibetan medicine processing. The processing method comprises the following steps that sulfur by water extraction liquid of rhizoma acori tatarinowii and rhizome imperatae sespia esculenta is steamed and boiled for 2 to 4h; soaking is performed for 18 to 30h; then, the sulfur is covered into blocks; the blocks are flushed and soaked by water for 6 to 8 days; sulfur powder is obtained after drying and crushing; mercury subjected to rust removal and detoxifcation is mixed with the sulfur powder; grinding is performed for 18 to 30h; then, obtained materials are mixed with metal ash, biotite ash, seven-mineral ash and pomegranate water extraction liquid; grinding is performed for 18 to 30h; then, drying is performed. The processing method has the advantages that the process stability is high; the quality is controllable; the processing cost is low; the Zuotail obtained by the processing method has low toxicity and strong medicine effect; the problem of medication safety of the Zuotai is effectively solved; the clinic medication requirements are met.
Owner:青海省藏医院
Vagina effervescent tablets contg. callicarpa nudiflora, and its prepn. method
InactiveCN1724034AQuick releaseQuality improvementPill deliverySexual disorderEffervescent tabletGynecology
A vaginal effervescent tablet with high release speed and no irritation is prepared from achamydeous beautyberry leaf, effervescent agent and auxiliary. Its preparing process is also disclosed.
Owner:石药集团中诺药业(石家庄)有限公司
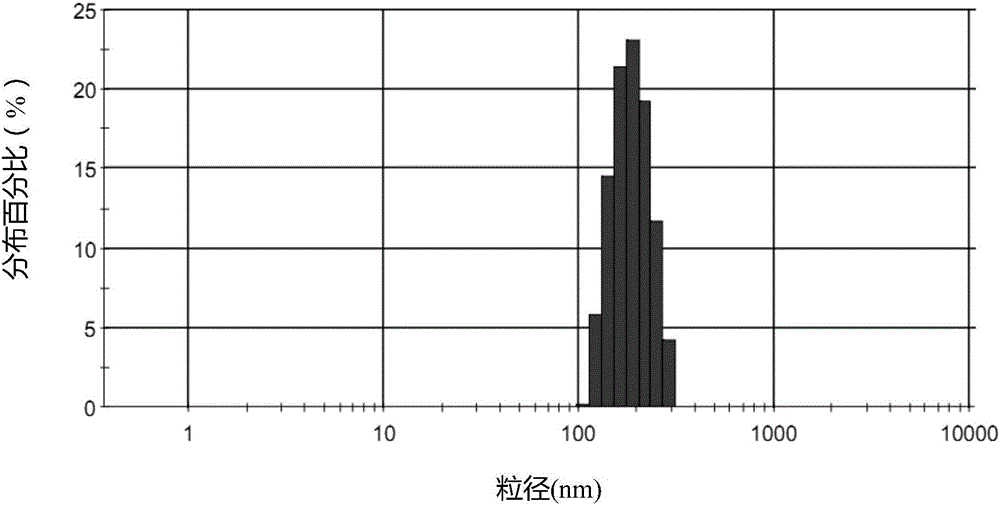
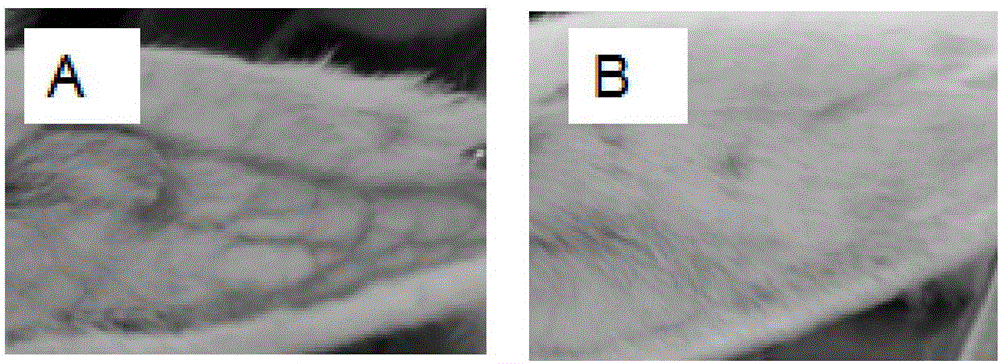
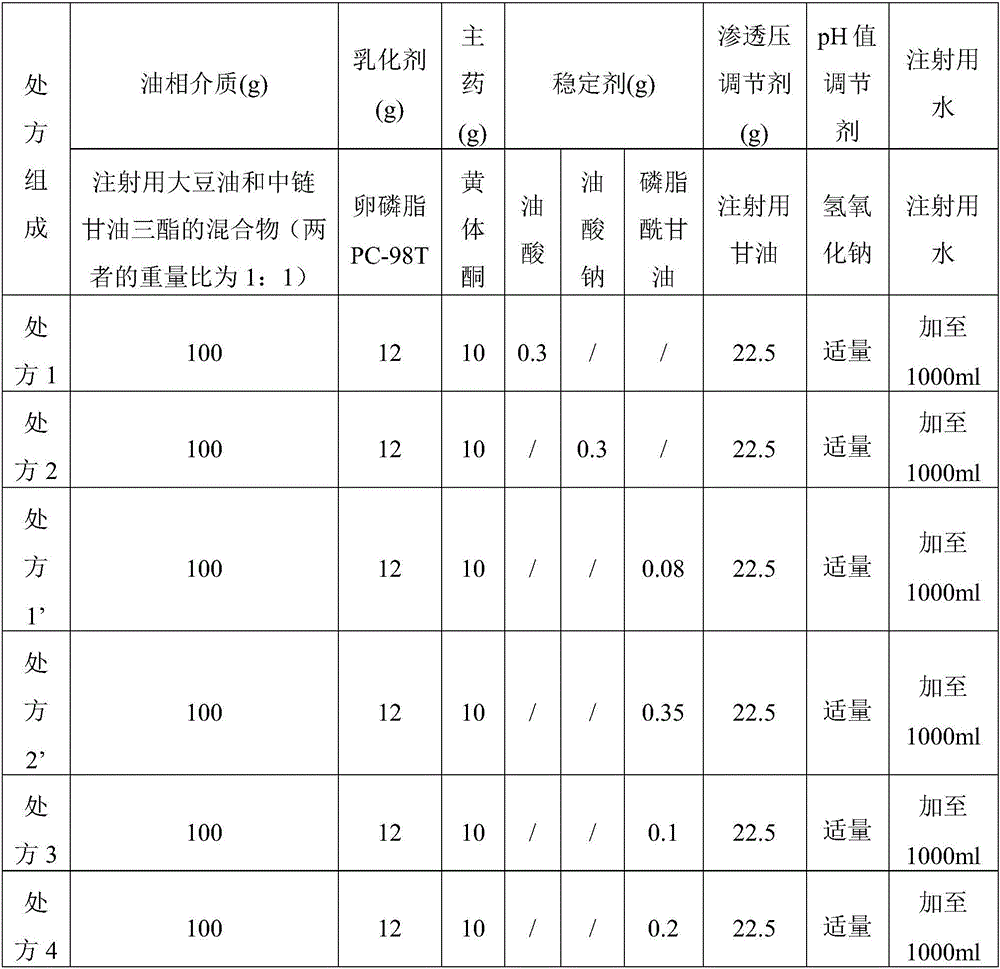
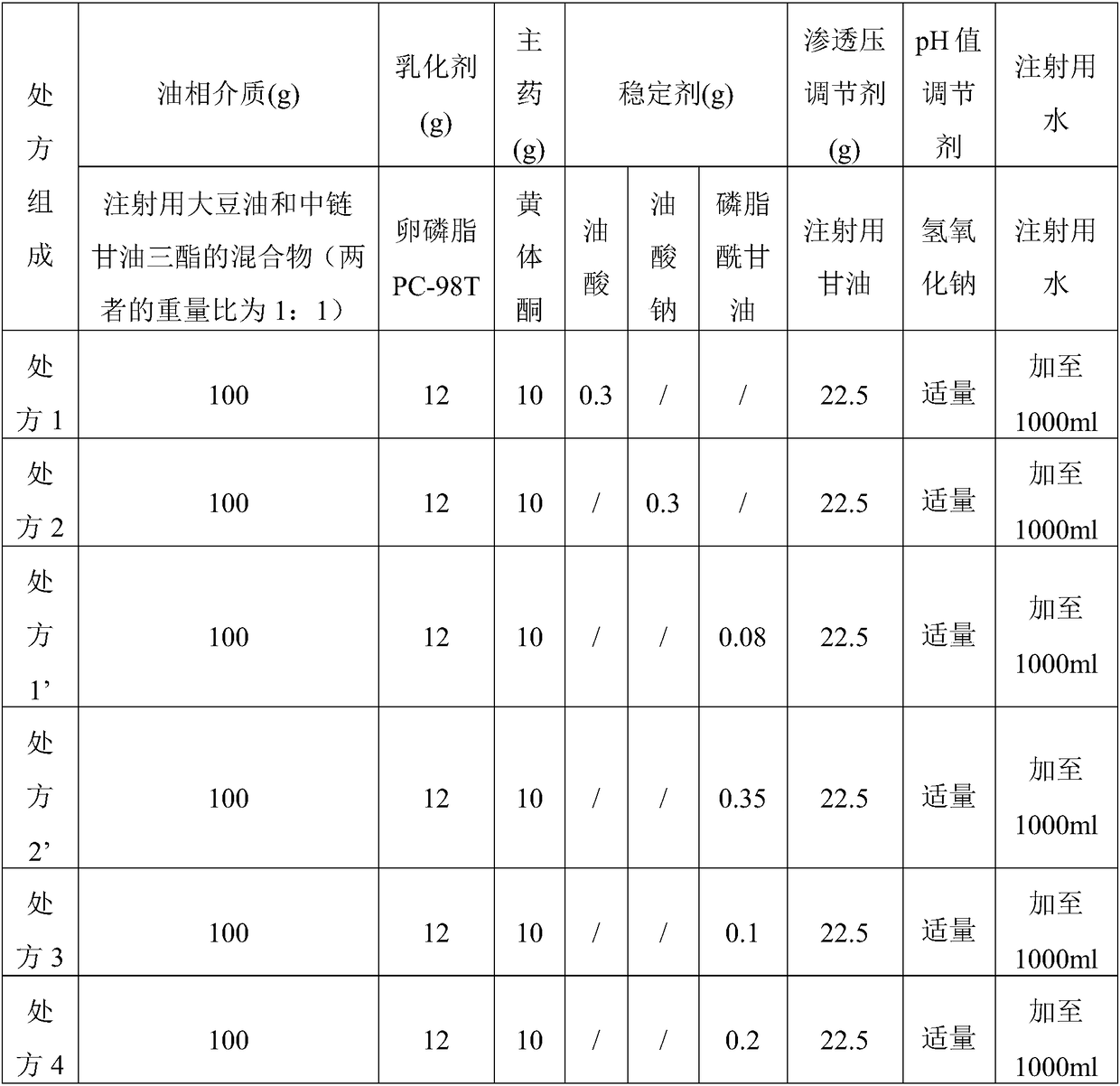
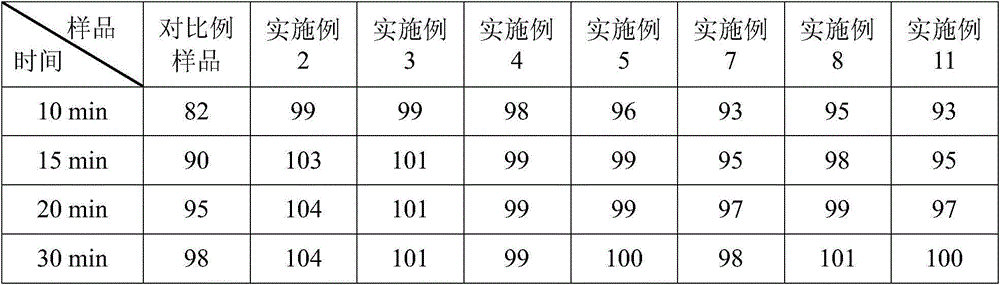
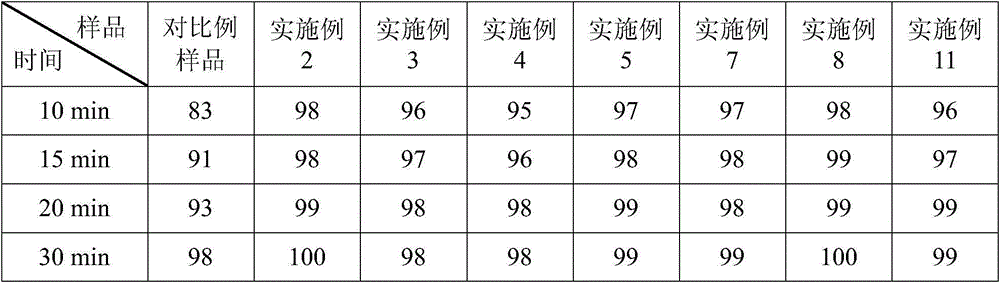
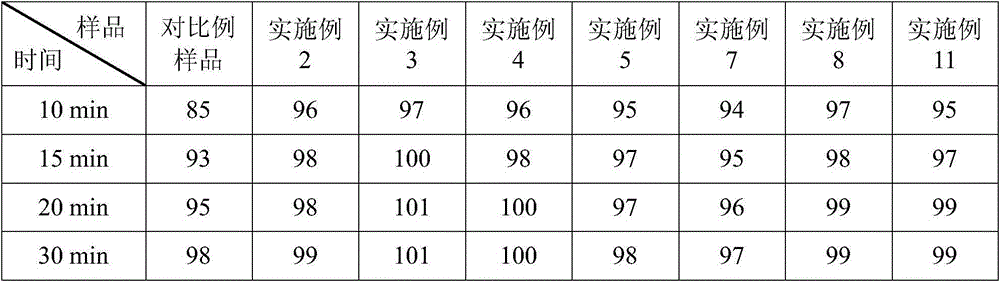
Lipid microballoon injection solution for progestational hormone drug and preparation method thereof
ActiveCN106074383ANon-irritatingImprove complianceOrganic active ingredientsEmulsion deliveryLipid formationIrritation
The invention discloses a lipid microballoon injection solution for a progestational hormone drug and a preparation method thereof. The lipid microballoon injection solution for the progestational hormone drug comprises progestational hormone drug progesterone as a primary drug and pharmaceutically acceptable drug excipient, wherein the drug excipient comprises oil phase, emulsifier, osmotic pressure adjusting agent, stabilizer, pH value modifier and injection water. The lipid microballoon injection solution for the progestational hormone drug is stable in quality, free from injection irritation, high in safety, applicable to local intramuscular injection and intravenous injection, and beneficial to promotion of compliance of clinical medication of patient and enriches clinical medication selection.
Owner:WUHAN CONFORM PHARMA CO LTD
Tibetan-medicine three-component Tibet inula soup-granula composition and preparation process thereof
The invention relates to a Tibetan medicine, and particularly relates to a Tibetan-medicine three-component Tibet inula soup-granula composition and a preparation process thereof. The composition disclosed by the invention is prepared from the following raw materials in parts by weight: Ramulus Rubi, Tinospora sinensis and Tibet inula in a proportion of 2:1:1. The preparation process comprises the following steps: 1) carrying out superfine grinding on the Tibet inula; 2) placing the Tinospora sinensis and the Ramulus Rubi in a multifunctional extraction pot to decoct, filtering and concentrating to obtain an aqueous extract, and drying the aqueous extract by using a centrifugal spray drier; 3) combining dry cream powder with micro-powder; and 4) adding ethanol into the obtained powder, preparing into a soft material, granulating the soft material by using a granulator, drying, and carrying out straightening granulation to obtain the composition. According to the composition disclosed by the invention, when the quality of Tibetan medicines is improved, due to the reference and introduction of some new processes, new techniques and new devices, the stability and bioavailability of preparations can be improved, and traditional Tibetan medicine and modern preparation methods are combined, thus a way for the rational development of Tibetan medicines is explored.
Owner:多杰 +3
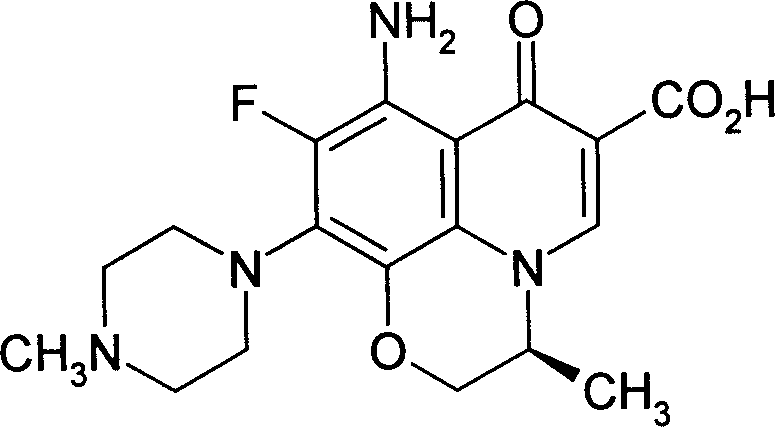
Carbostyrile genus antibiotic preparation took orally
InactiveCN101081216ABroad spectrum antibacterialImprove antibacterial propertiesAntibacterial agentsPowder deliveryCoated tabletsSide effect
The present invention provides one kind of orally taken quinolone antibiotic preparation, which contains quinolone antibiotic, its salt or their hydrate, and may be prepared into tablet, dispersed tablet, coated tablet, enteric coated tablet, slow released tablet, capsule or granule. The quinolone antibiotic preparation has dosage of 20-1000 mg, and contains stuffing in 10-60 wt% and disintegrant in 5-30 wt%. Quinolone antibiotic has phamacodynamic activity similar to that of levofloxacin, and possesses broad antibiotic spectrum, less toxic side effect and other advantages. The quinolone antibiotic preparation is stable, controllable, easy in taking, safe and effective.
Owner:严明
Method for extracting amygdalin from loquat cores by adopting supercritical CO2 extraction
InactiveCN104163837AHigh purityImprove extraction efficiencySugar derivativesSugar derivatives preparationAlcohol ethylAmygdalin
The invention relates to a method for extracting amygdalin from loquat cores by adopting supercritical CO2 extraction. The method comprises the following specific steps: grinding the loquat cores into loquat core powder; and performing supercritical CO2 extraction by taking absolute ethanol as an entrainer, wherein the amygdalin yield reaches more than 2.68% under the optimized conditions. Due to absolute ethanol taken as the entrainer, the selectivity and solubility of the amygdalin in the loquat cores in a supercritical fluid can be influenced; by virtue of the solubility difference of amygdalin and impurities such as protein and polysaccharide in the loquat cores in the ethanol entrainer, the alcohol-soluble amygdalin is pointedly extracted out and the extraction efficiency is high; an obtained amygdalin product is high in purity, free of harmful solvent residues and good in quality. The method is simple in process, low in cost and easy for industrial production.
Owner:HEFEI UNIV OF TECH
A kind of stable flurbiprofen axetil micro-nano emulsion and preparation method thereof
ActiveCN104188905BImprove stabilityHigh dependenceOrganic active ingredientsAntipyreticMicro nanoPolyethylene glycol
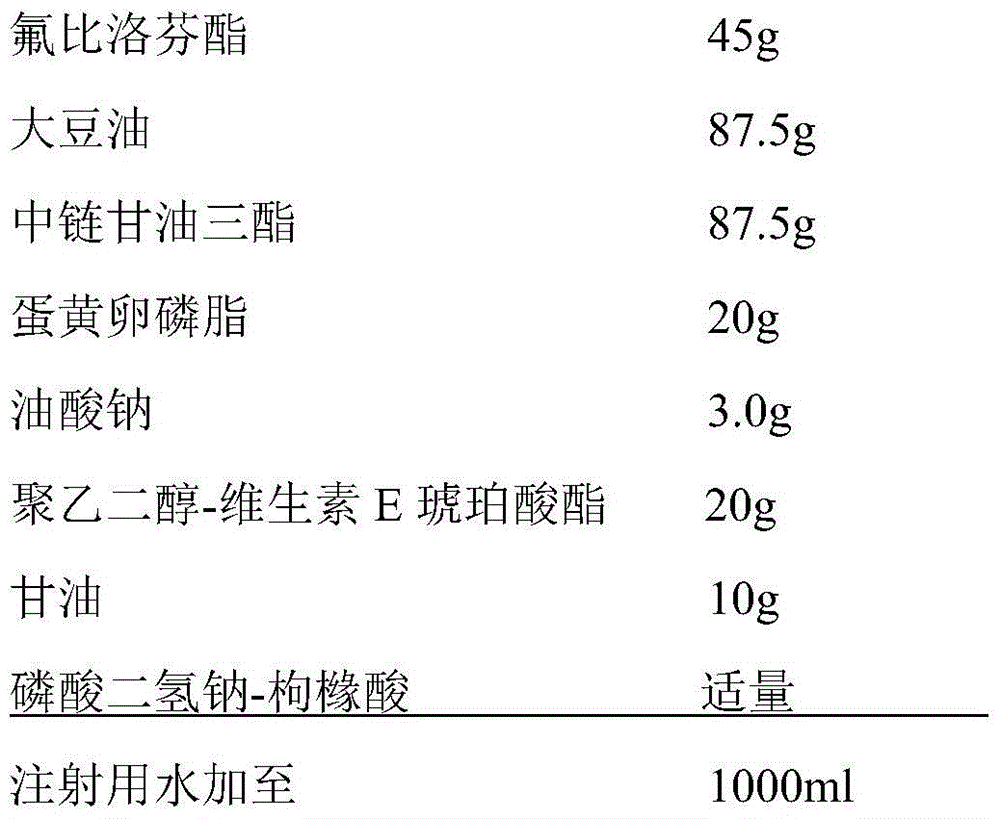
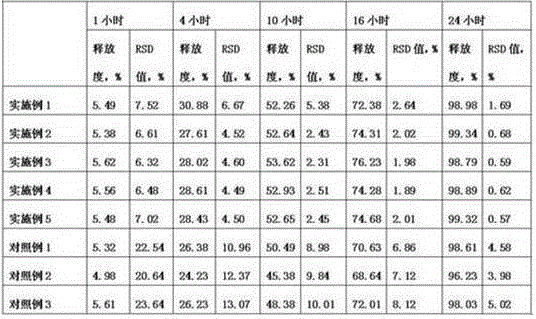
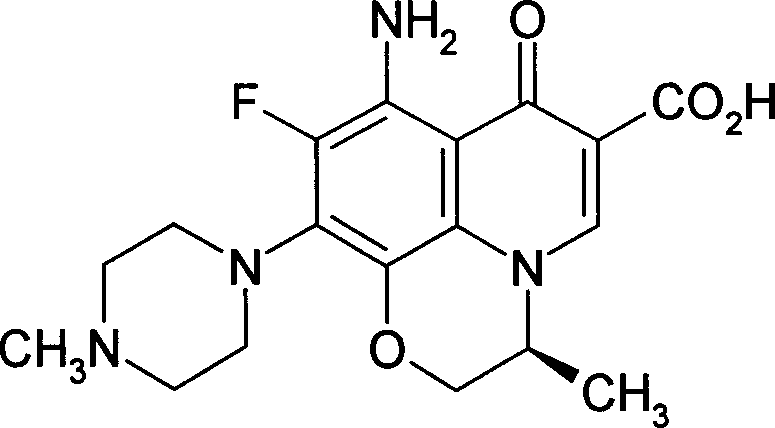
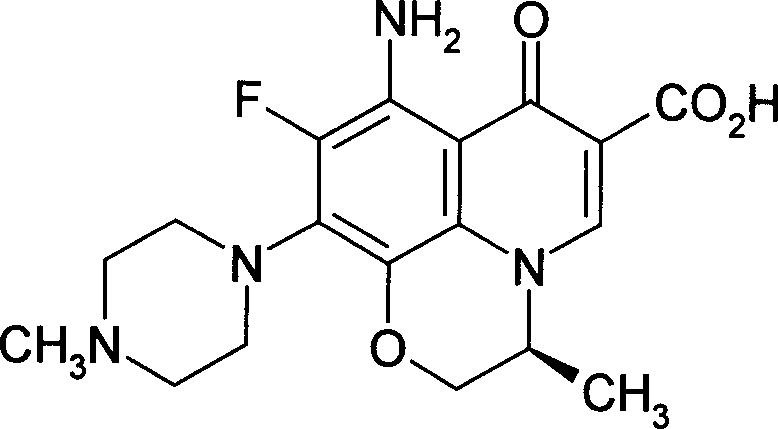
The invention relates to a stable flurbiprofen axetil micro-nano emulsion and a preparation method thereof, belonging to the technical field of pharmaceutical preparations. A stable flurbiprofen axetil micro-nano emulsion of the present invention contains flurbiprofen axetil, vegetable oil as a solvent, lecithin as a surfactant, polyethylene glycol derivatives, etc., has good stability, Quick effect, low toxicity and side effects.
Owner:HEBEI YIPIN PHARMA
Itopride hydrochloride composition
ActiveCN105267171AMeet clinical drug needsSolve the problem of large differences in releaseOrganic active ingredientsDigestive systemExtended release tabletsMethyl cellulose
The invention relates to an itopride hydrochloride sustained-release tablet composition. The technical scheme comprises that the cores of per 1000 tablets of itopride hydrochloride composition comprises 75 g of itopride hydrochloride, 45 g of Hydroxypropyl methyl cellulose, 15 g of sodium alginate, 40 g of microcrystalline cellulose, 8 g of aerosi, 7 g of polyvinylpyrrolidone K30, and 3 g of magnesium stearate. The beneficial effects comprise that the itopride hydrochloride sustained-release tablet according with clinic requirements is obtained through reasonable prescription adjusting, and the problem that the table release degree difference of the itopride hydrochloride sustained-release tablets is large is solved.
Owner:DISHA PHARMA GRP
Carbostyrile antibiotic injection preparations
InactiveCN101081210ABroad spectrum antibacterialSmall toxicityAntibacterial agentsPowder deliverySide effectFreeze-drying
The present invention provides one kind of quinolone antibiotic injection, which contains quinolone as antibiotic, its salt or their hydrate, and may be prepared into instant injection, concentrated solution for compounding injection or freeze dried powder for compounding injection. Quinolone antibiotic has phamacodynamic activity similar to that of levofloxacin, and possesses broad antibiotic spectrum, less toxic side effect and other advantages. The quinolone antibiotic injection is effective, safe and reliable.
Owner:严明
Altrenogest nanometer emulsion and method for preparing same
InactiveCN105878184AHigh thermodynamic stabilityGood storage stabilityOrganic active ingredientsEmulsion deliveryOral medicationHepatic first pass effect
The invention provides altrenogest nanometer emulsion and a method for preparing the same. The altrenogest nanometer emulsion comprises, by weight, 0.1-1 part of altrenogest, 15-35 parts of surfactants, 1.5-20 parts of auxiliary surfactants, 2-30 parts of oil and 26-45 parts of water for injection. The altrenogest nanometer emulsion and the method have the advantages that the altrenogest nanometer emulsion is good in thermodynamic stability, stable emulsion states can be completely recovered after the altrenogest nanometer emulsion is heated until the temperature of the altrenogest nanometer emulsion reaches 80 DEG C, then the altrenogest nanometer emulsion is allowed to stand still and then is sufficiently vibrated, the altrenogest nanometer emulsion is good in storage stability, layering can be prevented after the altrenogest nanometer emulsion is allowed to stand for a long time, the altrenogest nanometer emulsion not only can be orally administered by the aid of drinking water, but also can be administered after being mixed with materials, oral administration nano-emulsion substrates can effectively protect the altrenogest from being damaged by gastric acid, and the shortcoming of first-pass effects of livers can be overcome; the method for preparing the altrenogest nanometer emulsion is simple, and accordingly requirements of large-scale industrial production can be met.
Owner:TIANJIN ZHONGSHENG TIAOZHAN BIOTECH
Butyphthalide injection and preparation method thereof
ActiveCN107970208AEffectively mask special fragranceMask special fragrancePowder deliveryOrganic active ingredientsPolyethylene glycolHydroxystearic Acid
The invention relates to a butyphthalide injection and a preparation method of the butyphthalide injection. The butyphthalide injection disclosed by the invention contains butyphthalide or a derivative of butyphthalide, a surfactant and water for injection, wherein the surfactant is selected from one or more of phospholipid, polyethylene glycol-12-hydroxy stearate, polyethylene glycol-VE carbonate, polyethylene glycol-VE succinate, polyethylene glycol- distearoyl phosphatidyl ethanolamine, polyethylene glycol-cholesteryl succinate, polyethylene glycol-cholesterol methyl ester, polyethylene glycol- cholesterol sulfate, polyoxyethylene sorbitan fatty acid ester, and poloxamer. The butyphthalide injection disclosed by the invention can effectively mask the special fragrance of butyphthalide,the quality is stable, the content of butyphthalide is high, the butyphthalide injection can be sub-packaged into a preparation with small volume, can be used for intravenous injection, and also can be used for intramuscular injection, so that the requirements of clinical administration are met; a preparation process is simple, the operability is strong, and the industrialization is facilitated.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
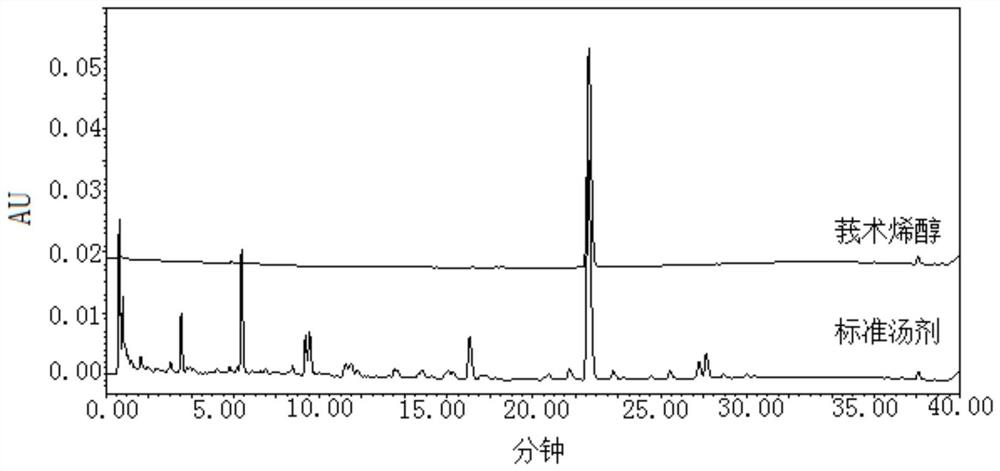
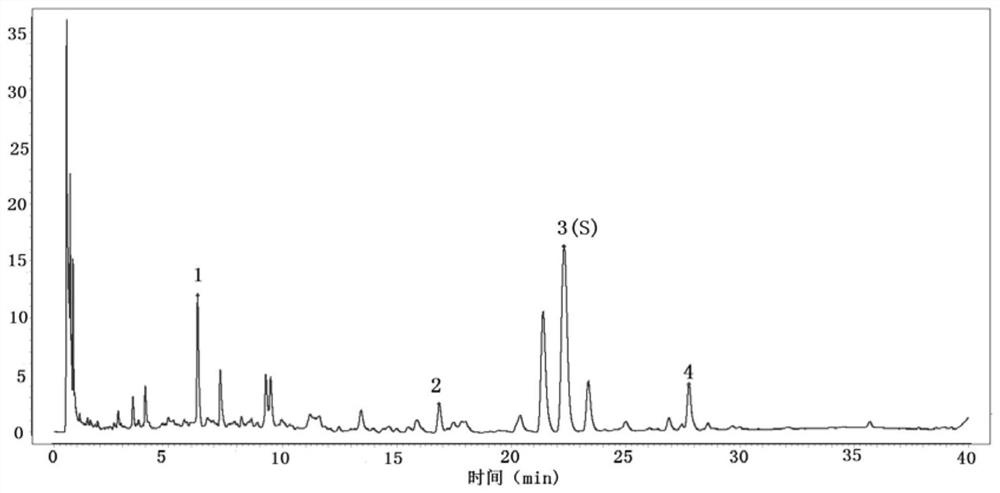
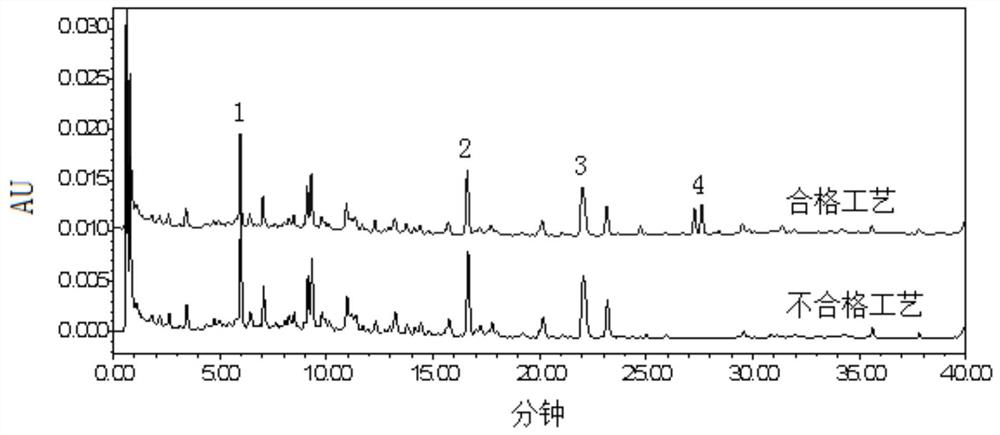
Quality control method of rhizoma wenyujin concisum formula granules
ActiveCN113759006ATo achieve the purpose of quality controlAccurate quality judgmentComponent separationFormularyFluid phase
The invention provides a quality control method of rhizoma wenyujin concisum formula granules, which comprises the following steps: obtaining a characteristic chromatogram of a test solution by adopting high performance liquid chromatography, calculating the relative retention time of each characteristic peak and the reference peak S by taking the chromatographic peak of curcumenol in the characteristic chromatogram as the reference peak S, wherein the relative retention time of at least three characteristic peaks is within + / -10% of specified values, and the specified values are 0.28, 0.76 and 1.25. The curcumenol which is relatively high in content and relatively stable is selected as a quality evaluation index component of the rhizoma wenyujin concisum formula granule, and the overall quality control of the rhizoma wenyujin concisum formula granule is realized in combination with common control of other quality evaluation index components; the method has the advantages that judgment is more accurate and objective, and clinical medication requirements are met.
Owner:BEIJING KANGRENTANG PHARMA
Formula for Tibetan medicines for treating gynecological qi diseases, medicinal liquor for treating same and process for preparing medicinal liquor
InactiveCN107854684ATibetan medicine works fastGood curative effectNervous disorderDispersion deliveryYeastDisease
The invention belongs to the field of technologies for preparing medicines and Tibetan medicines, and relates to a formula for Tibetan medicines for treating gynecological qi diseases, medicinal liquor for treating the same and a process for preparing the medicinal liquor. Medicinal materials, water and distiller's yeast in the formula are fermented, extracted and processed to obtain the medicinalliquor with the Tibetan medicines. The Tibetan medicines in the formula comprise, by weight, 3000-5000 parts of honey, 5-8 parts of gypsum rubrum, 2-4 parts of elettaria cardamomum, 2-4 parts of rhizoma podophylli emodi, 2-4 parts of fructus piperis longi, 2-5 parts of pimenta and 2-5 parts of rhizoma zingiberis. The process for preparing the medicinal liquor with the Tibetan medicines includes honey water preparing, distiller's yeast medicinal material package manufacturing, fermentation, extraction, disinfection and filling working procedures. The formula, the medicinal liquor and the process have the advantages that the medicinal liquor with the Tibetan medicines has functions of expelling wind, clearing heat, astringing yellow liquid and regulating menstruation and is applicable to endocrine disorder, irregular menstruation, insomnia and forgetfulness and cold backs due to the gynecological qi diseases and skin itch due to qi bone heat, kidney heat and yellow liquid in joints; theTibetan medicines and the medicinal liquor are stable in quality, and obvious curative effects can be realized.
Owner:索南扎西
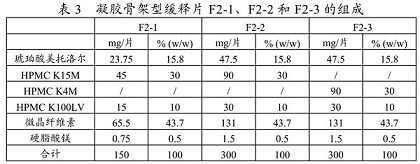
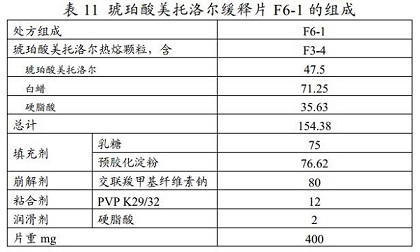
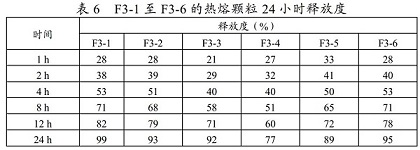
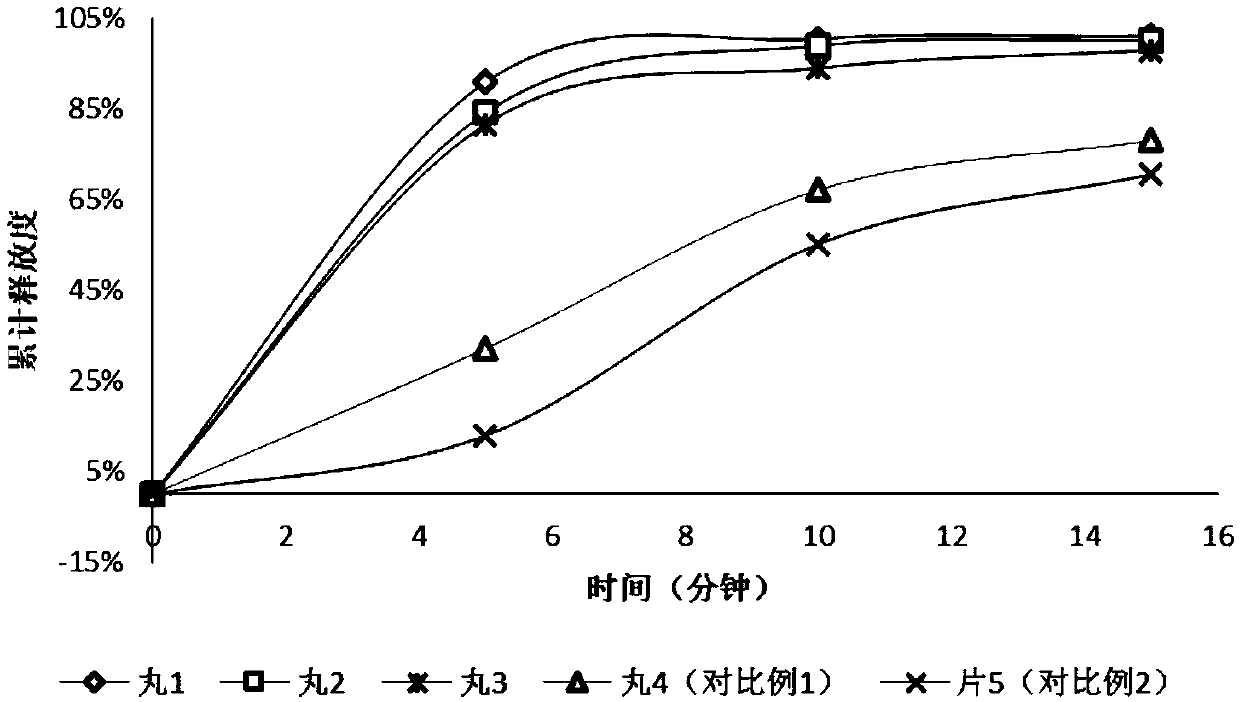
Metoprolol succinate sustained-release tablet and preparation method thereof
ActiveCN113925839ASimple production processReduce the difficulty of process amplificationOrganic active ingredientsPharmaceutical non-active ingredientsBiomedical engineeringPharmaceutical Aids
The present invention provides a metoprolol succinate sustained-release tablet and a preparation method thereof. Metoprolol succinate sustained-release particles are firstly prepared by adopting a hot melting process, and then the metoprolol succinate sustained-release tablet is prepared by granulating and tabletting the sustained-release particles, a disintegrating agent and other additional auxiliary materials. The metoprolol succinate sustained-release tablet provided by the invention has dissolution consistency before and after breaking under the condition that the metoprolol succinate sustained-release tablet is broken off by a patient according to the doctor's advice, can avoid the problem that a sustained-release particle coating layer in the existing sustained-release tablet is easy to break during tablet pressing, can significantly simplify the production process, and reduces the manufacturing cost of a unit preparation, and the clinical medication requirements under the condition of medical insurance cost control in China are met.
Owner:北京联嘉医药科技开发有限公司
Featherleaf rodgersflower and pyrola herb contained tablet for treating mammary disease and preparation processthereof
InactiveCN100342863CEasy to acceptQuality improvementUnknown materialsDrageesPyrolaTraditional medicine
The invention provides a featherleaf rodgersflower and pyrola herb tablet which is prepared from extract powder extracted from 400-500 parts of Yantuo, Chinese pyrola herb 55-65, deglued antler powder 5-10 and balancing tabletting adjuvant. The preparing process comprises the following steps, (1) subjecting Yantao 445 to ethanol backflow, merging the extracts, filtrating, reclaiming ethanol from the filtrate, concentrating to obtain clear grease with a specific gravity of 1.15-1.25, (2) disintegrating herba pyrolae 62.5, deerhorn cream 7.5, passing through 100 mesh sieve, (3) mixing herba pyrolae, deerhorn cream with Yantao clear cream, charging auxiliary materials, bed blending, obtaining granules, drying, granulating and tabletting.
Owner:石药集团中诺药业(石家庄)有限公司
Periplaneta americana extract enteric-coated preparation and preparation method thereof
ActiveCN111374962AImprove bioavailabilitySmooth releaseAnthropod material medical ingredientsDigestive systemMicrobiologyBioavailability
The invention discloses a periplaneta americana extract enteric-coated preparation and a preparation method thereof. Sequentially, the release amount of the enteric-coated preparation in artificial gastric juice within 2 hours is not more than 10%, the release amount of the enteric-coated preparation in artificial intestinal juice within 1 hour is not more than 10%, and the release amount of the enteric-coated preparation in artificial colonic juice within 1 hour is not less than 90%; the enteric-coated preparation comprises medicine-containing coated granules; and the outer surfaces of the medicine-containing coated granules are coated with enteric coatings. The enteric-coated preparation provided by the invention has the advantages of almost no release in the stomach, quick release in the intestinal tract environment, stable release, high bioavailability and the like, the requirements of existing clinical medication can be met, and the preparation process is simple and convenient.
Owner:INNER MONGOLIA JINGXIN PHARMACEUTICAL CO LTD +2
Pharmaceutical composition for treating respiratory system diseases and preparation method thereof
InactiveCN113018444AEasy to transportEasy to storeInorganic non-active ingredientsPharmaceutical delivery mechanismDiseaseAdrenergic receptor agonists
The invention discloses a pharmaceutical composition for treating respiratory system diseases and a preparation method thereof. The composition comprises the following components: a bronchiectasis agent, acetylcysteine, a stabilizer, an osmotic pressure regulator, a pH regulator, a dispersion medium and inert gas, wherein the bronchiectasis agent is a beta2 adrenergic receptor agonist or an anti-choline drug. The stability of the pharmaceutical composition is improved by adding the stabilizer and filling the inert gas in the composition of the pharmaceutical composition so that the pharmaceutical composition is convenient to store, transport and use.
Owner:HAINAN STAR PHARM CO LTD
A kind of taste-masking complex based on porous carrier and its preparation method and application
ActiveCN112641718BHigh drug loadingRelease behavior is controllableOrganic active ingredientsInorganic non-active ingredientsPediatric patientOrally disintegrating tablet
The invention provides a taste-masking compound based on a porous carrier and a preparation method and application thereof. The taste-masking compound is composed of a readily soluble bitter drug, a porous carrier and a release modifier; the release of the drug from the taste-masking compound can be regulated. , significantly reducing the contact chance of bitter drugs with taste buds in the oral environment, so as to achieve the purpose of taste masking. The present invention provides the formulation composition and preparation method of the above-mentioned taste-masking compound. The taste-masking compound is suitable for the taste-masking of high-dose bitter drugs, and the drug release can be regulated as required, and can be further prepared into suitable dosage forms for children such as granules, dry suspensions, orally disintegrating tablets, dispersible tablets and chewable tablets, etc. It can improve the compliance of children's medication and meet the clinical medication needs of pediatric patients.
Owner:SHANDONG UNIV +1
A bisphosphonate drug for inhalation, its preparation method and its use in chronic obstructive pulmonary disease
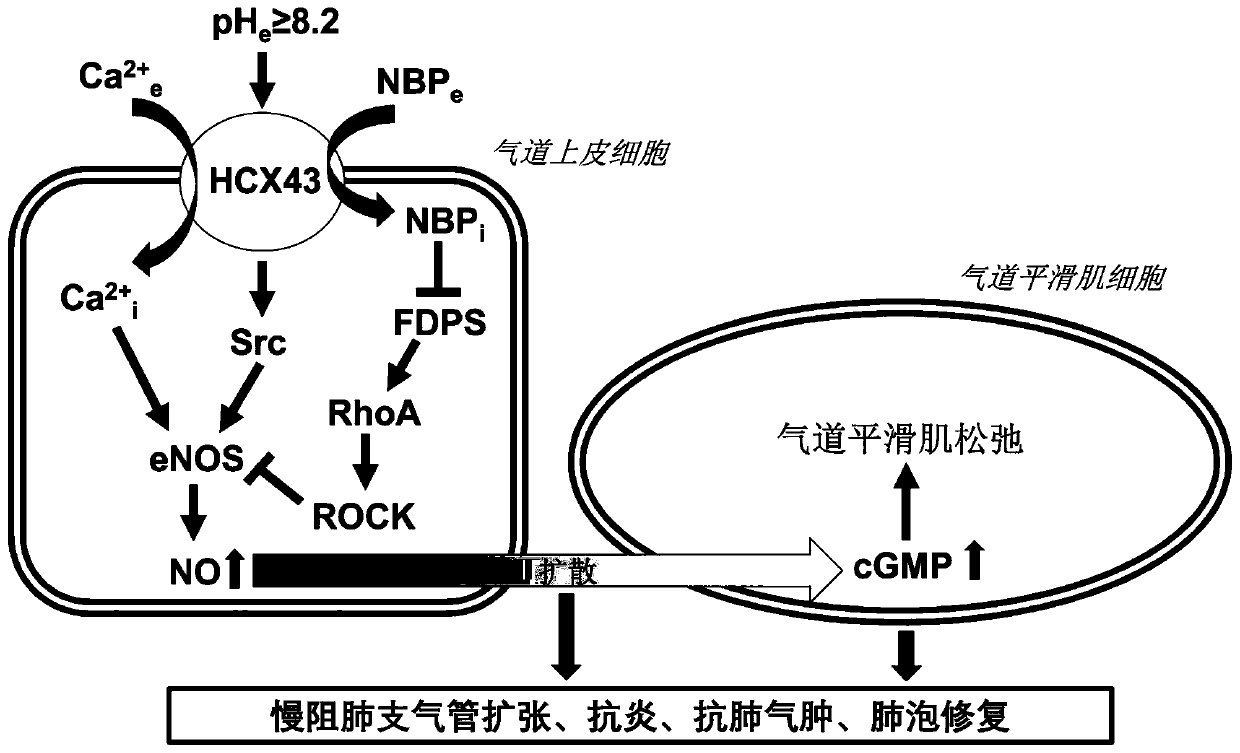
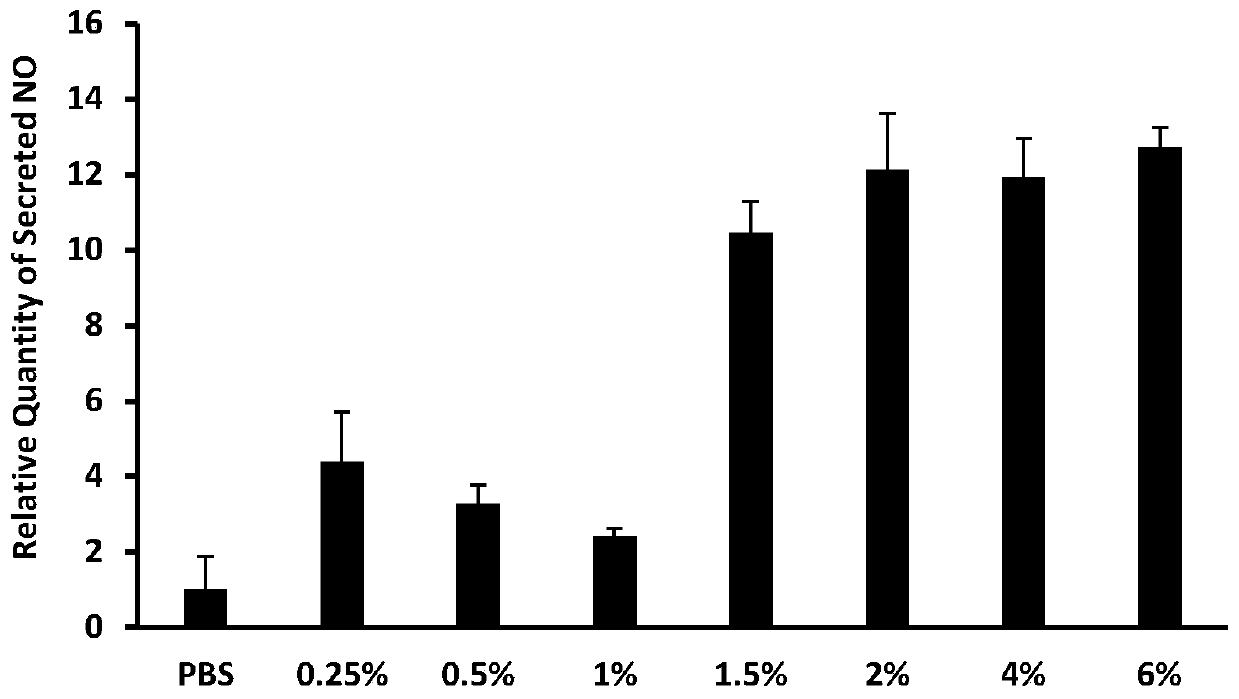
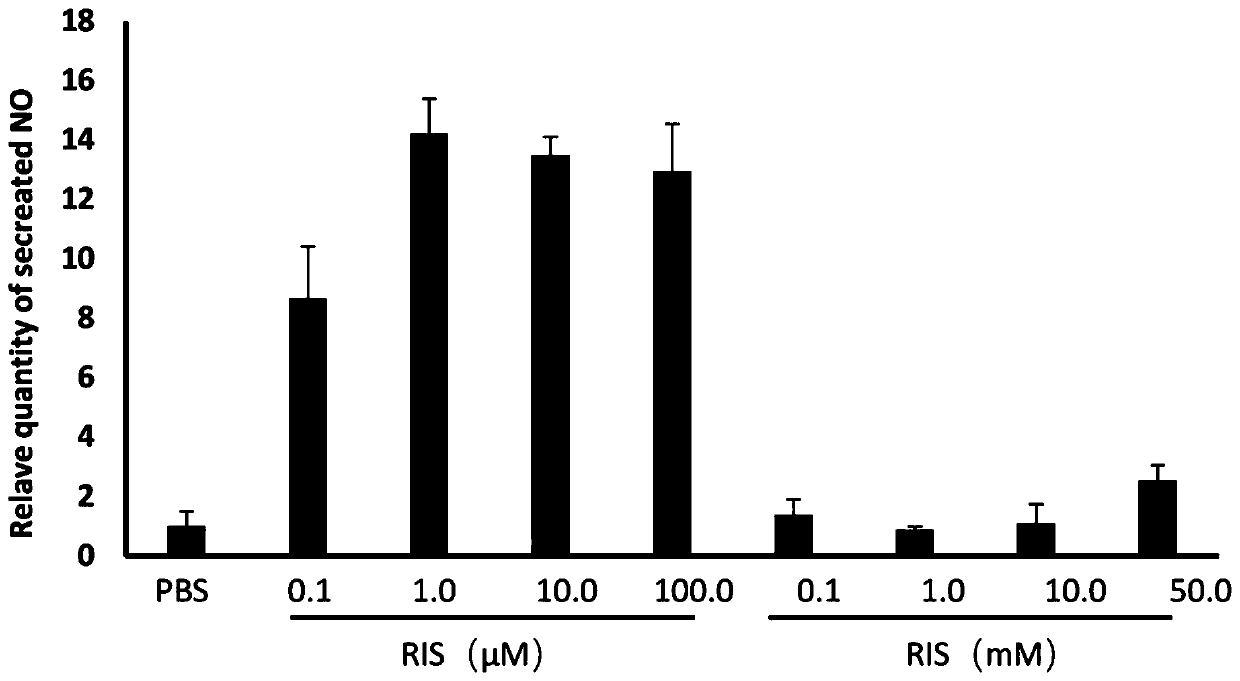
ActiveCN111053761BLow effective doseMeet clinical drug needsDispersion deliveryInorganic non-active ingredientsDrugs solutionFormulary
The invention discloses a bisphosphonic acid drug for inhalation, its preparation method and its application in chronic obstructive pulmonary disease. The formula of the drug solution includes: bisphosphonic acid drug, pH regulator, osmotic pressure regulator, pH The range is 8.2-8.9, and the osmotic pressure is isotonic or hypertonic; the preferred pH regulator and osmotic pressure regulator is bicarbonate, and the content of bisphosphonic acid drugs is 0.1μM-100μM in terms of extracellular application concentration, carbonic acid The content of the hydrogen salt is 1.5% (w / v)-4.0% (w / v); the drug dose developed by the invention is both effective and safe, and can meet the clinical medication needs of chronic obstructive pulmonary disease and other diseases.
Owner:HANGZHOU DC PHARM CO LTD
A kind of omeprazole nanoemulsion and preparation method thereof
ActiveCN103637986BHigh thermodynamic stabilityGood storage stabilityOrganic active ingredientsDigestive systemOral medicationIntramuscular injection
The invention provides an omeprazole nano emulsion and a preparation method thereof. The emulsion is respectively prepared from the following components by weight percent: 0.1-1% of omeprazole, 16-30% of surfactant, 0.15-20% of cosurfactant, 2-25% of oil and 34-50% of water for injection. The omeprazole nano emulsion is good in thermodynamic stability, good in storage stability, and free of layering after being placed for a long period of time, and can be subjected to oral medication and intramuscular injection; a stable emulsion state can be completely recovered by placement and sufficient oscillation after heating to 80 DEG C; especially, the omeprazole can be well prevented from being destroyed by gastric acid after a nano emulsion matrix is orally taken, and the first-pass effect of the liver can be overcome. Therefore, the omeprazole nano emulsion is simple in preparation method, and applicable to the requirements of large-scale industrial production.
Owner:TIANJIN ZHONGSHENG TIAOZHAN BIOTECH
Application of Fragrant Green Orchid Extract in the Preparation of Medicines for Lowering Uric Acid
ActiveCN109528819BRich clinical indicationsMeet clinical drug needsSkeletal disorderPlant ingredientsSerum uric acidBULK ACTIVE INGREDIENT
Owner:WUHAN JIANMIN ZHONGWEI PHARMA CO LTD
Diphosphonic acid drug for inhalation and preparation method thereof, and application of diphosphonic acid drug in chronic obstructive pulmonary diseases
ActiveCN111053761AGood bronchiectasisReduce airway resistanceDispersion deliveryInorganic non-active ingredientsBicarbonateDisease
The invention discloses a diphosphonic acid drug for inhalation and a preparation method thereof, and application of the diphosphonic acid drug in chronic obstructive pulmonary diseases. A formula ofa drug solution comprises the diphosphonic acid drug, a pH regulator and an osmotic pressure regulator, wherein a pH value is in a range of 8.2-8.9; osmotic pressure is isoosmotic or hyperosmotic pressure; preferably, the pH regulator and the osmotic pressure regulator are bicarbonate; and based on extracellular application concentration, the content of the diphosphonic acid drug is 0.1-100 [mu]M,and the content of the bicarbonate is 1.5% (w / v)-4.0% (w / v). A drug dose researched by the invention is effective and safe, and can meet the clinical medication requirements of diseases such as the chronic obstructive pulmonary diseases.
Owner:HANGZHOU DC PHARM CO LTD
Preparation method for blood-activation stasis-dissipation orifices-opening pain-relieving traditional Chinese medicine composition
InactiveCN102793744BThe production process is simple and easy to controlFast extractionAntipyreticAnalgesicsSalvia miltiorrhizaToad skin
A preparation method for a blood-activation stasis-dissipation orifices-opening pain-relieving traditional Chinese medicine composition includes the following steps: crushing salvia miltiorrhiza coarsely, extracting effective active ingredients by using a supercritical carbon dioxide fluid, precipitating for separation, drying under vacuum to get a dry extract, crushing the dry extract into a fine powder, crushing panax notoginseng, ginseng fibrous root and safflower into a fine powder by adopting a traditional Chinese medicine ultrafine pulverizer, grinding cultivated bezoar or in-vitro cultivated bezoar, cake of toad skin secretion and borneol into a fine powder, mixing the obtained three fine powders with a cornu bubali concentrated powder, carrying out granulation and screening with 14 mesh sieve, drying at 50-60 DEG C, finishing granules, filling into capsules, and packaging. By adopting the preparation method, the production process is controllable, extraction speed is fast, the product has no residual toxicity, the product is high in purity, the disadvantages of large extraction rate and lots of impurities in conventional water extraction method are overcome, and at the same time using cultivated bezoar or in-vitro cultivated bezoar can make up for the lack of a raw material of natural bezoar to meet the needs of clinical medication.
Owner:湖南恒伟药业股份有限公司
Vagina effervescent tablets containing callicarpa nudiflora and its preparation method
InactiveCN100463671CQuick releaseQuality improvementPill deliverySexual disorderEffervescent tabletIrritation
A vaginal effervescent tablet with high release speed and no irritation is prepared from achamydeous beautyberry leaf, effervescent agent and auxiliary. Its preparing process is also disclosed.
Owner:石药集团中诺药业(石家庄)有限公司
A kind of metoprolol succinate slow-release tablet and preparation method thereof
ActiveCN113925839BDissolution ConsistencySmooth releaseOrganic active ingredientsPill deliveryProlonged-release tabletSuccinic acid
The application provides a metoprolol succinate sustained-release tablet and its preparation method. First, the metoprolol succinate sustained-release granules are prepared by using a hot-melt process, and then the sustained-release granules are granulated with disintegrants and other excipients. 1. Preparation of compressed tablets. The metoprolol succinate sustained-release tablet provided by the present invention has the dissolution consistency before and after breaking apart when the patient follows the doctor's order to take it apart, and can avoid the sustained-release granules in the existing sustained-release tablet. The problem that the coating layer is easy to break during tablet compression can significantly simplify the production process, reduce the manufacturing cost of the unit preparation, and meet the clinical drug needs under the condition of my country's medical insurance fee control.
Owner:北京联嘉医药科技开发有限公司
Progesterone drug lipid microsphere injection and preparation method thereof
ActiveCN106074383BNon-irritatingImprove securityOrganic active ingredientsEmulsion deliveryLipid formationMicrosphere
The invention discloses a lipid microballoon injection solution for a progestational hormone drug and a preparation method thereof. The lipid microballoon injection solution for the progestational hormone drug comprises progestational hormone drug progesterone as a primary drug and pharmaceutically acceptable drug excipient, wherein the drug excipient comprises oil phase, emulsifier, osmotic pressure adjusting agent, stabilizer, pH value modifier and injection water. The lipid microballoon injection solution for the progestational hormone drug is stable in quality, free from injection irritation, high in safety, applicable to local intramuscular injection and intravenous injection, and beneficial to promotion of compliance of clinical medication of patient and enriches clinical medication selection.
Owner:WUHAN CONFORM PHARMA CO LTD
Qi benefiting and viscosity breaking particle and preparation method thereof
InactiveCN101537035AReduce viscosityGood thrombolytic effectAnthropod material medical ingredientsMetabolism disorderSide effectAdditive ingredient
The invention relates a qi benefiting and viscosity breaking particle and a preparation method thereof. The particle comprises accessory dextrin and also the ingredients of 50-65 percent of radix astragali, 20-30 percent of ligusticus wallichii franchet, and 10-20 percent of earthworm according to weight percentage. The preparation steps include: decocting the three crude drugs of radix astragali, ligusticus wallichii franchet and earthworm with water added for extraction for three times, wherein in the first time, adding water 10 times of the amount of the drugs, decocting for 1-2h, and adding water 8 times of the amount of the drugs and decocting for 0.5-2h in the second and third times; combining and filtering the decoction liquid, precipitating statically for 24-72h, and sucking supernatant fluid, conducting reduced pressure concentration till the extract with the specific weight of 1.18 is obtained; adding a proper amount of dextrin to make soft material which then passes through a 12-mesh sieve, drying, conducting size stabilization, making the material into finished product, packing and obtaining the qi benefiting and viscosity breaking particle. The particle has no toxic or side effect and can reduce blood viscosity while lowering lipid.
Owner:ANHUI RONGYUANTANG BIO TECH
Ubenimex capsule medicament composition and preparation method thereof
ActiveCN103610663BOmit to useImprove securityOrganic active ingredientsPharmaceutical non-active ingredientsMedicineMedical prescription
The invention provides an ubenimex capsule medicament composition and a preparation method thereof. Compared with the prior art, the prescription and process for preparing the ubenimex capsule, which are disclosed by the invention has the advantages that auxiliary materials, such as a binder and a disintegrating agent in the prescription are not used, the prescription is simple, and the safety is greatly improved. The preparation technology is easy to operate, and very suitable for large-scale industrial production. The prepared product can be completely dissolved out within a short period of time, and good in dissolving effect, relatively low in content of related substances, and stable in quality, and has a remarkable progress in comparison with the prior art.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com