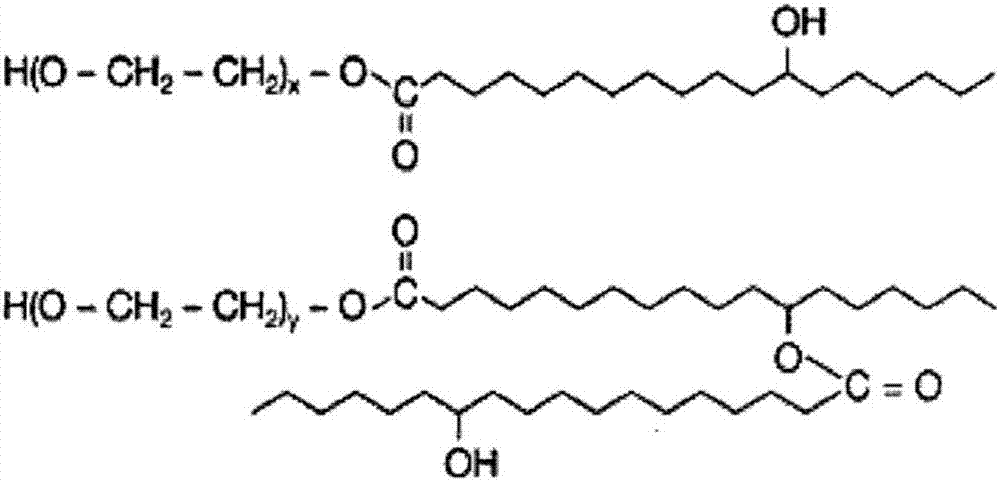
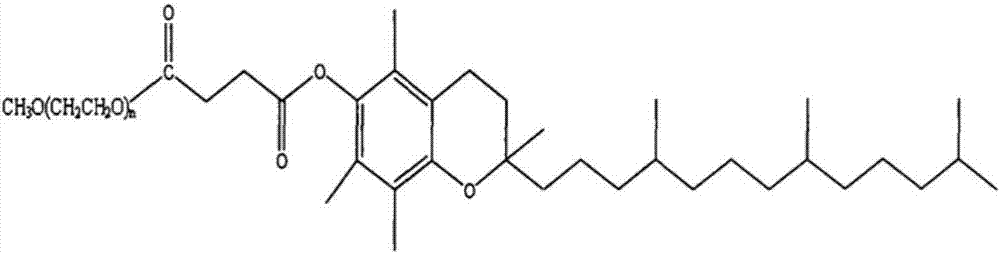
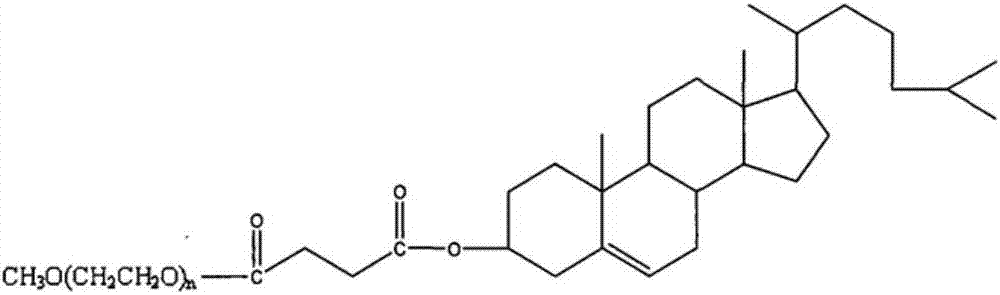
Butyphthalide injection and preparation method thereof
A technology of butylphthalide and injection, applied in the field of medicine, can solve the problems of unstable quality, inability to meet clinical drug requirements, difficult operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Embodiment 1 Butylphthalide injection (water injection): contains butylphthalide, soybean lecithin, and water for injection, and the prescription dosage of the three is shown in the following table:
[0114] Based on 100mL:
[0115] Material name
[0116] Preparation process: Dissolve soybean lecithin in about 90ml of water for injection at 40-60°C, slowly add butylphthalide dropwise, stir well, break up with a high-pressure homogenizer, control the particle size to be less than 50 nanometers, obtain a clear liquid, replenish water To 100ml, add 0.1% activated carbon and stir for 30 minutes, filter to remove charcoal, sterilize, pack in 10ml each, and sterilize.
Embodiment 2
[0117] Embodiment 2 Butylphthalide injection (water injection): contains butylphthalide, polyethylene glycol-12 hydroxystearate and water for injection, and the prescription dosage of the three is shown in the following table:
[0118] Material name
[0119] Preparation process: Dissolve polyethylene glycol-12 hydroxystearate in about 90ml of water for injection at 20-30°C, slowly add butylphthalide dropwise, stir well, no oil droplets appear on the liquid surface, and obtain a clear liquid, replenish water To 100ml, add 0.2% activated carbon and stir for 30 minutes, filter to remove charcoal, sterilize, pack in 10ml each, and sterilize.
Embodiment 3
[0120] Example 3 Butylphthalide injection (freeze-dried powder injection): contains butylphthalide, polyethylene glycol-12 hydroxystearate and water for injection, and the prescription dosage of the three is shown in the following table:
[0121] Material name
[0122] Preparation process: Dissolve polyethylene glycol-12 hydroxystearate in about 90ml of water for injection at 20-30°C, slowly add butylphthalide dropwise, stir well, until no oil drops appear on the liquid surface, add mannitol and glucose to dissolve , to obtain a clear liquid, add water to 100ml, add 0.1% activated carbon and stir for 30 minutes, filter to remove carbon, sterilize, subpackage, each 5ml, freeze-dried.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com